Professional Documents
Culture Documents
Thermodynamics of Solidification 2: 9.1 Entropy
Thermodynamics of Solidification 2: 9.1 Entropy
Uploaded by
Anonymous T02GVGzBCopyright:
Available Formats
You might also like
- Fluid Mechanics ReportDocument13 pagesFluid Mechanics ReportChan Jiun Haur100% (3)
- Thermodynamics: 2.1 The First Law of ThermodynamicsDocument7 pagesThermodynamics: 2.1 The First Law of ThermodynamicsAnonymous T02GVGzBNo ratings yet
- Solution Set 7Document15 pagesSolution Set 7Jean AraúzNo ratings yet
- Lecture 7 Quasichemical Solution ModelsDocument7 pagesLecture 7 Quasichemical Solution ModelsakshukNo ratings yet
- Graphite Oxide (GO) Decomposition Kinetics: Electronic Supplementary InformationDocument7 pagesGraphite Oxide (GO) Decomposition Kinetics: Electronic Supplementary Informationbai tap hoa vo coNo ratings yet
- CBE3508 Sp21 FinalDocument6 pagesCBE3508 Sp21 Finalsasuke uchihaNo ratings yet
- Saponification Rate Constant of An EsterDocument3 pagesSaponification Rate Constant of An EsterVikasH's Digital LibraryNo ratings yet
- MP4-3 .KCJDLKNKLDNCLDKSJDocument12 pagesMP4-3 .KCJDLKNKLDNCLDKSJAmanpreet Singh ParhwaggaNo ratings yet
- HW 3Document3 pagesHW 3JungHyunParkNo ratings yet
- ch8 ProbsDocument4 pagesch8 ProbsEkrem GüldesteNo ratings yet
- Rate Equations of Solid State Reactions. Euclidean and Fractal ModelsDocument4 pagesRate Equations of Solid State Reactions. Euclidean and Fractal ModelsNadyaZulfaniNo ratings yet
- MIT10 626S11 Lec07Document7 pagesMIT10 626S11 Lec07Naveen NaviNo ratings yet
- 3.012 PS 7 Thermo Solutions 3.012 Fall 2003Document11 pages3.012 PS 7 Thermo Solutions 3.012 Fall 2003Sanjeev SahuNo ratings yet
- Chapter 13Document38 pagesChapter 13Lucy BrownNo ratings yet
- Lippincot 1955Document2 pagesLippincot 1955Enrique PugaNo ratings yet
- Thermodynamics of Metallic SolutionDocument34 pagesThermodynamics of Metallic SolutionJaswant Singh ChauhanNo ratings yet
- Huckel Molecular Orbital Theory: Sapan Kumar Jain Assistant Professor, JMIDocument45 pagesHuckel Molecular Orbital Theory: Sapan Kumar Jain Assistant Professor, JMIMayank GuptNo ratings yet
- Spontaneous Generation of Photons in Transmission of Quantum Fields in PT-symmetric Optical SystemsDocument4 pagesSpontaneous Generation of Photons in Transmission of Quantum Fields in PT-symmetric Optical SystemsKenan QuNo ratings yet
- Phsv03i02p0186 PDFDocument7 pagesPhsv03i02p0186 PDFphysicsjournalNo ratings yet
- Radiative Capture of Protons by DeuteronsDocument9 pagesRadiative Capture of Protons by DeuteronsWolfgang SchadowNo ratings yet
- Chem350 Notes-8-20111Document14 pagesChem350 Notes-8-20111comeondudeffNo ratings yet
- Heterogeneous Nucleation Wetting AngleDocument7 pagesHeterogeneous Nucleation Wetting Anglemarthin_nielsenNo ratings yet
- Phsv02i02p0126 PDFDocument6 pagesPhsv02i02p0126 PDFphysicsjournalNo ratings yet
- Kinetic Theory of Gases: Addendum To Chapter 6 Che 505Document15 pagesKinetic Theory of Gases: Addendum To Chapter 6 Che 505Saravana ChandranNo ratings yet
- Four-nucleon: α-type correlations and proton-neutron pairing away from the N = Z lineDocument4 pagesFour-nucleon: α-type correlations and proton-neutron pairing away from the N = Z linekibur1No ratings yet
- How To FixDocument21 pagesHow To FixefgsdgergNo ratings yet
- MIT8 333F13 ExamReviewFinlDocument18 pagesMIT8 333F13 ExamReviewFinlHenry De AriesNo ratings yet
- Pathria Solution1Document4 pagesPathria Solution1Mario Mede RiteNo ratings yet
- Nonequilibrium Contributions To The Rate of Reaction. I. Perturbation of The Velocity Distribution FunctionDocument18 pagesNonequilibrium Contributions To The Rate of Reaction. I. Perturbation of The Velocity Distribution FunctionCarolina RibeiroNo ratings yet
- Interaction of Light and Matter: 6.1 The Two-Level ModelDocument21 pagesInteraction of Light and Matter: 6.1 The Two-Level ModelefgsdgergNo ratings yet
- First-Order Quantum Correction To The Ground-State Energy Density of Two-Dimensional Hard-Sphere Bose AtomsDocument9 pagesFirst-Order Quantum Correction To The Ground-State Energy Density of Two-Dimensional Hard-Sphere Bose AtomsSarvraj Singh RtNo ratings yet
- Convergence of The Self-Dual Ginzburg-Landau Gradient Flow: 1 Introduction and Statement of ResultsDocument15 pagesConvergence of The Self-Dual Ginzburg-Landau Gradient Flow: 1 Introduction and Statement of ResultsGonzalo Hernán Barría PérezNo ratings yet
- Solid State SatyabhamaDocument106 pagesSolid State Satyabhamawname384No ratings yet
- Discretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsDocument12 pagesDiscretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsPassammNo ratings yet
- Exam 2017 SoutionDocument15 pagesExam 2017 SoutionSushil AcharyaNo ratings yet
- KJM4120 Ch3 Defect EquilibriaDocument31 pagesKJM4120 Ch3 Defect EquilibriaMohamed Elsherif100% (1)
- "Diffusive" Heat and Mass Transfer: NPTEL, IIT Kharagpur, Prof. Saikat Chakraborty, Department of Chemical EngineeringDocument8 pages"Diffusive" Heat and Mass Transfer: NPTEL, IIT Kharagpur, Prof. Saikat Chakraborty, Department of Chemical EngineeringShanmukShannuNo ratings yet
- Jobs MethodDocument8 pagesJobs MethodJayli Caren RiveraNo ratings yet
- Pelton Bale1986 ModifiedInteractionParameterFDocument5 pagesPelton Bale1986 ModifiedInteractionParameterFmanish pandeNo ratings yet
- Kinetics: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Document9 pagesKinetics: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Anonymous T02GVGzBNo ratings yet
- Small Excitonic Complexes in A Disk-Shaped Quantum Dot: Ricardo Perez, Augusto GonzalezDocument8 pagesSmall Excitonic Complexes in A Disk-Shaped Quantum Dot: Ricardo Perez, Augusto GonzalezJairofisico JaramilloNo ratings yet
- Diq1m w8jrhDocument10 pagesDiq1m w8jrhMohsin MuhammadNo ratings yet
- Introduction To Electronic Structure Theory: X X X X XDocument10 pagesIntroduction To Electronic Structure Theory: X X X X XAnonymous UrVkcdNo ratings yet
- The Thermochemistry of A Reacting Mixture of Elastic Materials With DiffusionDocument31 pagesThe Thermochemistry of A Reacting Mixture of Elastic Materials With DiffusionSantiago Peña ClavijoNo ratings yet
- Statistical Physics. Solutions Sheet 10.: Hi, Ji I JDocument8 pagesStatistical Physics. Solutions Sheet 10.: Hi, Ji I JJuan MondáNo ratings yet
- Chapter 16 - Section B - Non-Numerical SolutionsDocument3 pagesChapter 16 - Section B - Non-Numerical SolutionsHiLda HayatiiNo ratings yet
- Problem Set 6 SolutionDocument4 pagesProblem Set 6 SolutionRod De GuzmanNo ratings yet
- Mid Exam SolutionsDocument3 pagesMid Exam SolutionsTime TravellerNo ratings yet
- Generalized Chemical Reactivity of Curved Surfaces: Carbon NanotubesDocument5 pagesGeneralized Chemical Reactivity of Curved Surfaces: Carbon NanotubesemediageNo ratings yet
- Thermodynamics Lecture 2Document18 pagesThermodynamics Lecture 2Rashaq AL-HeetyNo ratings yet
- Spin Orbit InteractionDocument6 pagesSpin Orbit InteractionJoanofRockNo ratings yet
- Appendix BDocument4 pagesAppendix BguiguiNo ratings yet
- Exam 2: P Is A Universal Function For All TheDocument10 pagesExam 2: P Is A Universal Function For All TheUday RameshNo ratings yet
- 2005 Ms LiquidDocument6 pages2005 Ms LiquidkhabiranNo ratings yet
- HW Pso201 2020Document6 pagesHW Pso201 2020Shashi KumarNo ratings yet
- You May Not Start To Read The Questions Printed On The Subsequent Pages of This Question Paper Until Instructed That You May Do So by The InvigilatorDocument3 pagesYou May Not Start To Read The Questions Printed On The Subsequent Pages of This Question Paper Until Instructed That You May Do So by The InvigilatorSpringOrchidNo ratings yet
- c1Document23 pagesc1akashNo ratings yet
- MIT8 333F13 Pset6Document10 pagesMIT8 333F13 Pset6Henry De AriesNo ratings yet
- MIT8 333F13 ExamReview3Document10 pagesMIT8 333F13 ExamReview3Henry De AriesNo ratings yet
- Reviews in Computational ChemistryFrom EverandReviews in Computational ChemistryAbby L. ParrillNo ratings yet
- Reactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFrom EverandReactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFranz-Josef SchmittNo ratings yet
- Lecture 12 BDocument1 pageLecture 12 BAnonymous T02GVGzBNo ratings yet
- Burdukova Et Al. 2006. Effect of CMC and PH On The Rheology of Suspensions of Isotropic and Anisotropic MineralsDocument15 pagesBurdukova Et Al. 2006. Effect of CMC and PH On The Rheology of Suspensions of Isotropic and Anisotropic MineralsAnonymous T02GVGzBNo ratings yet
- Copper Flotation: Metals and Minerals IndustryDocument2 pagesCopper Flotation: Metals and Minerals IndustryAnonymous T02GVGzBNo ratings yet
- Lecture13 14Document2 pagesLecture13 14Anonymous T02GVGzBNo ratings yet
- Lecture 12 ADocument1 pageLecture 12 AAnonymous T02GVGzBNo ratings yet
- Lecture 12Document1 pageLecture 12Anonymous T02GVGzBNo ratings yet
- Lecture 7Document2 pagesLecture 7Anonymous T02GVGzBNo ratings yet
- Chapter Outline: Failure Fracture: How Do Materials Break?Document9 pagesChapter Outline: Failure Fracture: How Do Materials Break?Anonymous T02GVGzBNo ratings yet
- Chapter 14 CDocument8 pagesChapter 14 CAnonymous T02GVGzBNo ratings yet
- Exploration Analysis: Evaluation of Geochemical Data C. JDocument2 pagesExploration Analysis: Evaluation of Geochemical Data C. JAnonymous T02GVGzBNo ratings yet
- GJmatDocument2 pagesGJmatAnonymous T02GVGzBNo ratings yet
- Kinetics of Solidification 3: 13.1 Solid-Liquid Interface StructureDocument7 pagesKinetics of Solidification 3: 13.1 Solid-Liquid Interface StructureAnonymous T02GVGzBNo ratings yet
- Viscosity and Thermodynamics 2004Document14 pagesViscosity and Thermodynamics 2004Anonymous T02GVGzBNo ratings yet
- Heat Transfer: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Document8 pagesHeat Transfer: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Anonymous T02GVGzBNo ratings yet
- Lecture 11Document18 pagesLecture 11Anonymous T02GVGzBNo ratings yet
- Case Study: Flow Models: 16.1 Lattice-Gas Automata ModelDocument9 pagesCase Study: Flow Models: 16.1 Lattice-Gas Automata ModelAnonymous T02GVGzBNo ratings yet
- Solidification Processing: Lecture 10: Thermodynamics 3Document17 pagesSolidification Processing: Lecture 10: Thermodynamics 3Anonymous T02GVGzBNo ratings yet
- Fluid Flow in Solidification 1Document7 pagesFluid Flow in Solidification 1Anonymous T02GVGzBNo ratings yet
- Phase Field Models: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Document10 pagesPhase Field Models: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Anonymous T02GVGzBNo ratings yet
- ENGG1500 Module 8 Tute SolutionsDocument15 pagesENGG1500 Module 8 Tute SolutionsKevin MalarkeyNo ratings yet
- Chemical Potential and Gibbs Distribution: Anders Malthe-SørenssenDocument24 pagesChemical Potential and Gibbs Distribution: Anders Malthe-SørenssenNingsihNo ratings yet
- Quiz Fluid LabDocument3 pagesQuiz Fluid LabMuhammad BilalNo ratings yet
- Quiz 2 PDFDocument7 pagesQuiz 2 PDFRuth Montebon0% (1)
- 〈911〉 VISCOSITY-CAPILLARY METHODSDocument5 pages〈911〉 VISCOSITY-CAPILLARY METHODSVieno Gino CruzNo ratings yet
- Line Sizing1Document20 pagesLine Sizing1Srihari KodimelaNo ratings yet
- Mind Map Physical ChemDocument1 pageMind Map Physical ChemhusnaNo ratings yet
- Discussion 3Document2 pagesDiscussion 3Vinod KrishnanNo ratings yet
- Final Exam 2015Document6 pagesFinal Exam 2015Kiran JojiNo ratings yet
- Behavior of GasesDocument13 pagesBehavior of GasesSharalyn P. SiaNo ratings yet
- Flash Point TutorialDocument8 pagesFlash Point TutorialS. GreenNo ratings yet
- 13 NumberDocument1 page13 NumberDonNo ratings yet
- U-2-Turbulent Flow in Pipes and Channels PDFDocument23 pagesU-2-Turbulent Flow in Pipes and Channels PDFLaxmi PrasannaNo ratings yet
- Constantes Físicas FundamentalesDocument1 pageConstantes Físicas FundamentalesAlejandro GafNo ratings yet
- Orifice Meter: Observation and Calculation DataDocument10 pagesOrifice Meter: Observation and Calculation DataabhishekkandoiNo ratings yet
- Pre Heater Design CalculationsDocument4 pagesPre Heater Design CalculationsFahad KhokharNo ratings yet
- Distillation IndtroductionDocument35 pagesDistillation IndtroductionRidha Cessar GozaliNo ratings yet
- Sci3 Q1mod5 Changes in Materials Brenda Quilladas Bgo v1Document25 pagesSci3 Q1mod5 Changes in Materials Brenda Quilladas Bgo v1MArkNo ratings yet
- Practise Questions 2019Document8 pagesPractise Questions 2019Sehar IshtiaqNo ratings yet
- Study - Guide - States - of - Matter - Student - Editable c12Document9 pagesStudy - Guide - States - of - Matter - Student - Editable c12Zoe MarokoNo ratings yet
- Chemical Engineering Thermodynamics: Volumetric Properties of Pure FluidDocument20 pagesChemical Engineering Thermodynamics: Volumetric Properties of Pure FluidThurgah VshinyNo ratings yet
- Adiabatic and Isothermal ProcessesDocument2 pagesAdiabatic and Isothermal ProcessesphydotsiNo ratings yet
- Soal Fisika AnswerDocument3 pagesSoal Fisika AnswerHimawan EkaNo ratings yet
- 8.334: StatMech II Problem Set #2Document2 pages8.334: StatMech II Problem Set #2bidsitlovNo ratings yet
- Pump Sizing ParametersDocument1 pagePump Sizing ParametersBramJanssen76No ratings yet
- Lecture 1 Choke PerformanceDocument45 pagesLecture 1 Choke PerformancearvNo ratings yet
- FORM 5 Chapter 2 PressureDocument6 pagesFORM 5 Chapter 2 PressureKishenraj RajendranNo ratings yet
- Rexroth HABDocument20 pagesRexroth HABeleceng1979No ratings yet
- Lampiran A Data Litelatur: A.1 Densitas Air Pada Berbagai Temperatur Tabel A.1 Densitas Air Pada Berbagai TemperaturDocument2 pagesLampiran A Data Litelatur: A.1 Densitas Air Pada Berbagai Temperatur Tabel A.1 Densitas Air Pada Berbagai Temperaturjoshua2alexander-2No ratings yet
Thermodynamics of Solidification 2: 9.1 Entropy
Thermodynamics of Solidification 2: 9.1 Entropy
Uploaded by
Anonymous T02GVGzBOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermodynamics of Solidification 2: 9.1 Entropy
Thermodynamics of Solidification 2: 9.1 Entropy
Uploaded by
Anonymous T02GVGzBCopyright:
Available Formats
Graduate Institute of Ferrous Technology, POSTECH
Rongshan Qin (R. S. Qin)
9. Thermodynamics of solidification 2
9.1 Entropy
Entropy is defined as T q S / = where q is the amount of heat absorbed or
evolved in an isothermal and reversible process in which the system goes from
one state to another, and T is the absolute temperature at which the process is
occurring. 0 =
S when system passes through a series of reversible,
equilibrium and isothermal steps and arriving back to the initial state.
This thermodynamic definition of entropy is only valid for a system in equilibrium
(because temperature is defined only for a system in equilibrium), while statistical
definition of entropy applies to any system. Thus the statistical definition is
usually considered fundamental definition of entropy.
Kelvin and Boltzmann considered that the entropy is related to the probability of a
system by
W k S ln = (8.1)
where k=1.3806502x10
-23
Joule/Kelvin is Boltzmann constant and W is the
possibility (a measure of disorder). Eq. (8.1) means that the maximum entropy is
associated with the greatest disorder. When the temperature of the system
approached absolute zero the entropy of the system would approach zero.
The 3
rd
law of thermodynamics: The entropy of a condensed system would be
zero at a temperature of absolute zero.
9.2 Entropy of solution at ideal mixing
Alloy is solution which is made up of components. There is solubility between the
various components. For an alloy in which there is no repulsive and attractive
interactions between various components, atoms (or molecules) can be mixed up
Graduate Institute of Ferrous Technology, POSTECH
Rongshan Qin (R. S. Qin)
ideally. Suppose that n atom or molecule of component A is mixed up with N-n
number of component B, the possibility is given by
)! ( !
!
n N n
N
W
= (8.2)
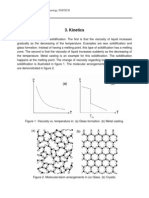
The possibility is demonstrated in figure 1 for a binary crystal (N=9, n=1).
Figure 1. Possibility
Inserting eq. (8.2) into eq. (8.1) gives the entropy
|
|
\
|
=
)! ( !
!
ln
n N n
N
k S (8.3)
Using Stirling approximation N N N N = ln ) ! ln( eq (8.3) becomes
[ ]
(
\
|
+
|
\
|
=
=
N
n N
N
n N
N
n
N
n
Nk
n N n N n n N N k S
ln ln
) ln( ) ( ln ln
(8.4)
Let N n x
A
/ = and ( ) N n N x
B
/ = ,
A
x and
B
x are molar fraction of components A
and B in the solution. The molar entropy in ideal solution is
( )
B B A A
x x x x R S ln ln + = (8.5)
If there are no repulsive or attractive interactions between atoms A and B the
solution is called ideal and the Gibbs energy of mixing is given by
Graduate Institute of Ferrous Technology, POSTECH
Rongshan Qin (R. S. Qin)
( )
B B A A
ideal
mix
x x x x RT G ln ln + = (8.6)
Generally, it takes the following format for multicomponent solution
=
i
i i
ideal
mix
x x RT G ln (8.7)
where
i
x is the molar fraction of component i .
9.3 Entropy of solution at non-ideal mixing
Normally there are interactions between different atoms or molecules of various
components. The excess mixing energy,
xs
mix
G , should be considered. The
simplest way to consider is via the regular solution model where
=
B A
xe
mix
x x G (8.8)
where is the regular solution interaction energy parameter and is related to the
energy of bonds between A and B atoms or molecules. >0 corresponds to
repulsive interaction, such as Cu-Ag. <0 corresponds to the attractive
interaction, such as Fe-Ni. The total mixing Gibbs energy of binary alloy is given
by combination of eqs. (8.6) and (8.8).
( ) + + =
B A B B A A mix
x x x x x x RT G ln ln (8.9)
(a) (b)
-0.9
-0.8
-0.7
-0.6
-0.5
-0.4
-0.3
-0.2
-0.1
0
0 0.2 0.4 0.6 0.8 1
MOLAR FRACTION B
G
I
B
B
S
M
I
X
I
N
G
E
N
E
R
G
Y
G-ideal
G-xe
G-mix
-0.4
-0.3
-0.2
-0.1
0
0.1
0.2
0.3
0.4
0.5
0.6
0 0.2 0.4 0.6 0.8 1
MOLAR FRACTION B
G
I
B
B
S
M
I
X
I
N
G
E
N
E
R
G
Y
G-ideal
G-xe
G-mix
Figure 2. Gibbs mixing energy at RT=1 (a) =-2 and (b) =2
Graduate Institute of Ferrous Technology, POSTECH
Rongshan Qin (R. S. Qin)
Eq. (8.9) is plotted with parameters of RT=1 (which means that the energy unit is
in RT ), =-2 (figure 2a) and =2 (figure 2b). It can be found from figure 2b that
the two-phase structure will be formed to reduce the system free energy. One is
A-rich and another is B-rich phases.
In general,
xs
mix
G is expressed by a expression of following
[ ]
=
+
+ =
+ + + =
n
i
n
i j
j i ij j i ij ij j i
xe
mix
x x x x x x G
1
1
1
2 2 1 0
... ) ( ) ( (8.10)
9.4 Gibbs energy of solution
To describe the Gibbs energy of solution, the reference states must be added to
the mixing Gibbs energy. The reference Gibbs energy is
=
i
i i ref
G x G (8.11)
where
i
G is the Gibbs energy of the phase at pure i . The Gibbs energy of
solution, therefore, is expressed at
[ ]
=
+
+ =
+ + + + + =
n
i
n
i j
j i ij j i ij ij j i
i
i i
i
i i
x x x x x x x x RT G x G
1
1
1
2 2 1 0
... ) ( ) ( ln (8.12)
-1
-0.8
-0.6
-0.4
-0.2
0
0.2
0.4
0 0.2 0.4 0.6 0.8 1
MOLAR FRACTION B
G
I
B
B
S
M
I
X
I
N
G
E
N
E
R
G
Y
G-ideal
G-xe
G-mix
G
Figure 3. Gibbs energy ( 3 . 0 05 . 0 = =
b A
G G , other parameters are same as in figure 2a)
Graduate Institute of Ferrous Technology, POSTECH
Rongshan Qin (R. S. Qin)
Equation (8.12) is plotted for a binary alloy with following parameters
RT=1
G
A
=0.05
G
B
=0.3
AB
0
=-2
AB
1
=
AB
2
==0
The result is the blue line in figure 3.
9.5 Solidification of binary alloy
Alloy solidification should follow the routine that free energy goes to minimum. In
equilibrium, the solute concentration in solid should be
E
S
x and in liquid should be
E
L
x . The free energy minimization is demonstrated in figure 4. The chemical
potentials of components A and B are defined as
B
x P T
A
A
x
G
, ,
|
|
\
|
= and
A
x P T
B
B
x
G
, ,
|
|
\
|
= (8.13)
At equilibrium, the free energy achieves minimum and the chemical potentials of
all components are equivalent. Here
B A
=
Figure 4. Free energy minimization during solidification
Solid
Liquid
x
0
x
S
E
x
L
E
Graduate Institute of Ferrous Technology, POSTECH
Rongshan Qin (R. S. Qin)
E
L
E
S
x
x
k = is called equilibrium partition ratio. The solute concentration profile in
steady state equilibrium solidification is illustrated in Figure 5. The method of
calculation for the solution distribution profile has been given in lecture 7.
Figure 5. Solute concentration distribution in binary solidification
In non-equilibrium casting such as rapid quench, free energy minimization is
restricted by kinetics and metastable solid forms. For example the metal glasses.
REFERENCES
1. M.C. Flemings, Solidification processing, 1974.
2. N. Saunders and A.P. Miodownik, CALPHAD, 1998.
x
S
E
x
L
E
K<1
x
S
E
x
L
E
K>1
You might also like
- Fluid Mechanics ReportDocument13 pagesFluid Mechanics ReportChan Jiun Haur100% (3)
- Thermodynamics: 2.1 The First Law of ThermodynamicsDocument7 pagesThermodynamics: 2.1 The First Law of ThermodynamicsAnonymous T02GVGzBNo ratings yet
- Solution Set 7Document15 pagesSolution Set 7Jean AraúzNo ratings yet
- Lecture 7 Quasichemical Solution ModelsDocument7 pagesLecture 7 Quasichemical Solution ModelsakshukNo ratings yet
- Graphite Oxide (GO) Decomposition Kinetics: Electronic Supplementary InformationDocument7 pagesGraphite Oxide (GO) Decomposition Kinetics: Electronic Supplementary Informationbai tap hoa vo coNo ratings yet
- CBE3508 Sp21 FinalDocument6 pagesCBE3508 Sp21 Finalsasuke uchihaNo ratings yet
- Saponification Rate Constant of An EsterDocument3 pagesSaponification Rate Constant of An EsterVikasH's Digital LibraryNo ratings yet
- MP4-3 .KCJDLKNKLDNCLDKSJDocument12 pagesMP4-3 .KCJDLKNKLDNCLDKSJAmanpreet Singh ParhwaggaNo ratings yet
- HW 3Document3 pagesHW 3JungHyunParkNo ratings yet
- ch8 ProbsDocument4 pagesch8 ProbsEkrem GüldesteNo ratings yet
- Rate Equations of Solid State Reactions. Euclidean and Fractal ModelsDocument4 pagesRate Equations of Solid State Reactions. Euclidean and Fractal ModelsNadyaZulfaniNo ratings yet
- MIT10 626S11 Lec07Document7 pagesMIT10 626S11 Lec07Naveen NaviNo ratings yet
- 3.012 PS 7 Thermo Solutions 3.012 Fall 2003Document11 pages3.012 PS 7 Thermo Solutions 3.012 Fall 2003Sanjeev SahuNo ratings yet
- Chapter 13Document38 pagesChapter 13Lucy BrownNo ratings yet
- Lippincot 1955Document2 pagesLippincot 1955Enrique PugaNo ratings yet
- Thermodynamics of Metallic SolutionDocument34 pagesThermodynamics of Metallic SolutionJaswant Singh ChauhanNo ratings yet
- Huckel Molecular Orbital Theory: Sapan Kumar Jain Assistant Professor, JMIDocument45 pagesHuckel Molecular Orbital Theory: Sapan Kumar Jain Assistant Professor, JMIMayank GuptNo ratings yet
- Spontaneous Generation of Photons in Transmission of Quantum Fields in PT-symmetric Optical SystemsDocument4 pagesSpontaneous Generation of Photons in Transmission of Quantum Fields in PT-symmetric Optical SystemsKenan QuNo ratings yet
- Phsv03i02p0186 PDFDocument7 pagesPhsv03i02p0186 PDFphysicsjournalNo ratings yet
- Radiative Capture of Protons by DeuteronsDocument9 pagesRadiative Capture of Protons by DeuteronsWolfgang SchadowNo ratings yet
- Chem350 Notes-8-20111Document14 pagesChem350 Notes-8-20111comeondudeffNo ratings yet
- Heterogeneous Nucleation Wetting AngleDocument7 pagesHeterogeneous Nucleation Wetting Anglemarthin_nielsenNo ratings yet
- Phsv02i02p0126 PDFDocument6 pagesPhsv02i02p0126 PDFphysicsjournalNo ratings yet
- Kinetic Theory of Gases: Addendum To Chapter 6 Che 505Document15 pagesKinetic Theory of Gases: Addendum To Chapter 6 Che 505Saravana ChandranNo ratings yet
- Four-nucleon: α-type correlations and proton-neutron pairing away from the N = Z lineDocument4 pagesFour-nucleon: α-type correlations and proton-neutron pairing away from the N = Z linekibur1No ratings yet
- How To FixDocument21 pagesHow To FixefgsdgergNo ratings yet
- MIT8 333F13 ExamReviewFinlDocument18 pagesMIT8 333F13 ExamReviewFinlHenry De AriesNo ratings yet
- Pathria Solution1Document4 pagesPathria Solution1Mario Mede RiteNo ratings yet
- Nonequilibrium Contributions To The Rate of Reaction. I. Perturbation of The Velocity Distribution FunctionDocument18 pagesNonequilibrium Contributions To The Rate of Reaction. I. Perturbation of The Velocity Distribution FunctionCarolina RibeiroNo ratings yet
- Interaction of Light and Matter: 6.1 The Two-Level ModelDocument21 pagesInteraction of Light and Matter: 6.1 The Two-Level ModelefgsdgergNo ratings yet
- First-Order Quantum Correction To The Ground-State Energy Density of Two-Dimensional Hard-Sphere Bose AtomsDocument9 pagesFirst-Order Quantum Correction To The Ground-State Energy Density of Two-Dimensional Hard-Sphere Bose AtomsSarvraj Singh RtNo ratings yet
- Convergence of The Self-Dual Ginzburg-Landau Gradient Flow: 1 Introduction and Statement of ResultsDocument15 pagesConvergence of The Self-Dual Ginzburg-Landau Gradient Flow: 1 Introduction and Statement of ResultsGonzalo Hernán Barría PérezNo ratings yet
- Solid State SatyabhamaDocument106 pagesSolid State Satyabhamawname384No ratings yet
- Discretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsDocument12 pagesDiscretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsPassammNo ratings yet
- Exam 2017 SoutionDocument15 pagesExam 2017 SoutionSushil AcharyaNo ratings yet
- KJM4120 Ch3 Defect EquilibriaDocument31 pagesKJM4120 Ch3 Defect EquilibriaMohamed Elsherif100% (1)
- "Diffusive" Heat and Mass Transfer: NPTEL, IIT Kharagpur, Prof. Saikat Chakraborty, Department of Chemical EngineeringDocument8 pages"Diffusive" Heat and Mass Transfer: NPTEL, IIT Kharagpur, Prof. Saikat Chakraborty, Department of Chemical EngineeringShanmukShannuNo ratings yet
- Jobs MethodDocument8 pagesJobs MethodJayli Caren RiveraNo ratings yet
- Pelton Bale1986 ModifiedInteractionParameterFDocument5 pagesPelton Bale1986 ModifiedInteractionParameterFmanish pandeNo ratings yet
- Kinetics: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Document9 pagesKinetics: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Anonymous T02GVGzBNo ratings yet
- Small Excitonic Complexes in A Disk-Shaped Quantum Dot: Ricardo Perez, Augusto GonzalezDocument8 pagesSmall Excitonic Complexes in A Disk-Shaped Quantum Dot: Ricardo Perez, Augusto GonzalezJairofisico JaramilloNo ratings yet
- Diq1m w8jrhDocument10 pagesDiq1m w8jrhMohsin MuhammadNo ratings yet
- Introduction To Electronic Structure Theory: X X X X XDocument10 pagesIntroduction To Electronic Structure Theory: X X X X XAnonymous UrVkcdNo ratings yet
- The Thermochemistry of A Reacting Mixture of Elastic Materials With DiffusionDocument31 pagesThe Thermochemistry of A Reacting Mixture of Elastic Materials With DiffusionSantiago Peña ClavijoNo ratings yet
- Statistical Physics. Solutions Sheet 10.: Hi, Ji I JDocument8 pagesStatistical Physics. Solutions Sheet 10.: Hi, Ji I JJuan MondáNo ratings yet
- Chapter 16 - Section B - Non-Numerical SolutionsDocument3 pagesChapter 16 - Section B - Non-Numerical SolutionsHiLda HayatiiNo ratings yet
- Problem Set 6 SolutionDocument4 pagesProblem Set 6 SolutionRod De GuzmanNo ratings yet
- Mid Exam SolutionsDocument3 pagesMid Exam SolutionsTime TravellerNo ratings yet
- Generalized Chemical Reactivity of Curved Surfaces: Carbon NanotubesDocument5 pagesGeneralized Chemical Reactivity of Curved Surfaces: Carbon NanotubesemediageNo ratings yet
- Thermodynamics Lecture 2Document18 pagesThermodynamics Lecture 2Rashaq AL-HeetyNo ratings yet
- Spin Orbit InteractionDocument6 pagesSpin Orbit InteractionJoanofRockNo ratings yet
- Appendix BDocument4 pagesAppendix BguiguiNo ratings yet
- Exam 2: P Is A Universal Function For All TheDocument10 pagesExam 2: P Is A Universal Function For All TheUday RameshNo ratings yet
- 2005 Ms LiquidDocument6 pages2005 Ms LiquidkhabiranNo ratings yet
- HW Pso201 2020Document6 pagesHW Pso201 2020Shashi KumarNo ratings yet
- You May Not Start To Read The Questions Printed On The Subsequent Pages of This Question Paper Until Instructed That You May Do So by The InvigilatorDocument3 pagesYou May Not Start To Read The Questions Printed On The Subsequent Pages of This Question Paper Until Instructed That You May Do So by The InvigilatorSpringOrchidNo ratings yet
- c1Document23 pagesc1akashNo ratings yet
- MIT8 333F13 Pset6Document10 pagesMIT8 333F13 Pset6Henry De AriesNo ratings yet
- MIT8 333F13 ExamReview3Document10 pagesMIT8 333F13 ExamReview3Henry De AriesNo ratings yet
- Reviews in Computational ChemistryFrom EverandReviews in Computational ChemistryAbby L. ParrillNo ratings yet
- Reactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFrom EverandReactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFranz-Josef SchmittNo ratings yet
- Lecture 12 BDocument1 pageLecture 12 BAnonymous T02GVGzBNo ratings yet
- Burdukova Et Al. 2006. Effect of CMC and PH On The Rheology of Suspensions of Isotropic and Anisotropic MineralsDocument15 pagesBurdukova Et Al. 2006. Effect of CMC and PH On The Rheology of Suspensions of Isotropic and Anisotropic MineralsAnonymous T02GVGzBNo ratings yet
- Copper Flotation: Metals and Minerals IndustryDocument2 pagesCopper Flotation: Metals and Minerals IndustryAnonymous T02GVGzBNo ratings yet
- Lecture13 14Document2 pagesLecture13 14Anonymous T02GVGzBNo ratings yet
- Lecture 12 ADocument1 pageLecture 12 AAnonymous T02GVGzBNo ratings yet
- Lecture 12Document1 pageLecture 12Anonymous T02GVGzBNo ratings yet
- Lecture 7Document2 pagesLecture 7Anonymous T02GVGzBNo ratings yet
- Chapter Outline: Failure Fracture: How Do Materials Break?Document9 pagesChapter Outline: Failure Fracture: How Do Materials Break?Anonymous T02GVGzBNo ratings yet
- Chapter 14 CDocument8 pagesChapter 14 CAnonymous T02GVGzBNo ratings yet
- Exploration Analysis: Evaluation of Geochemical Data C. JDocument2 pagesExploration Analysis: Evaluation of Geochemical Data C. JAnonymous T02GVGzBNo ratings yet
- GJmatDocument2 pagesGJmatAnonymous T02GVGzBNo ratings yet
- Kinetics of Solidification 3: 13.1 Solid-Liquid Interface StructureDocument7 pagesKinetics of Solidification 3: 13.1 Solid-Liquid Interface StructureAnonymous T02GVGzBNo ratings yet
- Viscosity and Thermodynamics 2004Document14 pagesViscosity and Thermodynamics 2004Anonymous T02GVGzBNo ratings yet
- Heat Transfer: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Document8 pagesHeat Transfer: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Anonymous T02GVGzBNo ratings yet
- Lecture 11Document18 pagesLecture 11Anonymous T02GVGzBNo ratings yet
- Case Study: Flow Models: 16.1 Lattice-Gas Automata ModelDocument9 pagesCase Study: Flow Models: 16.1 Lattice-Gas Automata ModelAnonymous T02GVGzBNo ratings yet
- Solidification Processing: Lecture 10: Thermodynamics 3Document17 pagesSolidification Processing: Lecture 10: Thermodynamics 3Anonymous T02GVGzBNo ratings yet
- Fluid Flow in Solidification 1Document7 pagesFluid Flow in Solidification 1Anonymous T02GVGzBNo ratings yet
- Phase Field Models: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Document10 pagesPhase Field Models: Graduate Institute of Ferrous Technology, POSTECH Rongshan Qin (R. S. Qin)Anonymous T02GVGzBNo ratings yet
- ENGG1500 Module 8 Tute SolutionsDocument15 pagesENGG1500 Module 8 Tute SolutionsKevin MalarkeyNo ratings yet
- Chemical Potential and Gibbs Distribution: Anders Malthe-SørenssenDocument24 pagesChemical Potential and Gibbs Distribution: Anders Malthe-SørenssenNingsihNo ratings yet
- Quiz Fluid LabDocument3 pagesQuiz Fluid LabMuhammad BilalNo ratings yet
- Quiz 2 PDFDocument7 pagesQuiz 2 PDFRuth Montebon0% (1)
- 〈911〉 VISCOSITY-CAPILLARY METHODSDocument5 pages〈911〉 VISCOSITY-CAPILLARY METHODSVieno Gino CruzNo ratings yet
- Line Sizing1Document20 pagesLine Sizing1Srihari KodimelaNo ratings yet
- Mind Map Physical ChemDocument1 pageMind Map Physical ChemhusnaNo ratings yet
- Discussion 3Document2 pagesDiscussion 3Vinod KrishnanNo ratings yet
- Final Exam 2015Document6 pagesFinal Exam 2015Kiran JojiNo ratings yet
- Behavior of GasesDocument13 pagesBehavior of GasesSharalyn P. SiaNo ratings yet
- Flash Point TutorialDocument8 pagesFlash Point TutorialS. GreenNo ratings yet
- 13 NumberDocument1 page13 NumberDonNo ratings yet
- U-2-Turbulent Flow in Pipes and Channels PDFDocument23 pagesU-2-Turbulent Flow in Pipes and Channels PDFLaxmi PrasannaNo ratings yet
- Constantes Físicas FundamentalesDocument1 pageConstantes Físicas FundamentalesAlejandro GafNo ratings yet
- Orifice Meter: Observation and Calculation DataDocument10 pagesOrifice Meter: Observation and Calculation DataabhishekkandoiNo ratings yet
- Pre Heater Design CalculationsDocument4 pagesPre Heater Design CalculationsFahad KhokharNo ratings yet
- Distillation IndtroductionDocument35 pagesDistillation IndtroductionRidha Cessar GozaliNo ratings yet
- Sci3 Q1mod5 Changes in Materials Brenda Quilladas Bgo v1Document25 pagesSci3 Q1mod5 Changes in Materials Brenda Quilladas Bgo v1MArkNo ratings yet
- Practise Questions 2019Document8 pagesPractise Questions 2019Sehar IshtiaqNo ratings yet
- Study - Guide - States - of - Matter - Student - Editable c12Document9 pagesStudy - Guide - States - of - Matter - Student - Editable c12Zoe MarokoNo ratings yet
- Chemical Engineering Thermodynamics: Volumetric Properties of Pure FluidDocument20 pagesChemical Engineering Thermodynamics: Volumetric Properties of Pure FluidThurgah VshinyNo ratings yet
- Adiabatic and Isothermal ProcessesDocument2 pagesAdiabatic and Isothermal ProcessesphydotsiNo ratings yet
- Soal Fisika AnswerDocument3 pagesSoal Fisika AnswerHimawan EkaNo ratings yet
- 8.334: StatMech II Problem Set #2Document2 pages8.334: StatMech II Problem Set #2bidsitlovNo ratings yet
- Pump Sizing ParametersDocument1 pagePump Sizing ParametersBramJanssen76No ratings yet
- Lecture 1 Choke PerformanceDocument45 pagesLecture 1 Choke PerformancearvNo ratings yet
- FORM 5 Chapter 2 PressureDocument6 pagesFORM 5 Chapter 2 PressureKishenraj RajendranNo ratings yet
- Rexroth HABDocument20 pagesRexroth HABeleceng1979No ratings yet
- Lampiran A Data Litelatur: A.1 Densitas Air Pada Berbagai Temperatur Tabel A.1 Densitas Air Pada Berbagai TemperaturDocument2 pagesLampiran A Data Litelatur: A.1 Densitas Air Pada Berbagai Temperatur Tabel A.1 Densitas Air Pada Berbagai Temperaturjoshua2alexander-2No ratings yet