Professional Documents
Culture Documents
1314 Lab - Precipitate Puzzle
1314 Lab - Precipitate Puzzle
Uploaded by
api-239578762Copyright:
Available Formats
You might also like
- 2-4 Lab AssignmentDocument5 pages2-4 Lab AssignmentMaxim100% (1)
- 6 Precipitation ReactionsDocument2 pages6 Precipitation ReactionsJacob DaughertyNo ratings yet
- 4.4.4 Lab: Precipitation Reactions: Points Possible:50Document3 pages4.4.4 Lab: Precipitation Reactions: Points Possible:50Sid Mathur67% (3)
- SFMS - 8th Grade - Chemistry Unit Lab Report - Example 02Document7 pagesSFMS - 8th Grade - Chemistry Unit Lab Report - Example 02OnlineEducatorNo ratings yet
- Chemistry StuffDocument3 pagesChemistry Stuffapi-239717554No ratings yet
- 1314 Lab - Precipitate PuzzleDocument3 pages1314 Lab - Precipitate Puzzleapi-239161053No ratings yet
- 1314 Lab - Precipitate PuzzleDocument4 pages1314 Lab - Precipitate Puzzleapi-239474882No ratings yet
- Precipitate PuzzleDocument2 pagesPrecipitate Puzzleapi-239474524No ratings yet
- Precipitate PuzzleDocument2 pagesPrecipitate Puzzleapi-239474541No ratings yet
- 1314 Lab - Precipitate Puzzle-1Document2 pages1314 Lab - Precipitate Puzzle-1api-239536821No ratings yet
- 1314 Lab - Precipitate Puzzle 1Document2 pages1314 Lab - Precipitate Puzzle 1api-239587463No ratings yet
- Chemistry Labwork: Lamia Sabry Marwa Al-Kandari Maya Zeini Nehl Karim Rasha Al-AssmiDocument11 pagesChemistry Labwork: Lamia Sabry Marwa Al-Kandari Maya Zeini Nehl Karim Rasha Al-Assmideepheat_008No ratings yet
- Mid 2nd Term Mathayom 1 ScienceDocument7 pagesMid 2nd Term Mathayom 1 ScienceAbdullohNo ratings yet
- Solubility LabDocument4 pagesSolubility Labe_gwen_buchananNo ratings yet
- Law of Conservation of Mass QuizDocument6 pagesLaw of Conservation of Mass QuizLeormhan Jacob Dela CruzNo ratings yet
- Exp 1 Chm361Document5 pagesExp 1 Chm361sabNo ratings yet
- 2-4: Precipitation Reactions: Data TableDocument2 pages2-4: Precipitation Reactions: Data TableSebastian Gomez LopezNo ratings yet
- Oxidation-Reduction Activity Series: Name: Hamad Naji Date: 8 April, 2019Document6 pagesOxidation-Reduction Activity Series: Name: Hamad Naji Date: 8 April, 2019irfanNo ratings yet
- SeparationsDocument8 pagesSeparationsHidayat KesumaNo ratings yet
- CHEM-1100 Exp 3, Group B Cation AnalysisDocument7 pagesCHEM-1100 Exp 3, Group B Cation AnalysisMUNEZERO Jean de DieuNo ratings yet
- CHEM 108 Lab Identification of Unknown Ionic Solutions F23Document6 pagesCHEM 108 Lab Identification of Unknown Ionic Solutions F23johnpaulnicklausNo ratings yet
- Sec 4 Chem Prelim Paper 1Document16 pagesSec 4 Chem Prelim Paper 1TeckluckyNo ratings yet
- Chem 1b Expt10Document5 pagesChem 1b Expt10حوراء جابر مزهرNo ratings yet
- Activity SeriesDocument7 pagesActivity SeriesAhmedSaad647No ratings yet
- Preproblems Al-BerunyiDocument22 pagesPreproblems Al-Berunyi13-11H-Nguyễn Thế KhangNo ratings yet
- Lab 8 CHM130LL Identification of Cations and AnionsDocument6 pagesLab 8 CHM130LL Identification of Cations and AnionsSJO1 G6- Escaro,Shaira JoyNo ratings yet
- E Redox IntroDocument5 pagesE Redox IntroJoshua GeddesNo ratings yet
- Lab 8 CHM130LL Identification of Cations and AnionsDocument6 pagesLab 8 CHM130LL Identification of Cations and AnionsFatimah AzzahrahNo ratings yet
- NSO 2 7th PYQ 13-14Document22 pagesNSO 2 7th PYQ 13-14ablegalconsultancyservicesNo ratings yet
- Ujian Selaras 1 F4 Chemistry 2021Document6 pagesUjian Selaras 1 F4 Chemistry 2021Michelle LambertNo ratings yet
- 9D Answer SheetDocument2 pages9D Answer SheetpikaryanyuNo ratings yet
- Diagram 1 Below Shows The Pathway of An Impulse For An ActionDocument19 pagesDiagram 1 Below Shows The Pathway of An Impulse For An ActionCik HidayahNo ratings yet
- Experiment 8: Separation of Cations: An ExampleDocument4 pagesExperiment 8: Separation of Cations: An ExampleRodrigoNo ratings yet
- Soal ToomDocument3 pagesSoal ToomAbdul Aziz SiregarNo ratings yet
- Single Replacement Reactions LabDocument2 pagesSingle Replacement Reactions Labapi-239386573No ratings yet
- CHEM 101 Midterm Exam 2 (Fa2023) RubricDocument10 pagesCHEM 101 Midterm Exam 2 (Fa2023) RubricJohn danielNo ratings yet
- KBAT Form 4Document13 pagesKBAT Form 4Arvin DiNozzo0% (1)
- Science Class X Sample Paper Test 02 For Board Exam 2023 AnswersDocument14 pagesScience Class X Sample Paper Test 02 For Board Exam 2023 Answershithesh365No ratings yet
- 1 Atomic Structure PDFDocument20 pages1 Atomic Structure PDFanilkumarsharma1969No ratings yet
- Exam1 f03Document4 pagesExam1 f03rasjerryNo ratings yet
- 2018 Y4 Prelim P2Document16 pages2018 Y4 Prelim P2Fangru CaoNo ratings yet
- Marithonchemper 8 SinglereplacementlabDocument2 pagesMarithonchemper 8 Singlereplacementlabapi-241156470No ratings yet
- 1 - REDOX Unit Exam STUDENT Studyguide 2015 - 8Document12 pages1 - REDOX Unit Exam STUDENT Studyguide 2015 - 8AYESHA NAAZNo ratings yet
- Final-Laboratory-Reportexperiment-no.1-inting FinalDocument5 pagesFinal-Laboratory-Reportexperiment-no.1-inting FinalChanie Baguio Pitogo100% (1)
- 30 Jan 23 Morning EnglishDocument14 pages30 Jan 23 Morning Englishcolada8216No ratings yet
- Finding Unknown Solutions: Sophie Woodcock, Sarah Bowler, Maud BeachDocument4 pagesFinding Unknown Solutions: Sophie Woodcock, Sarah Bowler, Maud BeachFrequentlyBespeckledNo ratings yet
- Name: Class: I/C NoDocument19 pagesName: Class: I/C NopermatasemarakNo ratings yet
- Soluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and DDocument4 pagesSoluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and DBEST OF ONE PIECENo ratings yet
- Qualitative AnalysisDocument16 pagesQualitative AnalysisGaneshNo ratings yet
- CSI - Chemistry Scene InvestigationDocument2 pagesCSI - Chemistry Scene InvestigationSourabh DasNo ratings yet
- Q.No Marks 1. 1: Month End Examination (2019-20) Time Allowed: 1.5 Hours Class - X (Science) (Set A) Maximum Marks: 40Document5 pagesQ.No Marks 1. 1: Month End Examination (2019-20) Time Allowed: 1.5 Hours Class - X (Science) (Set A) Maximum Marks: 40Kunal SharmaNo ratings yet
- AP Chemistry Mini Test Complete PDFDocument20 pagesAP Chemistry Mini Test Complete PDFEthan NguyenNo ratings yet
- Chem G10 First Term Exam20182019111Document12 pagesChem G10 First Term Exam20182019111Mona Against DampersNo ratings yet
- Periodic Table QDocument2 pagesPeriodic Table QSaihaan.ZNo ratings yet
- Anions SchemeDocument4 pagesAnions Schemewaee0565No ratings yet
- Electrolysis Worksheet 2Document11 pagesElectrolysis Worksheet 2Menaga A/P IlangkovanNo ratings yet
- Bo Tro Kien Thuc HoaDocument10 pagesBo Tro Kien Thuc Hoakilluahthanh0No ratings yet
- X CHEM CH-1 Worksheet 4Document2 pagesX CHEM CH-1 Worksheet 4Amrutha KNo ratings yet
- Materials - Metals and Non-Metals Worksheet 2Document2 pagesMaterials - Metals and Non-Metals Worksheet 2zakir soomroNo ratings yet
- Experiment RedoxDocument6 pagesExperiment RedoxJaaizah JaafarNo ratings yet
- Chemical Element Synthetic Metal Periodic Table Isotope: Technetium (TC)Document2 pagesChemical Element Synthetic Metal Periodic Table Isotope: Technetium (TC)Maria Patricia Angela MoslaresNo ratings yet
- University Institute of Architecture Subject - Building Material and Contruction - V Subject Code - 311 Topic - Ferrous and Non - Ferrous MetalsDocument34 pagesUniversity Institute of Architecture Subject - Building Material and Contruction - V Subject Code - 311 Topic - Ferrous and Non - Ferrous MetalsSaksham SharmaNo ratings yet
- Naming Ionic Compounds Worksheet IDocument3 pagesNaming Ionic Compounds Worksheet IrevieNo ratings yet
- Astm B265Document10 pagesAstm B265avinash bahadurNo ratings yet
- Catalogue Nova Luce 2021Document382 pagesCatalogue Nova Luce 2021Adrian TrandafirNo ratings yet
- Chemistry Brochure (Elements)Document1 pageChemistry Brochure (Elements)Maslina HereNo ratings yet
- June 2018 Question Paper 31 PDFDocument16 pagesJune 2018 Question Paper 31 PDFKazi Ahnaf SaadNo ratings yet
- ZG120 MN 13Document2 pagesZG120 MN 13Agam SanjayaNo ratings yet
- Chromium-Nickel Stainless SteelsDocument8 pagesChromium-Nickel Stainless SteelsRahul KhoslaNo ratings yet
- Chemistry 106, Chapter 16 Exercises: Concentrations of Ions in Solutions and KSPDocument3 pagesChemistry 106, Chapter 16 Exercises: Concentrations of Ions in Solutions and KSPrajNo ratings yet
- Extract Pages From Metallurgy and Materials Engineering - S. Ramachandran Et Al. (Air Walk Publications, 2016)Document31 pagesExtract Pages From Metallurgy and Materials Engineering - S. Ramachandran Et Al. (Air Walk Publications, 2016)Hunter NoVaNo ratings yet
- S - Block Elements PDFDocument14 pagesS - Block Elements PDFPankaj MauryaNo ratings yet
- Daftar Bahan Kimia Kode Kadocx PDFDocument4 pagesDaftar Bahan Kimia Kode Kadocx PDFAgoenk KertawijayaNo ratings yet
- Zwor Cwievwnzv I E'Y WZK We KølyDocument86 pagesZwor Cwievwnzv I E'Y WZK We KølyKhulna TravelsNo ratings yet
- Class-12 Chemistry Byjus Notes Chp-4 Topic - Important Compounds of Transition ElementsDocument107 pagesClass-12 Chemistry Byjus Notes Chp-4 Topic - Important Compounds of Transition ElementsJacintha .NNo ratings yet
- S - Block ElementsDocument23 pagesS - Block ElementsAnand MurugananthamNo ratings yet
- Element CompoundDocument25 pagesElement CompoundBhel San Pedro MarzanNo ratings yet
- 2019 Sec 4 Pure Chemistry SA2 Geylang MethodistDocument32 pages2019 Sec 4 Pure Chemistry SA2 Geylang MethodistErOn TaNNo ratings yet
- LOVE Rings - CartierDocument1 pageLOVE Rings - CartierenduenaNo ratings yet
- Gamma Energy (Kev) Nuclide Half-Life Percent Yield Per DecayDocument5 pagesGamma Energy (Kev) Nuclide Half-Life Percent Yield Per DecayFATIN YUNIARTINo ratings yet
- PT by MellorDocument16 pagesPT by MellorCesar Mera LlinasNo ratings yet
- Grease Compatibility Chart DomesticDocument1 pageGrease Compatibility Chart DomesticJeisson HolguinNo ratings yet
- Group 13 Occurrences, Extraction and Uses (Multiple Choice)Document3 pagesGroup 13 Occurrences, Extraction and Uses (Multiple Choice)kionaNo ratings yet
- Paper 2 November 2001Document4 pagesPaper 2 November 2001MSH50% (4)
- Csir Net Question PaperDocument41 pagesCsir Net Question PaperAanchal PathakNo ratings yet
- Rry'S Chemical Engineers' Handbook: Seventh EditionDocument2 pagesRry'S Chemical Engineers' Handbook: Seventh EditionRamaNo ratings yet
- MCX Margin 03.09Document6 pagesMCX Margin 03.09PANKAJ SHETENo ratings yet
- p1 ChemistryDocument15 pagesp1 ChemistryHumza SohailNo ratings yet
1314 Lab - Precipitate Puzzle
1314 Lab - Precipitate Puzzle
Uploaded by
api-239578762Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1314 Lab - Precipitate Puzzle
1314 Lab - Precipitate Puzzle
Uploaded by
api-239578762Copyright:
Available Formats
Chemistry
Name: Orla Deignan Period: 2 Date: November 12th 2013
Precipitate Puzzle Lab
U n i t 3
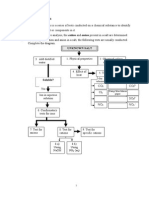
As a secret agent, your assignment is to pick up some chemicals and deliver them to the Special Chemicals Unit. You then undertake this assignment with all the adventures that would make OO7 proud of you. Following the pick up, you notice that the bottles are labeled A,B,C, X, Y, Z only. You are given two papers, which identify the bottles. One paper contains the following information: Bottles A, B, C X, Y, Z The other paper contains the following information Bottle Contains A xxx B xxx C xxx X xxx Y xxx Z xxx You realize that the second sheet is smudged (due to your escapades in attaining the chemicals.) In order to deliver the substances, you need to fix the second paper. Your task is to identify each of A, B, C, X, Y and Z, using their solubility tables, and then write formula, complete ionic and net ionic equations corresponding to each precipitate formed. Draw up a data table and show detailed observations for the tests that you conduct: A B X ObservationsObservations- White precipitate - Clear, no precipitate formed formation - Particularly cloudy (Corresponds to equation 1) Y ObservationsObservations- Yellow/white - White precipitate precipitate formed formed - Little bit of pink - Not very strong (Corresponds to equation color 2) (Corresponds to equation 4) Z ObservationsObservations- Red precipitate - Turned yellow in color, formed but no precipitate - Very deep color formation (Corresponds to Equation 3)
Contains In no particular order: Mg(NO3)2, AgNO3 and Ba(NO3)2 In no particular order: Na2CrO4, NaCl and Na2CO3.
C Observations- Clear, no precipitate formation
Observations- White precipitate formed - Not very strong color (Corresponds to Equation 5) Observations- Yellow precipitate formed - Strong color, slightly white (Corresponds to equation 6)
Images from the Lab:
This is an image of the completed spot plate with all the solutions
This is an image of adding the mystery substances to the spot plate
Identity of Mystery Substances: A is: Silver Nitrate - AgNO3 B is: Magnesium Nitrate - Mg(NO3)2 C is: Barium Nitrate - Ba(NO3)2 X is: Sodium Chloride - NaCl Y is: Sodium Carbonate - Na2CO3 Z is: Sodium Chromate - Na2CrO4
Equations for each reaction: 1. AgNO3(aq)+NaCl(aq)AgCl(s)+NaNO3(aq) Net Ionic Equation: Ag1+(aq)+Cl1- (aq)AgCl(s) 2. 2AgNO3(aq)+Na2CO3(aq)Ag2CO3(s)+2NaNO3(aq) Net Ionic Equation: 2Ag1+(aq)+CO32-(aq)Ag2CO3(s) 3. 2AgNO3(aq)+ Na2CrO4(aq)Ag2CrO4(s)+2NaNO3(aq) Net Ionic Equation: 2Ag1+(aq)+CrO41-(aq)Ag2CrO4(s) 4. Mg(NO3)(aq)+Na2CO3(aq)MgCO3(s)+2NaNO3(aq) Net Ionic Equation: Mg2+(aq)+ CO32-(aq)MgCO3(s) 5. Ba(NO3)2(aq)+Na2CO3(aq)BaCO3(s)+2NaNO3(aq) Net Ionic Equation: Ba2+(aq)+CO32-(aq)BaCO3(s) 6. Ba(NO3)2(aq)+Na2CrO4BaCrO4(s)+2NaNO3(aq) Net Ionic Equation: Ba2+(aq)+CrO41-(aq)BaCrO4(s)
Conclusion: Through the knowledge of solubility rules, precipitates, and chemical equations, we were able to successfully identify each mystery substance.
You might also like
- 2-4 Lab AssignmentDocument5 pages2-4 Lab AssignmentMaxim100% (1)
- 6 Precipitation ReactionsDocument2 pages6 Precipitation ReactionsJacob DaughertyNo ratings yet
- 4.4.4 Lab: Precipitation Reactions: Points Possible:50Document3 pages4.4.4 Lab: Precipitation Reactions: Points Possible:50Sid Mathur67% (3)
- SFMS - 8th Grade - Chemistry Unit Lab Report - Example 02Document7 pagesSFMS - 8th Grade - Chemistry Unit Lab Report - Example 02OnlineEducatorNo ratings yet
- Chemistry StuffDocument3 pagesChemistry Stuffapi-239717554No ratings yet
- 1314 Lab - Precipitate PuzzleDocument3 pages1314 Lab - Precipitate Puzzleapi-239161053No ratings yet
- 1314 Lab - Precipitate PuzzleDocument4 pages1314 Lab - Precipitate Puzzleapi-239474882No ratings yet
- Precipitate PuzzleDocument2 pagesPrecipitate Puzzleapi-239474524No ratings yet
- Precipitate PuzzleDocument2 pagesPrecipitate Puzzleapi-239474541No ratings yet
- 1314 Lab - Precipitate Puzzle-1Document2 pages1314 Lab - Precipitate Puzzle-1api-239536821No ratings yet
- 1314 Lab - Precipitate Puzzle 1Document2 pages1314 Lab - Precipitate Puzzle 1api-239587463No ratings yet
- Chemistry Labwork: Lamia Sabry Marwa Al-Kandari Maya Zeini Nehl Karim Rasha Al-AssmiDocument11 pagesChemistry Labwork: Lamia Sabry Marwa Al-Kandari Maya Zeini Nehl Karim Rasha Al-Assmideepheat_008No ratings yet
- Mid 2nd Term Mathayom 1 ScienceDocument7 pagesMid 2nd Term Mathayom 1 ScienceAbdullohNo ratings yet
- Solubility LabDocument4 pagesSolubility Labe_gwen_buchananNo ratings yet
- Law of Conservation of Mass QuizDocument6 pagesLaw of Conservation of Mass QuizLeormhan Jacob Dela CruzNo ratings yet
- Exp 1 Chm361Document5 pagesExp 1 Chm361sabNo ratings yet
- 2-4: Precipitation Reactions: Data TableDocument2 pages2-4: Precipitation Reactions: Data TableSebastian Gomez LopezNo ratings yet
- Oxidation-Reduction Activity Series: Name: Hamad Naji Date: 8 April, 2019Document6 pagesOxidation-Reduction Activity Series: Name: Hamad Naji Date: 8 April, 2019irfanNo ratings yet
- SeparationsDocument8 pagesSeparationsHidayat KesumaNo ratings yet
- CHEM-1100 Exp 3, Group B Cation AnalysisDocument7 pagesCHEM-1100 Exp 3, Group B Cation AnalysisMUNEZERO Jean de DieuNo ratings yet
- CHEM 108 Lab Identification of Unknown Ionic Solutions F23Document6 pagesCHEM 108 Lab Identification of Unknown Ionic Solutions F23johnpaulnicklausNo ratings yet
- Sec 4 Chem Prelim Paper 1Document16 pagesSec 4 Chem Prelim Paper 1TeckluckyNo ratings yet
- Chem 1b Expt10Document5 pagesChem 1b Expt10حوراء جابر مزهرNo ratings yet
- Activity SeriesDocument7 pagesActivity SeriesAhmedSaad647No ratings yet
- Preproblems Al-BerunyiDocument22 pagesPreproblems Al-Berunyi13-11H-Nguyễn Thế KhangNo ratings yet
- Lab 8 CHM130LL Identification of Cations and AnionsDocument6 pagesLab 8 CHM130LL Identification of Cations and AnionsSJO1 G6- Escaro,Shaira JoyNo ratings yet
- E Redox IntroDocument5 pagesE Redox IntroJoshua GeddesNo ratings yet
- Lab 8 CHM130LL Identification of Cations and AnionsDocument6 pagesLab 8 CHM130LL Identification of Cations and AnionsFatimah AzzahrahNo ratings yet
- NSO 2 7th PYQ 13-14Document22 pagesNSO 2 7th PYQ 13-14ablegalconsultancyservicesNo ratings yet
- Ujian Selaras 1 F4 Chemistry 2021Document6 pagesUjian Selaras 1 F4 Chemistry 2021Michelle LambertNo ratings yet
- 9D Answer SheetDocument2 pages9D Answer SheetpikaryanyuNo ratings yet
- Diagram 1 Below Shows The Pathway of An Impulse For An ActionDocument19 pagesDiagram 1 Below Shows The Pathway of An Impulse For An ActionCik HidayahNo ratings yet
- Experiment 8: Separation of Cations: An ExampleDocument4 pagesExperiment 8: Separation of Cations: An ExampleRodrigoNo ratings yet
- Soal ToomDocument3 pagesSoal ToomAbdul Aziz SiregarNo ratings yet
- Single Replacement Reactions LabDocument2 pagesSingle Replacement Reactions Labapi-239386573No ratings yet
- CHEM 101 Midterm Exam 2 (Fa2023) RubricDocument10 pagesCHEM 101 Midterm Exam 2 (Fa2023) RubricJohn danielNo ratings yet
- KBAT Form 4Document13 pagesKBAT Form 4Arvin DiNozzo0% (1)
- Science Class X Sample Paper Test 02 For Board Exam 2023 AnswersDocument14 pagesScience Class X Sample Paper Test 02 For Board Exam 2023 Answershithesh365No ratings yet
- 1 Atomic Structure PDFDocument20 pages1 Atomic Structure PDFanilkumarsharma1969No ratings yet
- Exam1 f03Document4 pagesExam1 f03rasjerryNo ratings yet
- 2018 Y4 Prelim P2Document16 pages2018 Y4 Prelim P2Fangru CaoNo ratings yet
- Marithonchemper 8 SinglereplacementlabDocument2 pagesMarithonchemper 8 Singlereplacementlabapi-241156470No ratings yet
- 1 - REDOX Unit Exam STUDENT Studyguide 2015 - 8Document12 pages1 - REDOX Unit Exam STUDENT Studyguide 2015 - 8AYESHA NAAZNo ratings yet
- Final-Laboratory-Reportexperiment-no.1-inting FinalDocument5 pagesFinal-Laboratory-Reportexperiment-no.1-inting FinalChanie Baguio Pitogo100% (1)
- 30 Jan 23 Morning EnglishDocument14 pages30 Jan 23 Morning Englishcolada8216No ratings yet
- Finding Unknown Solutions: Sophie Woodcock, Sarah Bowler, Maud BeachDocument4 pagesFinding Unknown Solutions: Sophie Woodcock, Sarah Bowler, Maud BeachFrequentlyBespeckledNo ratings yet
- Name: Class: I/C NoDocument19 pagesName: Class: I/C NopermatasemarakNo ratings yet
- Soluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and DDocument4 pagesSoluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and DBEST OF ONE PIECENo ratings yet
- Qualitative AnalysisDocument16 pagesQualitative AnalysisGaneshNo ratings yet
- CSI - Chemistry Scene InvestigationDocument2 pagesCSI - Chemistry Scene InvestigationSourabh DasNo ratings yet
- Q.No Marks 1. 1: Month End Examination (2019-20) Time Allowed: 1.5 Hours Class - X (Science) (Set A) Maximum Marks: 40Document5 pagesQ.No Marks 1. 1: Month End Examination (2019-20) Time Allowed: 1.5 Hours Class - X (Science) (Set A) Maximum Marks: 40Kunal SharmaNo ratings yet
- AP Chemistry Mini Test Complete PDFDocument20 pagesAP Chemistry Mini Test Complete PDFEthan NguyenNo ratings yet
- Chem G10 First Term Exam20182019111Document12 pagesChem G10 First Term Exam20182019111Mona Against DampersNo ratings yet
- Periodic Table QDocument2 pagesPeriodic Table QSaihaan.ZNo ratings yet
- Anions SchemeDocument4 pagesAnions Schemewaee0565No ratings yet
- Electrolysis Worksheet 2Document11 pagesElectrolysis Worksheet 2Menaga A/P IlangkovanNo ratings yet
- Bo Tro Kien Thuc HoaDocument10 pagesBo Tro Kien Thuc Hoakilluahthanh0No ratings yet
- X CHEM CH-1 Worksheet 4Document2 pagesX CHEM CH-1 Worksheet 4Amrutha KNo ratings yet
- Materials - Metals and Non-Metals Worksheet 2Document2 pagesMaterials - Metals and Non-Metals Worksheet 2zakir soomroNo ratings yet
- Experiment RedoxDocument6 pagesExperiment RedoxJaaizah JaafarNo ratings yet
- Chemical Element Synthetic Metal Periodic Table Isotope: Technetium (TC)Document2 pagesChemical Element Synthetic Metal Periodic Table Isotope: Technetium (TC)Maria Patricia Angela MoslaresNo ratings yet
- University Institute of Architecture Subject - Building Material and Contruction - V Subject Code - 311 Topic - Ferrous and Non - Ferrous MetalsDocument34 pagesUniversity Institute of Architecture Subject - Building Material and Contruction - V Subject Code - 311 Topic - Ferrous and Non - Ferrous MetalsSaksham SharmaNo ratings yet
- Naming Ionic Compounds Worksheet IDocument3 pagesNaming Ionic Compounds Worksheet IrevieNo ratings yet
- Astm B265Document10 pagesAstm B265avinash bahadurNo ratings yet
- Catalogue Nova Luce 2021Document382 pagesCatalogue Nova Luce 2021Adrian TrandafirNo ratings yet
- Chemistry Brochure (Elements)Document1 pageChemistry Brochure (Elements)Maslina HereNo ratings yet
- June 2018 Question Paper 31 PDFDocument16 pagesJune 2018 Question Paper 31 PDFKazi Ahnaf SaadNo ratings yet
- ZG120 MN 13Document2 pagesZG120 MN 13Agam SanjayaNo ratings yet
- Chromium-Nickel Stainless SteelsDocument8 pagesChromium-Nickel Stainless SteelsRahul KhoslaNo ratings yet
- Chemistry 106, Chapter 16 Exercises: Concentrations of Ions in Solutions and KSPDocument3 pagesChemistry 106, Chapter 16 Exercises: Concentrations of Ions in Solutions and KSPrajNo ratings yet
- Extract Pages From Metallurgy and Materials Engineering - S. Ramachandran Et Al. (Air Walk Publications, 2016)Document31 pagesExtract Pages From Metallurgy and Materials Engineering - S. Ramachandran Et Al. (Air Walk Publications, 2016)Hunter NoVaNo ratings yet
- S - Block Elements PDFDocument14 pagesS - Block Elements PDFPankaj MauryaNo ratings yet
- Daftar Bahan Kimia Kode Kadocx PDFDocument4 pagesDaftar Bahan Kimia Kode Kadocx PDFAgoenk KertawijayaNo ratings yet
- Zwor Cwievwnzv I E'Y WZK We KølyDocument86 pagesZwor Cwievwnzv I E'Y WZK We KølyKhulna TravelsNo ratings yet
- Class-12 Chemistry Byjus Notes Chp-4 Topic - Important Compounds of Transition ElementsDocument107 pagesClass-12 Chemistry Byjus Notes Chp-4 Topic - Important Compounds of Transition ElementsJacintha .NNo ratings yet
- S - Block ElementsDocument23 pagesS - Block ElementsAnand MurugananthamNo ratings yet
- Element CompoundDocument25 pagesElement CompoundBhel San Pedro MarzanNo ratings yet
- 2019 Sec 4 Pure Chemistry SA2 Geylang MethodistDocument32 pages2019 Sec 4 Pure Chemistry SA2 Geylang MethodistErOn TaNNo ratings yet
- LOVE Rings - CartierDocument1 pageLOVE Rings - CartierenduenaNo ratings yet
- Gamma Energy (Kev) Nuclide Half-Life Percent Yield Per DecayDocument5 pagesGamma Energy (Kev) Nuclide Half-Life Percent Yield Per DecayFATIN YUNIARTINo ratings yet
- PT by MellorDocument16 pagesPT by MellorCesar Mera LlinasNo ratings yet
- Grease Compatibility Chart DomesticDocument1 pageGrease Compatibility Chart DomesticJeisson HolguinNo ratings yet
- Group 13 Occurrences, Extraction and Uses (Multiple Choice)Document3 pagesGroup 13 Occurrences, Extraction and Uses (Multiple Choice)kionaNo ratings yet
- Paper 2 November 2001Document4 pagesPaper 2 November 2001MSH50% (4)
- Csir Net Question PaperDocument41 pagesCsir Net Question PaperAanchal PathakNo ratings yet
- Rry'S Chemical Engineers' Handbook: Seventh EditionDocument2 pagesRry'S Chemical Engineers' Handbook: Seventh EditionRamaNo ratings yet
- MCX Margin 03.09Document6 pagesMCX Margin 03.09PANKAJ SHETENo ratings yet
- p1 ChemistryDocument15 pagesp1 ChemistryHumza SohailNo ratings yet