Professional Documents
Culture Documents
2,5-Dimethylheptane: Condensed Structural Formula (6 Points)
2,5-Dimethylheptane: Condensed Structural Formula (6 Points)
Uploaded by
Andra AlOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2,5-Dimethylheptane: Condensed Structural Formula (6 Points)
2,5-Dimethylheptane: Condensed Structural Formula (6 Points)
Uploaded by
Andra AlCopyright:
Available Formats
CHEM116 Fall 2000 Exam #1 Prof.
Thomas Poon Joint Science Department, The Claremont Colleges
ANSWER KEY
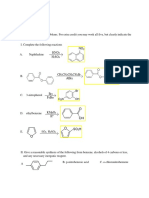
1) Translate the following structures to the representation requested above each box.
Condensed Structural Formula (6 points)
(CH3 )2 CH(CH 2)2CH(CH3 )CH 2CH3
Name the molecule shown above (4 points)
2,5-dimethylheptane
The most stable Newman Projection looking down the indicated bond (6 points) H H3 C CH3
CH3
H H CH3
H 3C CH
or H
H 3 C3
CH3 CH3 CH3 CH3
H
CH
2) The following drawing represents the most stable Lewis structure for methylvinyl ketone, a molecule used in commercial paint strippers. Methylvinyl ketone has been found to react with various Lewis bases. Using your knowledge of resonance theory, show the position or positions on the molecule where a Lewis base might react and provide a rationale for your selection(s) using both pictures and words. (10 points)
O C methylvinyl ketone
O C
O C
Lewis base reacts here Lewis bases are electron donors and usually negatively charged or partially negative. Their negative character causes them to be attracted to positive centers.
CHEM116 Fall 2000 Exam #1 Prof. Thomas Poon Joint Science Department, The Claremont Colleges
ANSWER KEY
3) For each pair of molecules shown below, select the one that best fits the accompanying description by circling it. Provide a concise rationale for each of your decisions. (6 points each) Rationale
The most acidic proton?
H2 C
CH CH3
vs.
H3 C
CH2 CH3
H2 C
CH CH2
H2 C
CH
CH2
H3 C
CH2 CH2
In the 1st acid, resonance can delocalize the negative charge formed in the conjugate base. This delocalization makes the conjugate base more stable. Therefore, the original acid is strengthened by its "desire" to populate the products side of the reaction.
The most stable molecule? H3 C CH3
vs.
H3 C
CH3 CH3
CH3
CH3 H3 C CH3
CH3 CH3 CH3
This isomer has an axial methyl group which leads to two unfavorable 1,3-diaxial interactions
The molecule with the highest melting point? (CH3 )4 C
vs.
CH3(CH2)3 CH3
The circled molecule is more symmetrical so it can pack more efficiently in the solid phase. This will make it harder to break the intermolecular forces and melt the molecule.
The carbon with the greatest "s" character?
vs.
The highly strained cyclopropane ring contains shorter C-C bonds. The shorter the bond, the greater the "s" character because s orbitals are closer to the nucleus and thus contribute to bringing atoms involved in such bonds closer to each other.
CHEM116 Fall 2000 Exam #1 Prof. Thomas Poon Joint Science Department, The Claremont Colleges
ANSWER KEY
4) Ozone (O3), a molecule found in the stratosphere, is vital in protecting the earths inhabitants from the suns harmful UV rays. Draw the Lewis structure of ozone in the box provided showing all lone pairs of electrons and charges where applicable. If any resonance contributors exist for ozone, draw those as well. Then answer the questions that follow:
Lewis Structure(s) for Ozone (6 points)
O O O O
O O
a) Circle the oxygen or oxygens in ozone that is/are most likely to react with a Lewis acid. (4 points) A Lewis acid is an electron acceptor. The negatively charged oxygen atoms in ozone are richest in electrons and thus most likely to donate electrons. See circled atoms above. b) Draw the orbital picture for ozone. Be sure to label the orbital types and bond types in your picture. (8 points) ! bond
p
sp 2
p sp 2 sp2
sp 2
sp 3
sp sp3
sp 2
sp3
"b
d on
" bond
sp2
c) Does ozone have a permanent dipole moment? Why or why not? (6 points)
!+ !-
O O O
!-
Yes. Based on the resonance picture of ozone, we can expect ozone to exist as a hybrid as shown in the diagram on the left. The partial charges cause individual dipoles to exist which in turn create a permanent dipole moment as drawn.
CHEM116 Fall 2000 Exam #1 Prof. Thomas Poon Joint Science Department, The Claremont Colleges
ANSWER KEY
5) Provide an example of a molecule that contains a 1, 2, 3 and 4 carbon. Label each carbon type in your drawing. (8 points)
1 4 2 3
6) Molecular modeling calculations show that molecule A is actually more stable when both substituents are axial whereas molecule B is more stable when both substituents are equatorial. Using words and at least one Newman projection, provide an explanation for this unusual result. (10 points)
more stable more stable
Br O Br A H H H H H H Br O H H H H O H H Br OH OH Br B
Br
OH
An increased electrostatic repulsion is created when the oxygen is deprotonated because one negatively charged atom is placed gauche to a partially negative bromine. This situation does not exist in compound B.
7) Medical evidence suggests that some speech deficiencies in children are caused by a lack of the amino acid histidine. Histidine is shown below in its uncharged form along with a table of pKas. Draw the predominant form of histidine at pH = 8 and indicate its overall charge (CHARGE=0). (8 points)
Functional Group O OH NH NH2 NH3 OH NH2 9.17 HN N pKa 6.04
The Henderson-Hasselbalch equation yields the following relationships between pH and pKa. When pKa > pH, an acid is in its protonated form. When pKa < pH, an acid is in its deprotonated form. O
N NH NH3 O
Histidine (Uncharged Form) O 1.82
You might also like
- Solution Manual For Chemistry An Atoms Focused Approach 3rd Edition Thomas R Gilbert Rein V Kirss Stacey Lowery Bretz Natalie FosterDocument37 pagesSolution Manual For Chemistry An Atoms Focused Approach 3rd Edition Thomas R Gilbert Rein V Kirss Stacey Lowery Bretz Natalie FosterChristianGonzalezsrybm100% (87)
- Cambridge Natural Sciences HandoutDocument55 pagesCambridge Natural Sciences Handout李超然100% (1)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- On-Bottom Stability AnalysisDocument42 pagesOn-Bottom Stability AnalysisYoungtae Kim67% (3)
- Resonance and Inductive Effects PresentationDocument36 pagesResonance and Inductive Effects Presentationeagl33yeNo ratings yet
- Organic ChemistryDocument3 pagesOrganic ChemistryMohammed AltahirNo ratings yet
- StickyquestionlabtedsDocument9 pagesStickyquestionlabtedsapi-287235370100% (4)
- Lithium I On IncidentsDocument12 pagesLithium I On IncidentsM8R-fsx9faNo ratings yet
- CHM 1321 Assignment #2 - : AnswersDocument11 pagesCHM 1321 Assignment #2 - : AnswersSara YuenNo ratings yet
- Problems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6Document6 pagesProblems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6JibrilAttawarahNo ratings yet
- BR BR BR: O-Bromotuloene P-Bromotuloene M-BromotuloeneDocument46 pagesBR BR BR: O-Bromotuloene P-Bromotuloene M-Bromotuloenejan100% (1)
- CHM 1321 Assignment 4 AnswersDocument12 pagesCHM 1321 Assignment 4 AnswersSara YuenNo ratings yet
- Organic Chemistry NotesDocument3 pagesOrganic Chemistry NotesHalimaNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- CHEM 33 Extra Practice 05 AnswersDocument7 pagesCHEM 33 Extra Practice 05 Answershuang.sundi3134No ratings yet
- Tutorial Letter 201/1/2016: General Chemistry 1BDocument22 pagesTutorial Letter 201/1/2016: General Chemistry 1BLeigh MakanNo ratings yet
- CBSE Class 11 Chemistry Chapter 12 - Organic Chemistry Important Questions 2022-23Document15 pagesCBSE Class 11 Chemistry Chapter 12 - Organic Chemistry Important Questions 2022-23Divye DasNo ratings yet
- Long Exam 1Document8 pagesLong Exam 1Allan DNo ratings yet
- CH 16Document42 pagesCH 16Kishore KishoreNo ratings yet
- Chemistry 10006Document27 pagesChemistry 10006Shai Shazza GrossNo ratings yet
- Important Questions For CBSE Class 11 Chemistry Chapter 12Document15 pagesImportant Questions For CBSE Class 11 Chemistry Chapter 12KrrishSPNo ratings yet
- 303 99 1stExKEY PDFDocument8 pages303 99 1stExKEY PDFaegaisNo ratings yet
- Workbook 01Document15 pagesWorkbook 01Kartik DhandNo ratings yet
- Chemistry-Orgo II Exam 1 Version A (UD) Answer KeyDocument8 pagesChemistry-Orgo II Exam 1 Version A (UD) Answer KeyNesrine LaradjiNo ratings yet
- Answer: (A) and (B)Document18 pagesAnswer: (A) and (B)Germaine Manangan100% (1)
- Problem Set 2Document8 pagesProblem Set 2Katrina Louise GonzalesNo ratings yet
- Chapter 1 Org ChemDocument18 pagesChapter 1 Org ChemBheaBylRiveraNo ratings yet
- 08exam 1keyDocument7 pages08exam 1keySeyi Martins ObandoNo ratings yet
- Chem 112A Homework D KeyDocument13 pagesChem 112A Homework D KeyShyam Bhakta100% (1)
- MotDocument21 pagesMotDheeraj PradeepNo ratings yet
- Organic Chemistry FHSC1124Document64 pagesOrganic Chemistry FHSC1124Hema Jothy100% (1)
- Chapter 11Document30 pagesChapter 11kanilkadianNo ratings yet
- ReactionsDocument30 pagesReactionskaloibestNo ratings yet
- ReactionsDocument5 pagesReactionsYeKwon Patrick HuhNo ratings yet
- NTU CB1117 Tutorial 1Document3 pagesNTU CB1117 Tutorial 1cassidychandidiNo ratings yet
- OchempractwrkshtDocument13 pagesOchempractwrkshtlabaileyNo ratings yet
- Acids and BasesDocument7 pagesAcids and BasesNurliana RoslanNo ratings yet
- 11 Cbse Chemistry Organic ChemistryDocument22 pages11 Cbse Chemistry Organic ChemistryKrish KakkarNo ratings yet
- Solution Manual For Chemistry An Atoms Focused Approach Second Edition Second EditionDocument37 pagesSolution Manual For Chemistry An Atoms Focused Approach Second Edition Second EditionAndrewMartinezjrqo100% (48)
- Chapter 10Document18 pagesChapter 10Khaled NaseerNo ratings yet
- 1 AllDocument18 pages1 AllMarcos ViníciusNo ratings yet
- 303 09FinalKEY PDFDocument15 pages303 09FinalKEY PDFaegaisNo ratings yet
- Organic Chemistry 2021Document26 pagesOrganic Chemistry 2021xapodi8776No ratings yet
- Chapter 1-Structure and Bonding:Acids and BasesDocument12 pagesChapter 1-Structure and Bonding:Acids and BasesANTON JOSHUA G. LAPINIGNo ratings yet
- Marks: D-Block Elements Have From 1 To 10 Electrons in The D-Orbitals. When FormingDocument15 pagesMarks: D-Block Elements Have From 1 To 10 Electrons in The D-Orbitals. When FormingdivaaaaaaaaaNo ratings yet
- Worksheet: Very Short Answer QuestionsDocument4 pagesWorksheet: Very Short Answer QuestionsssNo ratings yet
- CHEM 222 Chap 1 HW F21 KEYDocument3 pagesCHEM 222 Chap 1 HW F21 KEYJessicaNo ratings yet
- Answer Key: Chemistry 206 First Hour ExaminationDocument9 pagesAnswer Key: Chemistry 206 First Hour Examinationsudipta88No ratings yet
- Tutorial Letter 201/1/2017: General Chemistry 1BDocument23 pagesTutorial Letter 201/1/2017: General Chemistry 1BLeigh MakanNo ratings yet
- Organic ChemistryDocument30 pagesOrganic Chemistryj.obriain94No ratings yet
- Chemistry Shift 1 Nest 2023Document8 pagesChemistry Shift 1 Nest 2023Hardik JoshiNo ratings yet
- Compendium On Problems in Physical-Organic ChemistryDocument27 pagesCompendium On Problems in Physical-Organic ChemistrychemptnkNo ratings yet
- Chemistry 22: Some Notes On Chapter 10 Reactions: Y Y Y YDocument6 pagesChemistry 22: Some Notes On Chapter 10 Reactions: Y Y Y Yhairey947594No ratings yet
- Organic Chemistry Exam 1 (Practice) Chem 237Document3 pagesOrganic Chemistry Exam 1 (Practice) Chem 237Ngoc Minh NgoNo ratings yet
- Problems On Named ReactionsDocument103 pagesProblems On Named ReactionsBapu ThoratNo ratings yet
- Kvs Sample Paper Chemistry Page 2 - 6Document5 pagesKvs Sample Paper Chemistry Page 2 - 6Rohan BaghelNo ratings yet
- Chapter 2 Acid and BaseDocument8 pagesChapter 2 Acid and BaseKelsi Kyla PeraltaNo ratings yet
- Reaction MechanismDocument68 pagesReaction MechanismSiddarth Singh73% (11)
- 26 Petrucci10e CSMDocument44 pages26 Petrucci10e CSMAlexNo ratings yet
- Accepted Manuscript: Damiano Tanini, Veronica D'Esopo, Dan Chen, Giulia Barchielli, Antonella CapperucciDocument8 pagesAccepted Manuscript: Damiano Tanini, Veronica D'Esopo, Dan Chen, Giulia Barchielli, Antonella CapperucciAndra AlNo ratings yet
- 2004 - Current Medicinal Chemistry, 11, 1657-1669Document13 pages2004 - Current Medicinal Chemistry, 11, 1657-1669Andra AlNo ratings yet
- Cisplatin in Cancer TherapyDocument33 pagesCisplatin in Cancer TherapyAndra AlNo ratings yet
- Green Analytical Chemistry and Its Perspectives in Bulgaria: E. H. Ivanova, A. K. DetchevaDocument54 pagesGreen Analytical Chemistry and Its Perspectives in Bulgaria: E. H. Ivanova, A. K. DetchevaAndra AlNo ratings yet
- MF4212 Advanced Manufacturing LaboratoryDocument73 pagesMF4212 Advanced Manufacturing Laboratorybomb1 squadNo ratings yet
- Green Oxidation of Menthol Enantiomers and Analysis by Circular Dichroism Spectroscopy: An Advanced Organic Chemistry LaboratoryDocument3 pagesGreen Oxidation of Menthol Enantiomers and Analysis by Circular Dichroism Spectroscopy: An Advanced Organic Chemistry LaboratoryOscar Ramirez MartinezNo ratings yet
- Đánh Giá Phần Mềm Quản Lý Bất Động SảnDocument67 pagesĐánh Giá Phần Mềm Quản Lý Bất Động SảnNguyễn Thế PhongNo ratings yet
- BS 7671-2001 - 16th Edition IEE Wiring Regulations - Design & VerificationsDocument8 pagesBS 7671-2001 - 16th Edition IEE Wiring Regulations - Design & VerificationsAshwin DuhonarrainNo ratings yet
- Sae Technical Paper Series 2015-36-0353: Static and Dynamic Analysis of A Chassis of A Prototype CarDocument12 pagesSae Technical Paper Series 2015-36-0353: Static and Dynamic Analysis of A Chassis of A Prototype CarGanesh KCNo ratings yet
- Design and Development of Automatic Wheelchair Cum Patient BedDocument90 pagesDesign and Development of Automatic Wheelchair Cum Patient BedSRL MECHNo ratings yet
- Winac RTX 2010 Manual en-US en-US 0123Document296 pagesWinac RTX 2010 Manual en-US en-US 0123Riki PermanaNo ratings yet
- 132 KV SubstationDocument12 pages132 KV SubstationAnonymous HyOfbJ6No ratings yet
- GRDSLABDocument22 pagesGRDSLABCesar Rosas100% (1)
- Basics of Motor Starters and ContactorsDocument37 pagesBasics of Motor Starters and ContactorsTrifonas Krommidas100% (1)
- Kafd SationDocument21 pagesKafd SationmisharyNo ratings yet
- Stat 372 Midterm W14 SolutionDocument4 pagesStat 372 Midterm W14 SolutionAdil AliNo ratings yet
- Research Methodology MBADocument3 pagesResearch Methodology MBAYogesh KumarNo ratings yet
- Math T STPM Semester 1 2014Document2 pagesMath T STPM Semester 1 2014tchinhuat82No ratings yet
- Performance (COP) Analysis of A Vapour Compression Refrigeration System Component With Nano CoatingDocument4 pagesPerformance (COP) Analysis of A Vapour Compression Refrigeration System Component With Nano CoatingantonytechnoNo ratings yet
- Exploration of Geriatric Care Competencies In.4Document8 pagesExploration of Geriatric Care Competencies In.4Mesh CoyivNo ratings yet
- Och2016 17 PDFDocument28 pagesOch2016 17 PDFPayal SharmaNo ratings yet
- Activity No.6 FlowchartDocument1 pageActivity No.6 FlowchartKai GuzmanNo ratings yet
- IUPAC Provisional Recommendations: Table VII Ligand AbbreviationsDocument12 pagesIUPAC Provisional Recommendations: Table VII Ligand AbbreviationsSalih PaşaNo ratings yet
- Process Control Verification To Prevent Hydrogen Embrittlement in Plated or Coated FastenersDocument9 pagesProcess Control Verification To Prevent Hydrogen Embrittlement in Plated or Coated FastenersDarwin DarmawanNo ratings yet
- BenchmarkDocument35 pagesBenchmarkRk G MagzNo ratings yet
- Q SolvedDocument4 pagesQ SolvedAkhilvjohnNo ratings yet
- Learning With Kernels Support Vector Machines, Regularization, Optimization, and Beyond by Bernhard Schlkopf, Alexander J. SmolaDocument644 pagesLearning With Kernels Support Vector Machines, Regularization, Optimization, and Beyond by Bernhard Schlkopf, Alexander J. SmolaCyrus RayNo ratings yet
- Ye Tu19 Turning IDocument36 pagesYe Tu19 Turning IferNo ratings yet
- DHI-MXVR1004: H.265 Penta-Brid 2 SDDocument2 pagesDHI-MXVR1004: H.265 Penta-Brid 2 SDMiguel PradoNo ratings yet
- Wwkzii: Jan. 10, 1933. R. H. FarwellDocument3 pagesWwkzii: Jan. 10, 1933. R. H. Farwellmonem2014100% (1)
- 6 Construccion de Registro de ProductosDocument35 pages6 Construccion de Registro de Productostobi perezNo ratings yet