Professional Documents
Culture Documents
Tech Info Baume Scale
Tech Info Baume Scale
Uploaded by
bill_gator20059105Copyright:
Available Formats
You might also like
- Heat ExchangersDocument14 pagesHeat ExchangersAdrian StoicescuNo ratings yet
- Chapter 1: Boilers and ComponentsDocument12 pagesChapter 1: Boilers and ComponentseugeneNo ratings yet
- Survey On: "Real Time Water Quality Monitoring System Using Iot and Machine Learning"Document3 pagesSurvey On: "Real Time Water Quality Monitoring System Using Iot and Machine Learning"Snehal100% (1)
- Radiation Heat TransferDocument3 pagesRadiation Heat TransferAnonymous 2BJgxbxJNo ratings yet
- Lab Manuals 2CH403 IPC Jan 2020 PDFDocument47 pagesLab Manuals 2CH403 IPC Jan 2020 PDFSamriddha Das GuptaNo ratings yet
- Temperature Measuring Devices ExperimentDocument3 pagesTemperature Measuring Devices ExperimentNoman Iqbal BhattiNo ratings yet
- Determination of Chemical Oxygen DemandDocument9 pagesDetermination of Chemical Oxygen DemandAnand SolomonNo ratings yet
- Analysis Using Temperature Sensor DS18B20 HydroponicDocument9 pagesAnalysis Using Temperature Sensor DS18B20 Hydroponicjenixson tamondongNo ratings yet
- Electrical Machines Lab-II ManualDocument41 pagesElectrical Machines Lab-II Manualsuresh270No ratings yet
- Final Paper 1 Reg Set A20.04.2017 PDFDocument15 pagesFinal Paper 1 Reg Set A20.04.2017 PDFAnudeep ChittluriNo ratings yet
- Chapter 7 - Phase DiagramDocument52 pagesChapter 7 - Phase DiagramHoongNo ratings yet
- 1180 Exp 04, Density and Specific GravityDocument13 pages1180 Exp 04, Density and Specific GravityShaniCoolestNo ratings yet
- Equipments Regarding AbsorptionDocument7 pagesEquipments Regarding AbsorptionGerry Lou QuilesNo ratings yet
- Reaction Lab Manual PDFDocument25 pagesReaction Lab Manual PDFHasan AkhuamariNo ratings yet
- Arrangement of Atoms in MetalsDocument12 pagesArrangement of Atoms in MetalsNada AliaNo ratings yet
- Thermal Conductivity Detector (TCD) : Not As Sensitive Non-Specific Non-DestructiveDocument11 pagesThermal Conductivity Detector (TCD) : Not As Sensitive Non-Specific Non-DestructiveKajal Dhawde100% (1)
- Humidification and Cooling Towers FullDocument114 pagesHumidification and Cooling Towers FullHoorish NiaziNo ratings yet
- Heat Transfer MCQDocument167 pagesHeat Transfer MCQgaith.tw1997No ratings yet
- Tutorial SheetsDocument11 pagesTutorial SheetsKAMARAJU SAI VAMSHINo ratings yet
- Chap 012Document50 pagesChap 012Nguyễn TúNo ratings yet
- SCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteDocument41 pagesSCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteSivaSaiNo ratings yet
- 8 Vapour Power CycleDocument6 pages8 Vapour Power CycleSurendar PerumalNo ratings yet
- BODDocument7 pagesBODIrfan AhmedNo ratings yet
- Electric Circuits 3 QPDocument7 pagesElectric Circuits 3 QPPREM OfFiCiAlNo ratings yet
- Electrical SystemDocument75 pagesElectrical SystemS Bharadwaj Reddy75% (4)
- Grade 10 States of Matter Handout 2Document6 pagesGrade 10 States of Matter Handout 2Dexter TorringtonNo ratings yet
- CTDCHA2 - Learning Unit 3 2019Document39 pagesCTDCHA2 - Learning Unit 3 2019Brandon GreenwoodNo ratings yet
- Choking in A Convergent-Divergent DuctDocument11 pagesChoking in A Convergent-Divergent DuctDon CarlosNo ratings yet
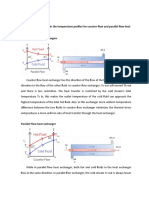
- Describe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat ExchangersDocument3 pagesDescribe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat Exchangersbyun baekNo ratings yet
- Basic Unit OperationsDocument2 pagesBasic Unit OperationsErwin CabangalNo ratings yet
- Gas Chromatography: ENVE 202 Dr. Aslıhan KerçDocument18 pagesGas Chromatography: ENVE 202 Dr. Aslıhan KerçlordniklausNo ratings yet
- IOT Based Water Quality Monitoring System PDFDocument6 pagesIOT Based Water Quality Monitoring System PDFsaknisurkaNo ratings yet
- Lecture 6 - Principle of PEDocument45 pagesLecture 6 - Principle of PEkurddoski28No ratings yet
- Chapter 4 Illumination1Document37 pagesChapter 4 Illumination1GurusumiNo ratings yet
- محاضرة في معالجة مياه الغلاياتDocument41 pagesمحاضرة في معالجة مياه الغلاياتCHE.ENG1734No ratings yet
- CHAPTER 2 Psychrometry and Air-ConditioningDocument14 pagesCHAPTER 2 Psychrometry and Air-ConditioningMdnor Rahim0% (1)
- Manufacture of Acetylene by Paraffin Hydrocarbons : Wulff ProcessDocument8 pagesManufacture of Acetylene by Paraffin Hydrocarbons : Wulff ProcessTones&Feels100% (1)
- Ferrite Processing: Powder Preparation-Raw Materials SelectionDocument66 pagesFerrite Processing: Powder Preparation-Raw Materials Selection吳尚謙No ratings yet
- Lecture 31 PDFDocument4 pagesLecture 31 PDFBhavesh Dilip ChanchlaniNo ratings yet
- The Basic Oxygen Steelmaking (BOS) ProcessDocument7 pagesThe Basic Oxygen Steelmaking (BOS) Processaecsuresh35No ratings yet
- Integral Method of Analysis of DataDocument15 pagesIntegral Method of Analysis of DataImran UnarNo ratings yet
- AbsorptionDocument42 pagesAbsorptionSumit Singh100% (1)
- Lecture 5Document44 pagesLecture 5lockas222No ratings yet
- Astm 4377-2011 Karl FischerDocument7 pagesAstm 4377-2011 Karl FischerManuel Peña BenavidesNo ratings yet
- Gas Chromotagraphy: B.HemakumarDocument46 pagesGas Chromotagraphy: B.HemakumarAkshayRMishraNo ratings yet
- Ferrous MaterialsDocument11 pagesFerrous MaterialsKing BatistaNo ratings yet
- 29 Ethyl Acetate LQDocument3 pages29 Ethyl Acetate LQAlisa MinasyanNo ratings yet
- Basic Principles Basic Transformer Parameters and Construction Construction Conclusion BibliographyDocument17 pagesBasic Principles Basic Transformer Parameters and Construction Construction Conclusion BibliographySHANKAR PRINTINGNo ratings yet
- Me Lab 2Document14 pagesMe Lab 2Lennin John CaguioaNo ratings yet
- Adsorption CoolingDocument7 pagesAdsorption Coolingnicholas bakaNo ratings yet
- Solid Fuel CharacteristicsDocument20 pagesSolid Fuel CharacteristicsMogahid OsmanNo ratings yet
- 14.0 Experiment On Determination of Total Solids in Water: Sl. NoDocument12 pages14.0 Experiment On Determination of Total Solids in Water: Sl. NoLely CasTroNo ratings yet
- Tia Eia 810 A FinalDocument66 pagesTia Eia 810 A Finaldaeng_ifNo ratings yet
- Fluid PropertiesDocument23 pagesFluid PropertiesKhiara Claudine EspinosaNo ratings yet
- Tutorial 3: Series Impedance and Shunt Admittance: J J J JDocument2 pagesTutorial 3: Series Impedance and Shunt Admittance: J J J JSouvikNo ratings yet
- LITERATURE REVIEW Exp 6Document3 pagesLITERATURE REVIEW Exp 6Meenakchi Anuradha100% (2)
- HumidificationDocument68 pagesHumidificationA AshokNo ratings yet
- Tech Info Baume Scale PDFDocument2 pagesTech Info Baume Scale PDFYoshuaOndangNo ratings yet
- Baume Scale HydrometerDocument3 pagesBaume Scale HydrometerSaro HNo ratings yet
- An Introductory Course of Quantitative Chemical Analysis: With Explanatory NotesFrom EverandAn Introductory Course of Quantitative Chemical Analysis: With Explanatory NotesNo ratings yet
- TrumpDocument1 pageTrumpbill_gator20059105No ratings yet
- Read MeDocument1 pageRead Mebill_gator20059105No ratings yet
- Funky Monkey Too9Document6 pagesFunky Monkey Too9bill_gator20059105No ratings yet
- Winky 5Document2 pagesWinky 5bill_gator20059105No ratings yet
- Two More Uses of Electrolysis: When Electrodes Are Not InertDocument2 pagesTwo More Uses of Electrolysis: When Electrodes Are Not InertShahid Ur RehmanNo ratings yet
- Parafin 3Document1 pageParafin 3Andrianna NastasyaNo ratings yet
- Rickers Et Al-2001-Journal of Metamorphic GeologyDocument20 pagesRickers Et Al-2001-Journal of Metamorphic GeologyAmitava SahaNo ratings yet
- Fluid Phase Equilibria: Muthanna J. Ahmed, Samar K. DhedanDocument6 pagesFluid Phase Equilibria: Muthanna J. Ahmed, Samar K. DhedanJijo RajendranNo ratings yet
- Ammonia and Urea Production.Document10 pagesAmmonia and Urea Production.180190105084rabadiyahenilNo ratings yet
- January 2018 MS - Paper 1C Edexcel Chemistry IGCSEDocument23 pagesJanuary 2018 MS - Paper 1C Edexcel Chemistry IGCSEVideesha AmunugamaNo ratings yet
- Adsorption of Tetracycline, Ofloxacin and Cephalexin Antibiotics On BoronDocument12 pagesAdsorption of Tetracycline, Ofloxacin and Cephalexin Antibiotics On Boronikhan1234No ratings yet
- Dupont Kalrez Perfluoroelastomer Parts: Chemical Process Industry (Cpi) Product Selector GuideDocument5 pagesDupont Kalrez Perfluoroelastomer Parts: Chemical Process Industry (Cpi) Product Selector GuideskullbasherNo ratings yet
- Purificiation and Characterisation of Organic CompoundsDocument15 pagesPurificiation and Characterisation of Organic CompoundsHari BabuNo ratings yet
- E-Book: Total Organic Carbon For Cleaning ValidationDocument21 pagesE-Book: Total Organic Carbon For Cleaning ValidationM. S. Chikkamani100% (2)
- Nano Select - 2023 - Mekuye - Nanomaterials An Overview of Synthesis Classification Characterization and ApplicationsDocument16 pagesNano Select - 2023 - Mekuye - Nanomaterials An Overview of Synthesis Classification Characterization and ApplicationsAmeer HamzaNo ratings yet
- Giving Out Energy As ElectricityDocument2 pagesGiving Out Energy As ElectricityShahid Ur RehmanNo ratings yet
- 9CH0 03 Que 20201020Document32 pages9CH0 03 Que 20201020Jovian AlvinoNo ratings yet
- Alcohol Jeemain - GuruDocument36 pagesAlcohol Jeemain - GuruSailost Jr.No ratings yet
- 2013 - Optimization of Composite Admixtures Used in Cementation Formula For Radioactive Evaporator ConcentratesDocument5 pages2013 - Optimization of Composite Admixtures Used in Cementation Formula For Radioactive Evaporator ConcentratesÉrica RodriguesNo ratings yet
- 1 s2.0 S0141813016327878 MainDocument27 pages1 s2.0 S0141813016327878 MainAlvaro Matus CNo ratings yet
- Drinking Water Quality Assessment and Its Effects On Residents Health in Wondo Genet Campus, Ethiopia - Environmental Systems Research - Full TextDocument23 pagesDrinking Water Quality Assessment and Its Effects On Residents Health in Wondo Genet Campus, Ethiopia - Environmental Systems Research - Full Textkim verdanaNo ratings yet
- Polysulphate Sales Sheet For UsaDocument2 pagesPolysulphate Sales Sheet For UsaEmatrix Sdn BhdNo ratings yet
- BSSA Passivation Stainless SteelDocument2 pagesBSSA Passivation Stainless SteelRuth KremerNo ratings yet
- CRYSTAL STRUCTURE of SpinelDocument5 pagesCRYSTAL STRUCTURE of SpinelRAM KUMARNo ratings yet
- MSDS PH. Acid 85%Document7 pagesMSDS PH. Acid 85%Charlie Coca NoeNo ratings yet
- W N 05 Bangur Power NewDocument1 pageW N 05 Bangur Power Newcricketsanju1811No ratings yet
- ISO-11469-2000 Material MarkingDocument8 pagesISO-11469-2000 Material MarkingGerald CelisNo ratings yet
- Chemistry PYQs (2016-2022)Document14 pagesChemistry PYQs (2016-2022)bhopalrajak786No ratings yet
- Solid State - Study Material - Yak9Document33 pagesSolid State - Study Material - Yak9Amrit Kumar BiswasNo ratings yet
- Debrecen-University-study Hungary Chemistry Sample Test 1Document5 pagesDebrecen-University-study Hungary Chemistry Sample Test 14ccqn96sr7No ratings yet
- Microbialites - Organosedimentary Deposits of Benthic Microbial CommunitiesDocument18 pagesMicrobialites - Organosedimentary Deposits of Benthic Microbial CommunitiesCarlos Alquinta PNo ratings yet
- Crodamol AB - FTDocument3 pagesCrodamol AB - FTalexanderNo ratings yet
- UntitledDocument330 pagesUntitledEduardo NascimentoNo ratings yet
- 2 - Akbar 2022 - Melamine-Based Porous Network Thin Film at Oil - Water Interface As Effective Catalyst For Reduction of PDocument17 pages2 - Akbar 2022 - Melamine-Based Porous Network Thin Film at Oil - Water Interface As Effective Catalyst For Reduction of Payuk novaNo ratings yet
Tech Info Baume Scale
Tech Info Baume Scale
Uploaded by
bill_gator20059105Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tech Info Baume Scale
Tech Info Baume Scale
Uploaded by
bill_gator20059105Copyright:
Available Formats
Technical Information:
Date: Subject: Topic : March 2006 Water Treatment - Degree Baume Scale The Term Degree Baume
Also referred to as Baum B, B, degrees Baum, Baume, Baum scale. Periodically we get calls with someone wanting to know what they should use with a chemical and give reference to a Degree Baume. E.g. 66 Baume Sulphuric Acid ? If asked most people would have no idea what this refers to. What does Baume mean with regards to chemical and concentration ? The Baume scale is an old antiquated method of measurement of a chemicals Specific Gravity. It was devised a long time ago and is a scaling on a hydrometer which measures the Specific Gravity of solutions. To really make things confusing there are 2 ways of using this scale, one for liquids that are more dense than water and one for liquids less dense than water. Liquids with lower S.G. than water, Liquids with higher S.G. than water S.G = 140 / ( B + 130 ) S.G = 145 / (145 B )
So, if you have the Baume of a known chemical, you could calculate the S.G. From this you can compare the S.G. of the particular chemical and see what the actual % concentration would be. This does seem cumbersome and normally the charts of % concentration and S.G. are not so readily available. The following 4 examples are how this conversion would be done, first 3 are for some typical chemicals used with a higher density than water and the fourth has a density less than that of water.
Example 1 66 B Sulphuric Acid. H2SO4 SG = 145 / ( 145 B ) = 145 / ( 145 66 ) = 145 / 79 = 1.835
If you look at the data of Sulphuric Acid you will see that a S.G. of 1.835 represents a concentration of approx. 93% Example 2 20 B Sodium Hydroxide NaOH SG = 145 / ( 145 B ) = 145 / ( 145 20 ) = 145 / 125 = 1.16 If you look at the data of Sodium Hydroxide you will see that a S.G of 1.16 represents a concentration of approx. 15 % Example 3 22 B Hydrochloric Acid HCl SG = 145 / (145 B ) = 145 / ( 145 22 ) = 145 / 123 = 1.1789 If you look at the data of Hydrochloric Acid you will see that a S.G. of 1.1789 represents a concentration of approx. 36% Example 4 26 B Ammonium Hydroxide NH4OH S.G. = 140 / (B + 130 ) = 140 / ( 26 + 130 ) = 140 / 156 = 0.897 If you look at the data of Ammonium Hydroxide you will see that a S.G of 0.897 represents a concentration of approx. 29%.
ProMinent Fluid Controls Ltd 490 Southgate Drive Guelph, Ontario N1G 4P5
Telephone: (519) 836-5692 Fax: (519) 836-5226 E-Mail: info@prominent.ca http://www.prominent.ca
You might also like
- Heat ExchangersDocument14 pagesHeat ExchangersAdrian StoicescuNo ratings yet
- Chapter 1: Boilers and ComponentsDocument12 pagesChapter 1: Boilers and ComponentseugeneNo ratings yet
- Survey On: "Real Time Water Quality Monitoring System Using Iot and Machine Learning"Document3 pagesSurvey On: "Real Time Water Quality Monitoring System Using Iot and Machine Learning"Snehal100% (1)
- Radiation Heat TransferDocument3 pagesRadiation Heat TransferAnonymous 2BJgxbxJNo ratings yet
- Lab Manuals 2CH403 IPC Jan 2020 PDFDocument47 pagesLab Manuals 2CH403 IPC Jan 2020 PDFSamriddha Das GuptaNo ratings yet
- Temperature Measuring Devices ExperimentDocument3 pagesTemperature Measuring Devices ExperimentNoman Iqbal BhattiNo ratings yet
- Determination of Chemical Oxygen DemandDocument9 pagesDetermination of Chemical Oxygen DemandAnand SolomonNo ratings yet
- Analysis Using Temperature Sensor DS18B20 HydroponicDocument9 pagesAnalysis Using Temperature Sensor DS18B20 Hydroponicjenixson tamondongNo ratings yet
- Electrical Machines Lab-II ManualDocument41 pagesElectrical Machines Lab-II Manualsuresh270No ratings yet
- Final Paper 1 Reg Set A20.04.2017 PDFDocument15 pagesFinal Paper 1 Reg Set A20.04.2017 PDFAnudeep ChittluriNo ratings yet
- Chapter 7 - Phase DiagramDocument52 pagesChapter 7 - Phase DiagramHoongNo ratings yet
- 1180 Exp 04, Density and Specific GravityDocument13 pages1180 Exp 04, Density and Specific GravityShaniCoolestNo ratings yet
- Equipments Regarding AbsorptionDocument7 pagesEquipments Regarding AbsorptionGerry Lou QuilesNo ratings yet
- Reaction Lab Manual PDFDocument25 pagesReaction Lab Manual PDFHasan AkhuamariNo ratings yet
- Arrangement of Atoms in MetalsDocument12 pagesArrangement of Atoms in MetalsNada AliaNo ratings yet
- Thermal Conductivity Detector (TCD) : Not As Sensitive Non-Specific Non-DestructiveDocument11 pagesThermal Conductivity Detector (TCD) : Not As Sensitive Non-Specific Non-DestructiveKajal Dhawde100% (1)
- Humidification and Cooling Towers FullDocument114 pagesHumidification and Cooling Towers FullHoorish NiaziNo ratings yet
- Heat Transfer MCQDocument167 pagesHeat Transfer MCQgaith.tw1997No ratings yet
- Tutorial SheetsDocument11 pagesTutorial SheetsKAMARAJU SAI VAMSHINo ratings yet
- Chap 012Document50 pagesChap 012Nguyễn TúNo ratings yet
- SCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteDocument41 pagesSCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteSivaSaiNo ratings yet
- 8 Vapour Power CycleDocument6 pages8 Vapour Power CycleSurendar PerumalNo ratings yet
- BODDocument7 pagesBODIrfan AhmedNo ratings yet
- Electric Circuits 3 QPDocument7 pagesElectric Circuits 3 QPPREM OfFiCiAlNo ratings yet
- Electrical SystemDocument75 pagesElectrical SystemS Bharadwaj Reddy75% (4)
- Grade 10 States of Matter Handout 2Document6 pagesGrade 10 States of Matter Handout 2Dexter TorringtonNo ratings yet
- CTDCHA2 - Learning Unit 3 2019Document39 pagesCTDCHA2 - Learning Unit 3 2019Brandon GreenwoodNo ratings yet
- Choking in A Convergent-Divergent DuctDocument11 pagesChoking in A Convergent-Divergent DuctDon CarlosNo ratings yet
- Describe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat ExchangersDocument3 pagesDescribe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat Exchangersbyun baekNo ratings yet
- Basic Unit OperationsDocument2 pagesBasic Unit OperationsErwin CabangalNo ratings yet
- Gas Chromatography: ENVE 202 Dr. Aslıhan KerçDocument18 pagesGas Chromatography: ENVE 202 Dr. Aslıhan KerçlordniklausNo ratings yet
- IOT Based Water Quality Monitoring System PDFDocument6 pagesIOT Based Water Quality Monitoring System PDFsaknisurkaNo ratings yet
- Lecture 6 - Principle of PEDocument45 pagesLecture 6 - Principle of PEkurddoski28No ratings yet
- Chapter 4 Illumination1Document37 pagesChapter 4 Illumination1GurusumiNo ratings yet
- محاضرة في معالجة مياه الغلاياتDocument41 pagesمحاضرة في معالجة مياه الغلاياتCHE.ENG1734No ratings yet
- CHAPTER 2 Psychrometry and Air-ConditioningDocument14 pagesCHAPTER 2 Psychrometry and Air-ConditioningMdnor Rahim0% (1)
- Manufacture of Acetylene by Paraffin Hydrocarbons : Wulff ProcessDocument8 pagesManufacture of Acetylene by Paraffin Hydrocarbons : Wulff ProcessTones&Feels100% (1)
- Ferrite Processing: Powder Preparation-Raw Materials SelectionDocument66 pagesFerrite Processing: Powder Preparation-Raw Materials Selection吳尚謙No ratings yet
- Lecture 31 PDFDocument4 pagesLecture 31 PDFBhavesh Dilip ChanchlaniNo ratings yet
- The Basic Oxygen Steelmaking (BOS) ProcessDocument7 pagesThe Basic Oxygen Steelmaking (BOS) Processaecsuresh35No ratings yet
- Integral Method of Analysis of DataDocument15 pagesIntegral Method of Analysis of DataImran UnarNo ratings yet
- AbsorptionDocument42 pagesAbsorptionSumit Singh100% (1)
- Lecture 5Document44 pagesLecture 5lockas222No ratings yet
- Astm 4377-2011 Karl FischerDocument7 pagesAstm 4377-2011 Karl FischerManuel Peña BenavidesNo ratings yet
- Gas Chromotagraphy: B.HemakumarDocument46 pagesGas Chromotagraphy: B.HemakumarAkshayRMishraNo ratings yet
- Ferrous MaterialsDocument11 pagesFerrous MaterialsKing BatistaNo ratings yet
- 29 Ethyl Acetate LQDocument3 pages29 Ethyl Acetate LQAlisa MinasyanNo ratings yet
- Basic Principles Basic Transformer Parameters and Construction Construction Conclusion BibliographyDocument17 pagesBasic Principles Basic Transformer Parameters and Construction Construction Conclusion BibliographySHANKAR PRINTINGNo ratings yet
- Me Lab 2Document14 pagesMe Lab 2Lennin John CaguioaNo ratings yet
- Adsorption CoolingDocument7 pagesAdsorption Coolingnicholas bakaNo ratings yet
- Solid Fuel CharacteristicsDocument20 pagesSolid Fuel CharacteristicsMogahid OsmanNo ratings yet
- 14.0 Experiment On Determination of Total Solids in Water: Sl. NoDocument12 pages14.0 Experiment On Determination of Total Solids in Water: Sl. NoLely CasTroNo ratings yet
- Tia Eia 810 A FinalDocument66 pagesTia Eia 810 A Finaldaeng_ifNo ratings yet
- Fluid PropertiesDocument23 pagesFluid PropertiesKhiara Claudine EspinosaNo ratings yet
- Tutorial 3: Series Impedance and Shunt Admittance: J J J JDocument2 pagesTutorial 3: Series Impedance and Shunt Admittance: J J J JSouvikNo ratings yet
- LITERATURE REVIEW Exp 6Document3 pagesLITERATURE REVIEW Exp 6Meenakchi Anuradha100% (2)
- HumidificationDocument68 pagesHumidificationA AshokNo ratings yet
- Tech Info Baume Scale PDFDocument2 pagesTech Info Baume Scale PDFYoshuaOndangNo ratings yet
- Baume Scale HydrometerDocument3 pagesBaume Scale HydrometerSaro HNo ratings yet
- An Introductory Course of Quantitative Chemical Analysis: With Explanatory NotesFrom EverandAn Introductory Course of Quantitative Chemical Analysis: With Explanatory NotesNo ratings yet
- TrumpDocument1 pageTrumpbill_gator20059105No ratings yet
- Read MeDocument1 pageRead Mebill_gator20059105No ratings yet
- Funky Monkey Too9Document6 pagesFunky Monkey Too9bill_gator20059105No ratings yet
- Winky 5Document2 pagesWinky 5bill_gator20059105No ratings yet
- Two More Uses of Electrolysis: When Electrodes Are Not InertDocument2 pagesTwo More Uses of Electrolysis: When Electrodes Are Not InertShahid Ur RehmanNo ratings yet
- Parafin 3Document1 pageParafin 3Andrianna NastasyaNo ratings yet
- Rickers Et Al-2001-Journal of Metamorphic GeologyDocument20 pagesRickers Et Al-2001-Journal of Metamorphic GeologyAmitava SahaNo ratings yet
- Fluid Phase Equilibria: Muthanna J. Ahmed, Samar K. DhedanDocument6 pagesFluid Phase Equilibria: Muthanna J. Ahmed, Samar K. DhedanJijo RajendranNo ratings yet
- Ammonia and Urea Production.Document10 pagesAmmonia and Urea Production.180190105084rabadiyahenilNo ratings yet
- January 2018 MS - Paper 1C Edexcel Chemistry IGCSEDocument23 pagesJanuary 2018 MS - Paper 1C Edexcel Chemistry IGCSEVideesha AmunugamaNo ratings yet
- Adsorption of Tetracycline, Ofloxacin and Cephalexin Antibiotics On BoronDocument12 pagesAdsorption of Tetracycline, Ofloxacin and Cephalexin Antibiotics On Boronikhan1234No ratings yet
- Dupont Kalrez Perfluoroelastomer Parts: Chemical Process Industry (Cpi) Product Selector GuideDocument5 pagesDupont Kalrez Perfluoroelastomer Parts: Chemical Process Industry (Cpi) Product Selector GuideskullbasherNo ratings yet
- Purificiation and Characterisation of Organic CompoundsDocument15 pagesPurificiation and Characterisation of Organic CompoundsHari BabuNo ratings yet
- E-Book: Total Organic Carbon For Cleaning ValidationDocument21 pagesE-Book: Total Organic Carbon For Cleaning ValidationM. S. Chikkamani100% (2)
- Nano Select - 2023 - Mekuye - Nanomaterials An Overview of Synthesis Classification Characterization and ApplicationsDocument16 pagesNano Select - 2023 - Mekuye - Nanomaterials An Overview of Synthesis Classification Characterization and ApplicationsAmeer HamzaNo ratings yet
- Giving Out Energy As ElectricityDocument2 pagesGiving Out Energy As ElectricityShahid Ur RehmanNo ratings yet
- 9CH0 03 Que 20201020Document32 pages9CH0 03 Que 20201020Jovian AlvinoNo ratings yet
- Alcohol Jeemain - GuruDocument36 pagesAlcohol Jeemain - GuruSailost Jr.No ratings yet
- 2013 - Optimization of Composite Admixtures Used in Cementation Formula For Radioactive Evaporator ConcentratesDocument5 pages2013 - Optimization of Composite Admixtures Used in Cementation Formula For Radioactive Evaporator ConcentratesÉrica RodriguesNo ratings yet
- 1 s2.0 S0141813016327878 MainDocument27 pages1 s2.0 S0141813016327878 MainAlvaro Matus CNo ratings yet
- Drinking Water Quality Assessment and Its Effects On Residents Health in Wondo Genet Campus, Ethiopia - Environmental Systems Research - Full TextDocument23 pagesDrinking Water Quality Assessment and Its Effects On Residents Health in Wondo Genet Campus, Ethiopia - Environmental Systems Research - Full Textkim verdanaNo ratings yet
- Polysulphate Sales Sheet For UsaDocument2 pagesPolysulphate Sales Sheet For UsaEmatrix Sdn BhdNo ratings yet
- BSSA Passivation Stainless SteelDocument2 pagesBSSA Passivation Stainless SteelRuth KremerNo ratings yet
- CRYSTAL STRUCTURE of SpinelDocument5 pagesCRYSTAL STRUCTURE of SpinelRAM KUMARNo ratings yet
- MSDS PH. Acid 85%Document7 pagesMSDS PH. Acid 85%Charlie Coca NoeNo ratings yet
- W N 05 Bangur Power NewDocument1 pageW N 05 Bangur Power Newcricketsanju1811No ratings yet
- ISO-11469-2000 Material MarkingDocument8 pagesISO-11469-2000 Material MarkingGerald CelisNo ratings yet
- Chemistry PYQs (2016-2022)Document14 pagesChemistry PYQs (2016-2022)bhopalrajak786No ratings yet
- Solid State - Study Material - Yak9Document33 pagesSolid State - Study Material - Yak9Amrit Kumar BiswasNo ratings yet
- Debrecen-University-study Hungary Chemistry Sample Test 1Document5 pagesDebrecen-University-study Hungary Chemistry Sample Test 14ccqn96sr7No ratings yet
- Microbialites - Organosedimentary Deposits of Benthic Microbial CommunitiesDocument18 pagesMicrobialites - Organosedimentary Deposits of Benthic Microbial CommunitiesCarlos Alquinta PNo ratings yet
- Crodamol AB - FTDocument3 pagesCrodamol AB - FTalexanderNo ratings yet
- UntitledDocument330 pagesUntitledEduardo NascimentoNo ratings yet
- 2 - Akbar 2022 - Melamine-Based Porous Network Thin Film at Oil - Water Interface As Effective Catalyst For Reduction of PDocument17 pages2 - Akbar 2022 - Melamine-Based Porous Network Thin Film at Oil - Water Interface As Effective Catalyst For Reduction of Payuk novaNo ratings yet