Professional Documents
Culture Documents
Acute Interstitial Pneumonitis: Current Understanding Regarding Diagnosis, Pathogenesis, and Natural History
Acute Interstitial Pneumonitis: Current Understanding Regarding Diagnosis, Pathogenesis, and Natural History
Uploaded by
Samuel HernandezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acute Interstitial Pneumonitis: Current Understanding Regarding Diagnosis, Pathogenesis, and Natural History
Acute Interstitial Pneumonitis: Current Understanding Regarding Diagnosis, Pathogenesis, and Natural History
Uploaded by
Samuel HernandezCopyright:
Available Formats
Acute Interstitial Pneumonitis: Current Understanding Regarding Diagnosis, Pathogenesis, and Natural History
Jason S. Vourlekis, M.D.,1,2 Kevin K. Brown, M.D.,1,2 and Marvin I. Schwarz, M.D.1,2
ABSTRACT
Acute interstitial pneumonitis (AIP) is a fulminant disease culminating in acute respiratory failure and often death.1 Originally described in 1935 by the pathologists Hamman and Rich, this rare syndrome is characterized by rapidly progressive pulmonary fibrosis, leading to frequent confusion with idiopathic pulmonary fibrosis.2 In fact, the eponym Hamman-Rich syndrome became synonymous with idiopathic pulmonary fibrosis despite clear differences in clinical presentation, radiography, pathology, and survival.3 In 1986, Katzenstein described eight patients with acute respiratory failure of unknown etiology.4 On biopsy, organizing diffuse alveolar damage was present in all specimens. Given the idiopathic nature of the disease, Katzenstein coined the phrase acute interstitial pneumonitis to distinguish it from the fibroproliferative stage of the acute respiratory distress syndrome (ARDS), which has an identical pathology.5 Olson et al retrospectively examined Hamman and Richs original cases, compared them to contemporary cases of AIP, and found the two identical.6 Since then, little progress into understanding this disease has been made. Many questions still linger regarding the epidemiology, pathogenesis, and outcome. We recently published our experience with AIP providing new information regarding natural history.7 This review summarizes the current literature on AIP emphasizing diagnostic criteria, pathogenesis, and natural history.
KEYWORDS: Acute interstitial pneumonitis, acute respiratory distress syndrome,
diffuse alveolar damage, Hamman-Rich syndrome, pulmonary fibrosis
Objectives: Upon completion of this article, the reader will be familiar with the most current information on acute interstitial pneumonitis, including and with special reference to diagnostic criteria, pathogenesis, and natural history. Accreditation: The University of Michigan is accredited by the Accreditation Council for Continuing Medical Education to sponsor continuing medical education for physicians. Credit: The University of Michigan designates this educational activity for a maximum of 1.0 hours in category one credit toward the AMA Physicians Recognition Award.
Seminars in Respiratory and Critical Care Medicine, volume 22, number 4, 2001. Address for correspondence and reprint requests: Jason S. Vourlekis, M.D., National Jewish Medical and Research Center, 1400 Jackson Street, F107, Denver, CO 80206. E-mail: vourlekisj@njc.org. 1National Jewish Medical and Research Center, Denver, Colorado; 2University of Colorado Health Sciences Center, Denver, Colorado. Copyright 2001 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. Tel: +1(212) 584-4662. 10693424,p;2001,22,04,399,408,ftx,en;srm00091x.
399
400
SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE/VOLUME 22, NUMBER 4 2001
CASE DEFINITION Table 1 lists the criteria for a diagnosis of acute interstitial pneumonitis (AIP). The salient clinical features are that of an acute respiratory illness in a previously healthy person associated with diffuse, bilateral infiltrates on chest radiography and without any known inciting event. A definitive diagnosis requires the demonstration of organizing diffuse alveolar damage (DAD) by surgical lung biopsy.3 Based on our literature review, we used a symptom duration of 60 days or less as the threshold to differentiate AIP from more insidious forms of fibrotic interstitial lung disease.7 This resulted in the exclusion of only one potential subject from our series and no cases from the medical literature. An additional three subjects in our series were excluded on the basis of a previously abnormal chest x-ray. The distinction is important because acute exacerbations of idiopathic pulmonary fibrosis clinically and pathologically mimic AIP.8,9
Figure 1 Photomicrograph (200 )of hematoxylin-eosin stained early diffuse alveolar damage. A hyaline membrane (arrowhead) rims an edematous alveolar septum (arrow).
PATHOGENESIS The earliest lung lesion in AIP is unknown. Based on the uniformity and extent of the injury present on lung biopsy, it is assumed that a single insult triggers the pathologic process.3 The presence of hyaline membrane remnants and organizing alveolar exudate suggests that AIP progresses from the exudative stage of DAD, shown in Figure 1, to the proliferative stage (Fig. 2).5 DAD is the same lesion present in ARDS.5 In early DAD, injury to the pulmonary capillary endothelium results in exudation of proteinaceous fluid into the alveolar space with formation of fibrin-rich hyaline membranes.10 Upregulation of cellular adhesion molecules on the endothelium with formation of gaps between cells allows the margination and migration of neutrophils from the capillary lumen into the alveolar septum and ultimately the alveolar space.11 These steps are associated with the activation of neutrophils and resident alveolar macrophages leading to the production of
proinflammatory cytokines and subsequent escalation of the intensity of the injury.12 Concomitant with the endothelial injury is injury to the alveolar epithelium.13,14 In the normal alveolus, the epithelium is composed of type I cells that cover approximately 90% of the alveolar surface area and type II cells that cover the remaining 10%.15 Type I cells are important to the maintenance of the alveolar septal barrier.12 Loss of this barrier enhances exudation of fluid from the vascular space. Further, it allows for the apposition of adjacent alveolar septa resulting in alveolar space collapse.13 Microscopic studies of DAD confirm the loss of the epithelial cell barrier.14 Type I cell loss likely occurs as a direct result of the initial injury.16 It is associated with disappearance of laminin, a basement membrane protein that serves to anchor the epithelium.14 Type II cells may sustain direct injury leading to necrosis but also undergo programmed cell death.13,17,18 In severe cases, the injured epithelial cells are replaced by cuboidal
Table 1 Case Definition of Acute Interstitial Pneumonitis
Required Criteria Acute lower respiratory tract illness of 60 days duration Diffuse bilateral radiographic infiltrates Organizing or proliferative diffuse alveolar damage on lung biopsy Absence of any known inciting event or predisposing condition including: infection, systemic inflammatory response syndrome (SIRS), environmental or toxic exposures, connective tissue disease, prior interstitial lung disease Absence of a previously abnormal chest x-ray
Figure 2 Photomicrograph (200 )of hematoxylin-eosin stained proliferative diffuse alveolar damage. Note the markedly thickened alveolar septum (white arrow) due to fibroblast proliferation with collagen production. There is near obliteration of air spaces (arrowhead) and type II cell hyperplasia (black arrow).
ACUTE INTERSTITIAL PNEUMONITIS/VOURLEKIS ET AL
401
epithelium of bronchiolar origin, termed bronchiolization of alveolar spaces. In other cases, type II cells proliferate and ultimately reestablish the epithelial barrier.19 Type II cells are metabolically active cells that, under normal physiological conditions, are responsible for surfactant production.15 However, they retain considerable regenerative capacity and in the event of injury to the alveolar epithelium will proliferate to restore the epithelial surface.20 They also may contribute to the inflammatory process by elaboration of cytokine molecules.2123 The orderly resolution of the fibrin-rich hyaline membranes may be crucial to lung healing in DAD.24 Hyaline membranes provide a scaffolding that allows migration of inflammatory cells, fibroblasts, and myofibroblasts into the alveolar space. In animal models of acute lung injury, increased fibrinolytic activity is associated with a protective phenotype against fibrosis, whereas decreased activity results in greater fibrosis.25 In healthy lung, the alveolar epithelium maintains a net fibrinolytic environment by production of both urokinase and plasminogen activator inhibitor.2628 ARDS is associated with a transition to a procoagulant environment, which is likely due to loss of the alveolar epithelial lining.29,30 Hence, the degree of injury to the epithelium and its ability to recover may be critical factors in determining whether lung healing or lung fibrosis develops.20 The steps marking the transition from the more inflammatory exudative stage of DAD to the fibrosing, proliferative stage are not fully elucidated. Tumor necrosis factor-alpha (TNF-) and interleukin-1-beta are manufactured by type II cells and alveolar macrophages in both stages of DAD.21,22 Animal data suggest that TNF- may be important for early activation of the fibrotic process. In a mouse model of asbestosinduced lung fibrosis, mice homozygous deficient for the TNF- receptor do not develop early fibroproliferative lesions.31 Further, inbred 129 mice, which are innately protected from asbestos-induced fibrosis, show little upregulation in TNF- protein over baseline following asbestos exposure and markedly less in comparison to animals that develop fibroproliferative lesions.32 TNF- signaling is important to upregulation and elaboration of the profibrotic cytokines: transforming growth factors, alpha and beta (TGF-, TGF-), and platelet-derived growth factor (PDGF).31,33 In a rat model of pulmonary fibrosis, overexpression of TNF- induces upregulation of TGF-, suggesting TGF- activation is a requisite step for the induction of fibrosis.33 TGF- stimulates the production of collagen and other extracellular matrix proteins while inhibiting the synthesis of proteases that degrade the extracellular matrix.34,35 Further, TGF- is necessary for stimulation of fibroblast proliferation and differentiation into myofibroblasts.36 TGF- and PDGF are potent inducers of fibroblast proliferation.37,38 PDGF is present in high
concentrations of bronchoalveolar lavage (BAL) fluid from patients with severe ARDS.39 The transition from exudative DAD to proliferative DAD is marked by interstitial thickening, organization of alveolar exudates with partial resolution of hyaline membranes, and early production of collagen.40 Resident pulmonary fibroblasts within the interstitium undergo proliferation and differentiation into myofibroblasts.40 Myofibroblasts are motile cells characterized by positive immunostaining for -smooth muscle actin and vimentin.40 Myofibroblast and alveolar epithelial cell elaboration of matrix metalloproteinases creates disruptions in the alveolar septal basement membrane through which the myofibroblasts are able to migrate into the airspaces.14,41 Therefore, a marked expansion of myofibroblast numbers within the alveolar septa and hyaline membranes of the air spaces occurs, which provides the framework for subsequent collagen production.40 There are two main mechanisms of interstitial thickening and fibrosis in AIP.13 The first mechanism involves fibroblast migration, proliferation, and differentiation into myofibroblasts, which occurs within the alveolar septa and extends into the alveolar exudates. Fibroblastic differentiation into myofibroblasts is associated with early production of collagen types III, IV, and VI and the extracellular matrix protein, fibronectin.4144 Fibronectin is a glycoprotein that stimulates the proliferation and migration of mesenchymal cells and may be important to the reestablishment of a basement membrane.45,46 Staining for collagen types III, IV, and VI is heaviest in areas of fibroblastic proliferation and colocalizes with myofibroblasts.41,42,44 These collagens are synthesized prior to type I collagen.42,44 Evidence that these same areas serve as the focus of type I collagen production is provided by immunostaining demonstrating colocalization of the intracellular propeptide of type I collagen and its molecular chaperone, heat shock protein 47, to areas of fibroblastic proliferation.43,47 Synthesis of collagen VI, which serves as an anchoring filament, may be a necessary prerequisite for type I collagen production.44 Type I collagen is the dominant collagen in end-stage fibrotic lung.44 Its production heralds a state of irreversible fibrosis due to its greater resistance to metalloproteinase digestion.48,49 The second mechanism of fibrosis relates to denudation of the alveolar epithelium and loss of laminin protein from the underlying basement membrane.13,14 Exposure of the naked basement membranes allows apposition of adjacent areas of alveolar septum, which invaginate into clefts.16 In some cases, this process is so extensive as to involve the entire alveolus. Subsequent reepitheliazation of the alveolar space covers the clefts, which are incorporated into alveolar septum. In more extensive cases, type II cell bridging of opposite septal
402
SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE/VOLUME 22, NUMBER 4 2001
walls functions as a glue leading to permanent collapse of the alveolar space.13 The process of lung injury with subsequent development of scar tissue in idiopathic pulmonary fibrosis has been likened to an abnormal wound healing model.50 A similar construct may be applied to AIP. In a normally healing wound, tissue injury is followed by migration and proliferation of fibroblasts, production of extracellular matrix, angiogenesis, reestablishment of the epithelial border, and subsequent fibroblast apoptosis with partial resorption of the extracellular matrix.5052 DAD myofibroblasts are protected from apoptosis by production of anti-apoptotic factors whereas type II cells appear to be at increased risk of apoptosis.1720 The consequences may be exuberant, uncontrolled fibroblastic proliferation and failure to reepithelialize the alveolus. Reepitheliazation of the air space is considered essential to normal healing.16,50 In cutaneous wounds, reepitheliazation occurs prior to complete reestablishment of the basement membrane and may provide important signals to guide tissue repair.51,52 The factors that determine successful tissue repair versus progressive fibrosis in AIP are unknown. The progression from the exudative to the proliferative stage of DAD is not absolute. In an autopsy study of ARDS, only 37.5% of patients mechanically ventilated for 2 or more weeks before death developed pulmonary fibrosis.53 Therefore, other factors, such as host susceptibility, may be necessary to progress to fibrosis.54 For example, polymorphisms in the TGF-1 gene regulate cellular production of TGF-1 and appear to predict the development of allograft fibrosis in lung transplant recipients.55 The role of genetic predisposition to AIP has not been examined directly. However, a similar lung disease occurs in association with rheumatoid arthritis, systemic lupus erythematosus, and polymyositis, diseases for which a genetic basis is well established.56,57
within the air spaces. Collagen staining demonstrates immature collagen bundles within the interstitium and focally, within air spaces. In advanced cases, honeycomb fibrosis may be seen.7 The degree of fibroblastic proliferation can be so exuberant as to resemble usual interstitial pneumonitis (idiopathic pulmonary fibrosis). The pathological differential diagnosis of AIP includes usual interstitial pneumonitis, cryptogenic organizing pneumonia, early DAD, and organizing DAD due to other etiologies.5 Table 2 provides a limited differential diagnosis of organizing DAD. AIP is distinguished from usual interstitial pneumonitis by the diffuse nature of the abnormalities and the relative uniformity of the lesions.3 Pathologically, usual interstitial pneumonitis is characterized by temporally heterogeneous lesions. Normal lung is adjacent to collagenproducing buds of fibroblastic proliferation (fibroblastic foci) and end-stage honeycomb fibrosis. Rarely, an AIPlike process may be superimposed on usual interstitial pneumonitis.59 In cryptogenic organizing pneumonia, the fibroblastic proliferation occurs within the bronchioles and alveolar ducts rather than the interstitium, leading to production of immature collagen bundles, known as Masson bodies.60 Early DAD is characterized by alveolar exudates, alveolar fibrin deposition with hyaline membrane formation, and interstitial edema but not interstitial fibrosis.5 At times, considerable overlap may be present between early and organizing DAD.
EPIDEMIOLOGY Table 3 summarizes the available demographic and outcome data of the five published case series of AIP.4,6,7,61,62 An additional reference was omitted because the cases were duplicated in a later publication.62,63 Most affected patients are adults. The mean age is 54 years but patients as young as 7 years and as old as 83 years have been reported. Forty-nine patients
PATHOLOGY The defining pathological characteristic of AIP is the extensive injury to the lung associated with marked interstitial fibrosis and obscuration of normal anatomic landmarks.4,6 Rarely, the lesions may be patchy, interspersed with areas of normal lung.7,58 The interstitial thickening results from a combination of edema, chronic inflammatory cell infiltrate, fibroblastic proliferation, and production of immature collagen.4 Architecturally, the air spaces are preserved although they may be reduced to slit-like openings. Complete effacement of air spaces sometimes occurs due to apposition of adjacent alveolar septa. Within the air spaces, organizing exudate, type II cell hyperplasia, and scattered hyaline membrane remnants are often present.6 Spindle-shaped fibroblasts with elongated nuclei are present within the alveolar septa and may be present
Table 2 Differential Diagnosis of Organizing Diffuse Alveolar Damage
Acute hypersensitivity pneumonitis Acute respiratory distress syndrome (fibroproliferative stage) Collagen-vascular disease (e.g., dermatomyositis/polymyositis, mixed connective tissue disease, microscopic polyarteritis, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis) Drug-induced lung disease (e.g., amiodarone, bleomycin, busulfan, carmustine, colchicine, crack cocaine, methotrexate, nitrofurantoin) Infection (e.g., Legionella pneumophilia, Mycoplasma pneumoniae, viruses) Inhalants/toxins (e.g., chlorine gas, nitrogen dioxide, oxygen toxicity, paraquat, phosgene)
ACUTE INTERSTITIAL PNEUMONITIS/VOURLEKIS ET AL
403
Table 3 Summary of Published Case Series of Acute Interstitial Pneumonitis
First Author Katzenstein Year Published 1986 Mean Age (Range) 28 (1350) Acute Case Fatality Ratio 62.5
Reference 4
Women 5
Men 3
Comments Two additional deaths within 6 months of hospital discharge, including one from pneumonia, making 1-year mortality 87 .5%. Four of six survivors with normal spirometry at follow-up One subject with systemic lupus erythematosus and 1 subject with idiopathic pulmonary fibrosis were eliminated Two subjects with HRCT improvement 3 and 18 months, respectively, after diagnosis of AIP Two subjects died of AIP recurrence, 2 subjects with progressive fibrosis
6 61
Olson Primack
1990 1993
15 1
14 6
50 (777) 65 (4683)
59 86
62
Johkoh
1999
16
20
61 (2283) 54 (3474) 54 (783)
89
7 Totals
Vourlekis
2000
7 44
6 49
50* 73
*Four subjects seen in outpatient consultation by the authors after discharge for AIP from an outside hospital were excluded from these calculations to eliminate a survivor bias.
were men and 44 were women, indicating there is no gender difference. Summary data on symptom duration is available for 73 patients.6,7,62 Forty-five percent of patients presented within 1 week or less of symptom onset. The mean duration of symptoms was 18.6 days with a range of 0 to 90 days but the median symptom duration was only 7 days. These data indicate a bimodal distribution related to symptom duration with 50% of patients presenting within the first week of symptoms but another 30% not presenting until at least 1 month after symptom onset.6,7
air-space densities, suggesting an atypical pneumonia.7 In most cases, as seen in Figure 3, the radiographic infiltrates progress to a diffuse, alveolar pattern involving all five lobes.61 High resolution computed tomography (HRCT), as seen in Figure 4, shows that most of the in-
CLINICAL FEATURES The rapid onset of symptoms is characteristic of AIP. The majority of patients describe a flulike prodrome, which may include symptoms of sore throat, headache, myalgia, and malaise.1 Cough is present in nearly all patients. Most patients complain of dyspnea at presentation, although occasionally it may be a late symptom.7 Fever was present in 75% in our series but only 35% in other series.7 The physical exam findings in AIP are not specific. Patients appear acutely ill and most will manifest tachycardia, tachypnea, and signs of hypoxemia at baseline. Both crackles and wheezes may be auscultated on chest examination. The presence of an exanthem, synovitis, or other signs of extrapulmonary disease is unusual and suggests an alternative diagnosis. The initial radiographic findings in AIP may be quite minimal. Early chest films often show only patchy
Figure 3 Standard posterior-anterior chest radiograph of a patient with acute interstitial pneumonitis. There is diffuse ground-glass abnormality present within all five lung lobes.
404
SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE/VOLUME 22, NUMBER 4 2001
filtrate is ground-glass, but consolidative infiltrates with air bronchograms are also seen.61,62 With progressive disease, traction bronchiectasis indicative of pulmonary fibrosis develops.62 Most patients will require mechanical ventilation for acute hypoxemic respiratory failure. In our series, the mean duration of mechanical ventilation was 20 days for both survivors and nonsurvivors.7 There is a small subset of AIP patients who have a subacute onset of symptoms. They do not develop acute respiratory failure and may not even require hospitalization. We identified one such patient and Robinson et al reported a second.7,64 Clinically, these patients more closely resemble those originally reported by Hamman and Rich.2,65 In their original series, despite the lack of availability of oxygen therapy and mechanical ventilation, 80% survived from 1 to 6 months after the onset of symptoms. In our experience, without such interventions, most patients would die within hours to days.
MANAGEMENT AND DIFFERENTIAL DIAGNOSIS Any acute lung disease that produces respiratory failure with diffuse radiographic infiltrates must be included in the differential diagnosis of AIP.60 In the absence of exposure associated with lung toxicity, patients should be treated initially for severe community-acquired pneumonia. We advocate early, aggressive diagnostic evaluation in suspected cases, with BAL initially. BAL is helpful in obtaining material for microbial culture, for assessment of possible diffuse alveolar hemorrhage, and
for measurement of cellular differential. In AIP, BAL neutrophilia is the expected finding.1 The identification of significant BAL lymphocytosis or eosinophilia suggests alternative diagnoses such as acute hypersensitivity pneumonitis or eosinophilic pneumonia respectively.60 While transbronchial biopsy is helpful for diagnosis of infection or eosinophilic pneumonia, the amount of tissue provided is usually insufficient to establish a diagnosis of either AIP or another acute interstitial lung disease. Should the results of BAL fail to provide a specific diagnosis, we generally proceed to surgical lung biopsy, which is safe even in mechanically ventilated patients.66 Table 4 lists the differential diagnosis of AIP. Critical to the evaluation of a patient with suspected AIP is a thorough medical history including prior diagnoses, medication and illicit drug use, and environmental/occupational exposures. Many of these conditions can produce the identical pathology of organizing DAD.60 Hence, the biopsy findings are supportive but not diagnostic and must be interpreted carefully in the context of the individual patient. For example, several of the connective tissue diseases are associated with an AIP-like pulmonary syndrome, which, mechanistically, is assumed to be a manifestation of the underlying disease and not idiopathic.6772 Further, the failure to recognize medication-induced pulmonary toxicity and appropriately discontinue the offending agent could have disastrous consequences for the patient. For confirmed cases of AIP, we initiate high doses of intravenous corticosteroids. Typically, methylprednisolone 250 mg every 6 hours for 3 days is given followed by a gradual taper. The efficacy of such treatment in AIP is unproven. In one retrospective study, corticosteroid treatment was not associated with a survival benefit.6 However, in fibroproliferative phase of ARDS, corticosteroids appear to have a role in hastening improvement and increasing survival.73 Alternative
Table 4 Differential Diagnosis of Acute Interstitial Pneumonitis
Acute eosinophilic pneumonia Acute respiratory distress syndrome Collagen-vascular disease Cryptogenic organizing pneumonia (acute variant) Diffuse alveolar hemorrhage Vasculitis-induced (e.g., antiphospholipid antibody syndrome, Behcets syndrome, Goodpastures syndrome, microscopic polyangiitis, Wegeners granulomatosis) Bland hemorrhage (e.g., coagulopathy, idiopathic pulmonary hemosiderosis, mitral stenosis) Drug-induced lung disease Hypersensitivity pneumonitis (acute variant) Infection Inhalation/toxic exposures
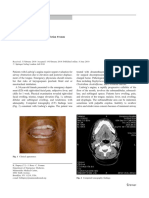
Figure 4 CT scan of a patient with biopsy-proven acute interstitial pneumonitis showing diffuse ground-glass infiltrate in both lungs and posterior consolidation on the right evidenced by air bronchograms. The pattern of abnormality is typical but not diagnostic of acute interstitial pneumonitis.
ACUTE INTERSTITIAL PNEUMONITIS/VOURLEKIS ET AL
405
therapies such as cyclophosphamide have not been adequately investigated and are not recommended. For patients who survive to hospital discharge, we generally taper corticosteroids over 4 to 6 weeks, though a much longer taper (36 months or more) may be necessary. The benefits of longer treatment are unproven.
severity of disease and are unlikely to be related to disease pathogenesis.78
SURVIVAL Table 3 shows the acute case fatality ratios of AIP for published series. In North American studies, the fatality ratio is 50 to 62% and is even higher in international studies.4,6,7,61,62 The mortality from AIP is greater than in patients with acute respiratory failure of any causation and also greater than mortality from ARDS.74,75 The greater mortality of AIP is often cited as evidence that AIP is fundamentally different from ARDS. However, when ARDS subjects are stratified according to presence or absence of fibrosis, survival in fibrotic ARDS is comparable to AIP.76 The difference may lie in the responsiveness of the disease to corticosteroid therapy. Where corticosteroids appear to substantially improve survival in fibroproliferative ARDS, they have not been shown to have any impact on survival in AIP.6,73 The overall estimates of mortality for AIP are similar to early data for ARDS, which approached 70% in the mid-1980s. With improvements in mechanical ventilation strategies and in the overall treatment of critically ill patients, mortality in ARDS declined to 31% in the recently concluded ARDS Network ventilator management study.75,77 Whether these improvements apply to AIP is unknown. Given the low number of reported cases of AIP, it is difficult to make any inferences about trends in survival. The greater survival of AIP patients in North American studies is, in part, due to an ascertainment bias. In North American studies, 88% of AIP patients are identified antemortem by performance of a surgical lung biopsy versus only 36% in international studies.4,6,7,61,62 In the remaining patients, the diagnosis is established at autopsy. Thus, the differences in survival reflect differences in practice style, particularly the likelihood of a patient with acute respiratory failure undergoing diagnostic lung biopsy. Because pathological assessment of lung tissue remains the gold standard for diagnosis of AIP, survivors of AIP not undergoing lung biopsy may remain undetected or may not be included in studies of AIP. Several groups have tried unsuccessfully to identify factors predictive of mortality in AIP. Olson et al examined several histopathologic features, including degree of interstitial fibrosis, and found no correlation with survival.6 We found that survivors, in comparison to nonsurvivors, have statistically significant differences in hematocrit and serum creatinine concentrations at baseline.7 However, these factors relate better to overall
LONG-TERM PROGNOSIS The natural history of AIP in survivors is variable. The current literature provides longitudinal data on 22 survivors.4,6,7,61,62,64 Survivors appear to follow one of four patterns: (1) complete recovery of lung function; (2) stable but persistent abnormalities in lung function; (3) progressive pulmonary fibrosis; (4) recurrent AIP. Eleven of 22 patients were clinically stable with either normal lung function (n = 6) or persistent but stable abnormalities (n = 5). Five patients experienced progressive pulmonary fibrosis, leading to lung transplantation in two. Recurrent acute respiratory failure developed in four patients, leading to death in three. Two patients died of unrelated causes. In contrast to ARDS survivors, AIP survivors appear to be at increased risk of subsequent respiratory events and death.7,79 In most ARDS survivors, maximal recovery of lung function is experienced by 6 months, and progressive lung disease does not occur.80 What factors influence either recurrence or progressive fibrosis in AIP subjects are unknown. We tested the hypothesis that the lung biopsy features of either honeycomb fibrosis or extent of lung injury influences long-term outcome and found no correlation with either variable.7
CONCLUSION AIP is a fulminant respiratory illness associated with high mortality. Much remains unknown about this rare disease. To gain greater insight into the cause(s) of AIP, substantial work needs to be done. Important steps toward achieving this end would include the adoption of a standard set of diagnostic criteria, sharing of valuable and limited patient materials amongst research centers to facilitate and enable experimental investigation, and the establishment of a registry for the reporting of cases.
REFERENCES
1. Bouros D, Nicholson AC, Polychronopoulos V, du Bois RM. Acute interstitial pneumonia. Eur Respir J 2000;15:412418 2. Hamman L, Rich AR. Fulminating diffuse interstitial fibrosis of the lungs. Trans Am Clin Climat Assoc 1935;51:154163 3. Katzenstein ALA, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998;157:13011315 4. Katzenstein ALA, Myers JL, Mazur MT. Acute interstitial pneumonia: a clinicopathologic, ultrastructural, and cell kinetic study. Am J Surg Pathol 1986;10(4):256267 5. Katzenstein AA. Acute Lung Injury Patterns: Diffuse Alveolar Damage and Bronchiolitis Obliterans-Organizing Pneumonia. Katzenstein and Askins Surgical Pathology of Non-neoplastic Lung Disease. 3rd ed. Philadelphia: WB Saunders; 1997:1447
406
SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE/VOLUME 22, NUMBER 4 2001
6. Olson J, Colby TV, Elliott CG. Hamman-Rich syndrome revisited. Mayo Clin Proc 1990;65:15381548 7. Vourlekis JS, Brown KK, Cool CD, et al. Acute interstitial pneumonitis: case series and review of the literature. Medicine 2000;79(6):369378 8. Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. Am J Roentgenol 1997;168:7983 9. Akira M. Computed tomography and pathologic findings in fulminant forms of idiopathic interstitial pneumonia. J Thor Imaging 1999;14:7684 10. Pugin J, Verghese G, Widmer M-C, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 1999;27:304312 11. Doerschuk CM, Mizgerd JP, Kubo H, Qin L, Kumasaka T. Adhesion molecules and cellular biomechanical changes in acute lung injury. Chest 1999;116(1):37S-43S 12. Ware LB, Matthay MA. The acute respiratory distress syndrome. New Engl J Med 2000;342(18):13341349 13. Katzenstein AA. Pathogenesis of fibrosis in interstitial pneumonia: an electron microscopic study. Hum Pathol 1985; 16:10151024 14. Matsubara O, Tamura A, Ohdama S, Mark EJ. Alveolar basement membrane breaks down in diffuse alveolar damage: an immunohistochemical study. Pathol Intl 1995;45:473482 15. Mason RJ, Shannon JM. Alveolar type II cells. In: Crystal RG, West JB, eds. The Lung: Scientific Foundations. Philadelphia, PA: Lippincott-Raven Publishers; 1997 16. Burkhardt A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Respir Dis 1989;140: 513524 17. Guinee D Jr, Fleming M, Hayashi T, et al. Association of p53 and WAF1 expression with apoptosis in diffuse alveolar damage. Am J Pathol 1996;149(2):531538 18. Guinee D Jr, Brambilla E, Fleming M, et al. The potential role of BAX and BCL-2 expression in diffuse alveolar damage. Am J Pathol 1997;151(4):9991007 19. Bardales RH, Xie SS, Schaefer RF, Hsu SM. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol 1996;149(3): 845852 20. Adamson A, Perkins S, Brambilla E, et al. Proliferation, Cmyc, and cyclin D11 expression in diffuse alveolar damage: potential roles in pathogenesis and implications for prognosis. Hum Pathol 1999;30:10501057 21. Nash JRG, McLaughlin PJ, Hoyle C, Roberts D. Immunolocalization of tumour necrosis factor a in lung tissue from patients dying with adult respiratory distress syndrome. Histopathol 1991;19:395402 22. Pan L, Ohtani H, Yamauchi K, Nagura H. Co-expression of TNF and IL-1 in human acute pulmonary fibrotic diseases: an immunohistochemical analysis. Pathol Intl 1996;46: 9199 23. McCormack FX. Role of pulmonary epithelium and surfactant in the pathogenesis of interstitial lung disease. In: Schwarz MI, King TE Jr, eds. Interstitial Lung Disease. 3rd ed. Hamilton-London: BC Decker Inc.; 1997:165205 24. Toews GB. Cellular alterations in fibroproliferative lung disease. Chest 1999;116:112S116S 25. Eitzman DT, McCoy RD, Zheng X, et al. Bleomycininduced pulmonary fibrosis in transgenic mice that either lack
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 1996;97(1):232237 Chapman HA, Allen CL, Stone OL. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis 1986;133:437443 Hasday JD, Bachwich PR, Lynch JP III, Sitrin RG. Procoagulant and plasminogen activator activities of bronchoalveolar fluid in patients with pulmonary sarcoidosis. Exp Lung Res 1988;14:261278 Gross TJ, Simon RH, Kelly CJ, Sitrin RG. Rat alveolar epithelial cells concomitantly express plasminogen activator inhibitor-1 and urokinase. Am J Physiol 1991;260(4): L286L295 Idell S, Gonzalez K, Bradford H, et al. Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome: contribution of tissue factor associated with factor VII. Am Rev Respir Dis 1987;136:14661474 Idell S, James KK, Levin EG, et al. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest 1989;84:695705 Liu J, Brass DM, Hoyle GW, Brody AR. TNF- receptor knockout mice are protected from the fibroproliferative effects of inhaled asbestos fibers. Am J Pathol 1998;153(6): 18391847 Brass DM, Hoyle GW, Poovey HG, Liu J, Brody AR. Reduced tumor necrosis factor-a and transforming growth factor-1 expression in the lungs of inbred mice that fail to develop fibroproliferative lesions consequent to asbestos exposure. Am J Pathol 1999;154(3):853862 Sime PJ, Marr RA, Gauldie D, et al. Transfer of tumor necrosis factor-a to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-b1 and myofibroblasts. Am J Pathol 1998;153(3):825832 Kahari V, Chen YQ, Su MW, Ramirez F, Uitto J. Tumor necrosis factor-a and interferon-g suppress the activation of human type I collagen gene expression by transforming growth factor-1: evidence for two distinct mechanisms of inhibition at the transcriptional and posttranscriptional levels. J Clin Invest 1990;86:14891495 Strieter RM, Keane MP, Standiford TJ, Kunkel SL. Cytokine Biology and the Pathogenesis of Interstitial Lung Disease. In: Schwarz MI, King TE Jr, eds. Interstitial Lung Disease. 3rd ed. Hamilton-London: BC Decker Inc.; 1997:181205 Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-1 induces a-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122: 103111 Chan ED, Worthen GS, Augustin A, Lapadat R, Riches DWH. Inflammation in the pathogenesis of interstitial lung diseases. In: Schwarz MI, King TE Jr, eds. Interstitial Lung Disease. 3rd ed. Hamilton-London: BC Decker Inc.; 1997:135 164 Thornton SC, Por SB, Wlash BJ, Penny R, Breit SN. Interaction of immune and connective tissue cells: the effect of lymphokines and monokines on fibroblast growth. J Leukoc Biol 1990;47:312320 Snyder LS, Hertz MI, Peterson MS, et al. Acute lung injury: pathogenesis of intraalveolar fibrosis. J Clin Invest 1991;88:663673 Pache J, Christakos PG, Gannon DE, Mitchell JJ, Low RB,
ACUTE INTERSTITIAL PNEUMONITIS/VOURLEKIS ET AL
407
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
Leslie KO. Myofibroblasts in diffuse alveolar damage of the lung. Mod Pathol 1998;11(11):10641070 Hayashi T, Stetler-Stevenson WG, Fleming M, et al. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol 1996;149(4):12411256 Raghu G, Striker LJ, Hudson LD, Striker GE. Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis 1985;131:281289 Kuhn C III, Boldt J, King TE Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989;140:16931703 Specks U, Nerlich A, Colby TV, Wiest I, Timpl R. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med 1995;151:19561964 McDonald JA, Kelley DG, Broekelmann TS. Role of fibronectin in collagen deposition: fab to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol 1982;92:485492 Marinelli WA, Henke CA, Harmon KR, Hertz MI, Bitterman PB. Mechanism of alveolar fibrosis after acute lung injury. Clin Chest Med 1990;11(4):657672 Razzaque MS, Nazneen A, Taguchi T. Immunolocalization of collagen and collagen-binding heat shock protein 47 in fibrotic lung diseases. Mod Pathol 1998;11(12):11831188 Zapol WM, Trelstad RL, Coffey JW, Tsai I, Salvador RA. Pulmonary fibrosis in severe acute respiratory failure. Am Rev Respir Dis 1979;119:547554 Meduri GU, Eltorky M, Winer-Muram HT. The Fibroproliferative phase of late adult respiratory distress syndrome. Seminars Respir Infections 1995;10(3):154175 Selman M, King TE Jr, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134: 136151 Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MWJ. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992;141(5):10851095 Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995;146(1): 5666 Collins JF, Smith JD, Coalson JJ, Johanson WG Jr. Variability in lung collagen amounts after prolonged support of acute respiratory failure. Chest 1984;85(5):641646 Raghu G, Mageto YN. Genetic predisposition of interstitial lung disease. In: Schwarz MI, King TE Jr, eds. Interstitial Lung Disease. 3rd ed. Hamilton-London: BC Decker Inc.; 1998:119134 Awad MR, El-Gamel A, Hasleton P, Turner DM, Sinnott PJ, Hutchinson IV. Genotypic variation in the transforming growth factor-1 gene. Transplantation 1998;66:10141020 Olsen NJ, Wortmann RL. Inflammatory and metabolic diseases of muscle. In: Klippel JH, ed. Primer on the Rheumatic Diseases. 11th ed. Atlanta: The Arthritis Foundation; 1997:276282 Criswell LA, Amos CI. Update on genetic risk factors for systemic lupus erythematosus and rheumatoid arthritis. Curr Opin Rheum 2000;12(2):8590
58. Yazdy AM, Tomashefski JFJ, Yagan R, Kleinerman J. Regional alveolar damage: a localized counterpart of diffuse alveolar damage. Am J Clin Pathol 1989;92:1015 59. Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbations in idiopathic pulmonary fibrosis: analysis of clinical and pathologic findings in three cases. Chest 1993;103:18081812 60. Schwarz MI. The acute (noninfectious) interstitial lung diseases. Comprehensive Therapy 1996;22(10):622630 61. Primack SL, Hartman TE, Ikezoe J, Akira M, Sakatani M, Muller NL. Acute interstitial pneumonia: radiographic and CT findings in nine patients. Radiology 1993;188: 817820 62. Johkoh T, Muller NL, Taniguchi H, et al. Acute interstitial pneumonia: thin-section CT findings in 36 patients. Radiology 1999;211:859863 63. Ichikado K, Johkoh T, Ikezoe J, et al. Acute interstitial pneumonia: high-resolution CT findings correlated with pathology. Am J Roentgenol 1997;168:333338 64. Robinson DS, Geddes DM, Hansell DM, et al. Partial resolution of acute interstitial pneumonia in native lung after single lung transplantation. Thorax 1996;51:11581159 65. Hamman L, Rich AR. Acute diffuse interstitial fibrosis of the lungs. Bull Hopkins Hosp 1944;74:177212 66. Warner DO, Warner MA, Divertie MB. Open lung biopsy in patients with diffuse pulmonary infiltrates and acute respiratory failure. Am Rev Respir Dis 1988;137:9094 67. Matthay RA, Schwarz MI, Petty TL, et al. Pulmonary manifestations of systemic lupus erythematosus: review of twelve cases of acute lupus pneumonitis. Medicine 1974;54(5): 397409 68. Pratt DS, Schwarz MI, May JJ, Dreisin RB. Rapidly fatal pulmonary fibrosis: the accelerated variant of interstitial pneumonitis. Thorax 1979;34:587593 69. Tazelaar HD, Viggiano RW, Pickersgill J, Colby TV. Interstitial lung disease in polymyositis and dermatomyositis: clinical features and prognosis as correlated with histologic findings. Am Rev Respir Dis 1990;141:727733 70. Kreidstein SH, Lytwyn A, Keystone EC. Takayasu arteritis with acute interstitial pneumonia and coronary vasculitis: expanding the spectrum. Arthr Rheum 1993;36(8):11751178 71. Akikusa B, Kondo y, Irabu N, Yamamoto S, Saiki S. Six cases of microscopic polyarteritis exhibiting acute interstitial pneumonia. Pathol Intl 1995;45:580588 72. Muir TE, Tazelaar HD, Colby TV, Myers JL. Organizing diffuse alveolar damage associated with progressive systemic sclerosis. Mayo Clin Proc 1997;72:639642 73. Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998;280(2):159165 74. Behrendt CE. Acute respiratory failure in the United States: incidence and 31-day survival. Chest 2000;118:11001105 75. Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 19831993. JAMA 1995;273(4):306309 76. Martin C, Papazian L, Payan M, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome: a study in mechanically ventilated patients. Chest 1995;107:196200 77. Brower RG, Matthay MA, Morris AH, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute
408
SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE/VOLUME 22, NUMBER 4 2001
lung injury and the acute respiratory distress syndrome. New Engl J Med 2000;342(18):13011308 78. Gunning K, Rowan K. ABC of intensive care: outcome data and scoring systems. BMJ 1999;319(7204):241244 79. Davidson TA, Rubenfeld GD, Caldwell ES, Hudson LD, Steinberg K. The effect of acute respiratory distress syndrome
on long-term survival. Am J Respir Crit Care Med 1999;160: 18381842 80. McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LJ. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1994;150:9094
You might also like
- ArdsDocument53 pagesArdsSophy Sony100% (3)
- Anatomy & Physiology SlidesDocument372 pagesAnatomy & Physiology Slidesnursereview100% (185)
- Interstitial Pneumonia Seminar PaperDocument9 pagesInterstitial Pneumonia Seminar PaperLamy SNo ratings yet
- Fpi Nejm Clincal Practice 2018Document13 pagesFpi Nejm Clincal Practice 2018cjonderedsonNo ratings yet
- Idiopathic Pulmonary FibrosisDocument13 pagesIdiopathic Pulmonary FibrosisRaul David Maldonado LearyNo ratings yet
- ICU Department ICU DepartmentDocument48 pagesICU Department ICU DepartmentEmad ElhusseinNo ratings yet
- Letter TO THE Editor: Secondary Organizing Pneumonia After Varicella-Zoster Virus Infection: A Rare AssociationDocument3 pagesLetter TO THE Editor: Secondary Organizing Pneumonia After Varicella-Zoster Virus Infection: A Rare AssociationfatimiahmastaNo ratings yet
- Parasitic Infections of The Lung: A Guide For The Respiratory PhysicianDocument9 pagesParasitic Infections of The Lung: A Guide For The Respiratory Physicianحسام الدين إسماعيلNo ratings yet
- Parasitic Infections of The Lung: A Guide For The Respiratory PhysicianDocument9 pagesParasitic Infections of The Lung: A Guide For The Respiratory PhysicianSuria KumarNo ratings yet
- A Rare Cause of Acute Respiratoryfailure: CysticpulmonarytuberculosisDocument2 pagesA Rare Cause of Acute Respiratoryfailure: CysticpulmonarytuberculosisIJAR JOURNALNo ratings yet
- Acute Respiratory Distress SyndromeDocument21 pagesAcute Respiratory Distress Syndromemisseve252100% (1)
- Comment: Lancet Infect Dis 2020Document2 pagesComment: Lancet Infect Dis 2020kayegi8666No ratings yet
- Ce Lu Last Ronco SaraDocument15 pagesCe Lu Last Ronco SaraEduardo SoaresNo ratings yet
- ARDS Causes Diffuse Alveolar Damage in The LungDocument5 pagesARDS Causes Diffuse Alveolar Damage in The LungArfyanda ZakariaNo ratings yet
- CCR Landoni120 June v6-2Document3 pagesCCR Landoni120 June v6-2Rara AuliaNo ratings yet
- Fibrosis QuisticaDocument23 pagesFibrosis QuisticaGabriel ArayaNo ratings yet
- Cryptogenic Organizing PneumoniaDocument12 pagesCryptogenic Organizing Pneumoniavalentina peñaNo ratings yet
- Proptosis UnilateralDocument5 pagesProptosis UnilateralCarlos NiveloNo ratings yet
- Pathophysiologyand Managementofacute Respiratorydistresssyndrome InchildrenDocument21 pagesPathophysiologyand Managementofacute Respiratorydistresssyndrome InchildrenKarín Sebastiàn AndradeNo ratings yet
- Di¡erential Adaptation of Microbial Pathogens To Airways of Patients With Cystic Brosis and Chronic Obstructive Pulmonary DiseaseDocument24 pagesDi¡erential Adaptation of Microbial Pathogens To Airways of Patients With Cystic Brosis and Chronic Obstructive Pulmonary DiseaseAbd Halim Gazali HNo ratings yet
- Lung Involvement in Childhood Measles: Severe Immune Dysfunction Revealed by Quantitative ImmunohistochemistryDocument10 pagesLung Involvement in Childhood Measles: Severe Immune Dysfunction Revealed by Quantitative Immunohistochemistryapi-286128974No ratings yet
- Acute Respiratory Distress Syndrome - Background, Pathophysiology, EtiologyDocument5 pagesAcute Respiratory Distress Syndrome - Background, Pathophysiology, EtiologyARHNo ratings yet
- Cryptogenic Organizing PneumoniaDocument12 pagesCryptogenic Organizing Pneumoniajerodussart.22No ratings yet
- Pulmonary FibrosisDocument5 pagesPulmonary FibrosisАлтыншаш АхметоваNo ratings yet
- N Engl J Med 2022 386 1058Document12 pagesN Engl J Med 2022 386 1058EvelynNo ratings yet
- Cryptogenic Organizing Pneumonia: Review ArticleDocument12 pagesCryptogenic Organizing Pneumonia: Review ArticleMatías Jesús Flamm ZamoranoNo ratings yet
- PJMS 36 S75Document8 pagesPJMS 36 S75smruti ghadigaonkarNo ratings yet
- ORENSTEIN 2002 - Cystic Fibrosis - A 2002 UpdateDocument9 pagesORENSTEIN 2002 - Cystic Fibrosis - A 2002 UpdateRafael JustinoNo ratings yet
- F - CMCRep 1 Assy Et Al - 1405Document2 pagesF - CMCRep 1 Assy Et Al - 1405faegrerh5ju75yu34yNo ratings yet
- COP - Cryptogenic Organizing Pneumonia - StatPearls - NCBI BookshelfDocument7 pagesCOP - Cryptogenic Organizing Pneumonia - StatPearls - NCBI Bookshelfrbatjun576No ratings yet
- Epithelial Barriers in Allergy and AsthmaDocument11 pagesEpithelial Barriers in Allergy and AsthmaFrancisco Baca DejoNo ratings yet
- FPI Progn TratDocument13 pagesFPI Progn TratTurcu AndreeaNo ratings yet
- Abses SubmentalDocument11 pagesAbses SubmentalSilviaSumintoNo ratings yet
- Case Reporttonsillar Tuberculosis Simulating A Cancer and Revealing Miliary Pulmonary TuberculosisDocument5 pagesCase Reporttonsillar Tuberculosis Simulating A Cancer and Revealing Miliary Pulmonary TuberculosisIJAR JOURNALNo ratings yet
- Pulmonary Immuno-Thrombosis in COVID-19 ARDS PathogenesisDocument4 pagesPulmonary Immuno-Thrombosis in COVID-19 ARDS PathogenesisXavier AbrilNo ratings yet
- Treatment of Slowly Growing Mycobacteria: Julie V. Philley,, David E. GriffithDocument12 pagesTreatment of Slowly Growing Mycobacteria: Julie V. Philley,, David E. Griffiththanhnhat5521No ratings yet
- Pulmonary Edema II: Noncardiogenic Pulmonary EdemaDocument31 pagesPulmonary Edema II: Noncardiogenic Pulmonary EdemaGita Helvia SariNo ratings yet
- Covid 19 and Risk of Pulmonary Fibrosis The Importance of Planning AheadDocument5 pagesCovid 19 and Risk of Pulmonary Fibrosis The Importance of Planning AheadaiNo ratings yet
- Pulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (ARDS) : A Case Report and Review of LiteratureDocument5 pagesPulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (ARDS) : A Case Report and Review of LiteratureCimol AgustinaNo ratings yet
- Lupus 1Document9 pagesLupus 1karina hernandezNo ratings yet
- ERS MONOGRAPH - PULMONARY MANIFESTATIONS OF SYSTEMIC DISEASES/ Artritis ReumatoideaDocument24 pagesERS MONOGRAPH - PULMONARY MANIFESTATIONS OF SYSTEMIC DISEASES/ Artritis ReumatoidearocioanderssonNo ratings yet
- CH ILDDocument3 pagesCH ILDLaith AL-GurayfiiNo ratings yet
- Makalah Radiologi TB BE v0.1Document15 pagesMakalah Radiologi TB BE v0.1giovannigyoNo ratings yet
- Lymphocyticinterstitial Pneumonia: Tanmay S. Panchabhai,, Carol Farver,, Kristin B. HighlandDocument12 pagesLymphocyticinterstitial Pneumonia: Tanmay S. Panchabhai,, Carol Farver,, Kristin B. HighlandaabigutNo ratings yet
- 965-The Effects of Coronavirus On Human NasalDocument6 pages965-The Effects of Coronavirus On Human NasalVenkatesh VelNo ratings yet
- 1 s2.0 S003139551730072XDocument21 pages1 s2.0 S003139551730072XvgmanjunathNo ratings yet
- Chronic Obstructive Pulmonary Disease: Iman Galal, MDDocument60 pagesChronic Obstructive Pulmonary Disease: Iman Galal, MDYan Sheng HoNo ratings yet
- ARDS Article AppendixDocument12 pagesARDS Article AppendixAvery DreamsNo ratings yet
- Induced Pluripotent Stem Cell-Derived Lung Alveolar Epithelial Type II Cells Reduce Damage in Bleomycin-Induced Lung FibrosisDocument12 pagesInduced Pluripotent Stem Cell-Derived Lung Alveolar Epithelial Type II Cells Reduce Damage in Bleomycin-Induced Lung FibrosisPercy SolisNo ratings yet
- Malformaciones Congenitas Neoreviews - 2016 - May - 17 (5) - E263Document10 pagesMalformaciones Congenitas Neoreviews - 2016 - May - 17 (5) - E263valentina rojasNo ratings yet
- Diagnosis and Treatment of Diffuse Interstitial Lung DiseasesDocument21 pagesDiagnosis and Treatment of Diffuse Interstitial Lung Diseaseswajeeh rehmanNo ratings yet
- Annotated BibliographyDocument9 pagesAnnotated BibliographyJoy AquinoNo ratings yet
- Necrotizing Pneumonia (Aetiology, Clinical Features and Management)Document8 pagesNecrotizing Pneumonia (Aetiology, Clinical Features and Management)pachomdNo ratings yet
- 0000542-200504000-00021Document17 pages0000542-200504000-00021Daniel Martinez HernándezNo ratings yet
- Submandibular Space Infection: A Potentially Lethal InfectionDocument7 pagesSubmandibular Space Infection: A Potentially Lethal InfectionAnonymous 9KcGpvNo ratings yet
- Silicosis - A Case Report Case Report: Address of The CorrespondenceDocument3 pagesSilicosis - A Case Report Case Report: Address of The CorrespondenceBunga RamadhaniNo ratings yet
- Ten Things We Learned About COVID-19: What'S New in Intensive CareDocument4 pagesTen Things We Learned About COVID-19: What'S New in Intensive CareDiego MerchánNo ratings yet
- Asthama - Pathology and PathophysiologyDocument5 pagesAsthama - Pathology and PathophysiologyTejal ParulkarNo ratings yet
- Study Guide 2Document16 pagesStudy Guide 2cNo ratings yet
- Ludwig 'S Angina: Clinical ImagesDocument2 pagesLudwig 'S Angina: Clinical Images송란다No ratings yet
- Pulmonary Manifestations of Primary Immunodeficiency DiseasesFrom EverandPulmonary Manifestations of Primary Immunodeficiency DiseasesSeyed Alireza MahdavianiNo ratings yet
- Recurrent Miscarriage EpidemiologymmmDocument7 pagesRecurrent Miscarriage EpidemiologymmmrhinaNo ratings yet
- Genetic Codes With No Dedicated Stop Codon Context Dependent Translation TerminationDocument13 pagesGenetic Codes With No Dedicated Stop Codon Context Dependent Translation TerminationAarcamNo ratings yet
- Book Chapter Physiology of Astigmatism 2012Document20 pagesBook Chapter Physiology of Astigmatism 2012Sebas H. MonsalveNo ratings yet
- ABO-RH IncompatiilityDocument6 pagesABO-RH IncompatiilitymarisonaningatNo ratings yet
- Psoriasis in UnaniDocument15 pagesPsoriasis in UnaniAbdul Jalil HridoyNo ratings yet
- Failure To ThriveDocument8 pagesFailure To ThriveFitria AhdiyantiNo ratings yet
- Prussian BlueDocument66 pagesPrussian BlueK AnjaliNo ratings yet
- Some Applications of Genetic EngineeringDocument9 pagesSome Applications of Genetic Engineeringshibi sharmaNo ratings yet
- Pernicious AnemiaDocument28 pagesPernicious AnemiaEncee MianNo ratings yet
- Plant PathologyDocument17 pagesPlant PathologyAlishba SherazNo ratings yet
- Immune Responses To Influenza VirusDocument12 pagesImmune Responses To Influenza Virusdavid onyangoNo ratings yet
- Cytokinin Cross-Talking During Biotic and Abiotic Stress ResponsesDocument11 pagesCytokinin Cross-Talking During Biotic and Abiotic Stress ResponsesasifNo ratings yet
- Nervous System NotesDocument8 pagesNervous System NotesGina ChunNo ratings yet
- The Milk Letter-NomilkDocument32 pagesThe Milk Letter-NomilkRinio SimeonidouNo ratings yet
- Introduction To Pathology: Yodit Getahun, MD PathologistDocument29 pagesIntroduction To Pathology: Yodit Getahun, MD PathologistWolderufael100% (1)
- Lacunar Stroke Guide - Causes, Symptoms and Treatment OptionsDocument6 pagesLacunar Stroke Guide - Causes, Symptoms and Treatment OptionsRismanto TorsioNo ratings yet
- Cambridge Ordinary LevelDocument20 pagesCambridge Ordinary Levelrania moomalNo ratings yet
- Cerebral Palsy An Overview 2018Document11 pagesCerebral Palsy An Overview 2018Yhuliana AcostaNo ratings yet
- Motofei 2019Document26 pagesMotofei 2019Paula AzevedoNo ratings yet
- Model Biologics IndicationsDocument25 pagesModel Biologics IndicationsVenkata Suryanarayana GorleNo ratings yet
- What Is Biochemistry? - Biochemistry - McGill UniversityDocument3 pagesWhat Is Biochemistry? - Biochemistry - McGill UniversityKunalraj SinghNo ratings yet
- Epilepsy Board Review, A Comprehensive Guide.2017 - Mohamad Z. KoubeissiDocument360 pagesEpilepsy Board Review, A Comprehensive Guide.2017 - Mohamad Z. Koubeissikaram aliNo ratings yet
- Anatomy of PeriodontiumDocument2 pagesAnatomy of PeriodontiumNana HindraNo ratings yet
- Guardant360 A0771104 v1 FinalDocument6 pagesGuardant360 A0771104 v1 FinaltraveltreatsteluguNo ratings yet
- Root BiomodificationDocument56 pagesRoot BiomodificationDr Jinal Desai50% (2)
- Links Library Find Out If You WonDocument10 pagesLinks Library Find Out If You WonusmleNo ratings yet
- ELS - Q2 - Week 4aDocument11 pagesELS - Q2 - Week 4aShekaina Faith Cuizon LozadaNo ratings yet
- Genetic Disorders SourcebookDocument748 pagesGenetic Disorders Sourcebookpaconscribd100% (1)
- Health IndicatorDocument30 pagesHealth IndicatorPawan KumarNo ratings yet