Professional Documents
Culture Documents
Sumber Anastesi
Sumber Anastesi
Uploaded by
Kemala IntanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sumber Anastesi
Sumber Anastesi
Uploaded by
Kemala IntanCopyright:
Available Formats
MERCURY NEPHROTOXICITY IN THE RAT
1. FACTORS INFLUENCING THE LOCALIZATION OF THE TUBULAR LESIONS
A. E. RODIN, M.D., M.Sc. (MED.), F.R.C.P.(C),* AND C. N. CROWSON, MA., M.D., PH.D.t
Winnipeg Manitoba, Canada
Since the first report on animal experimentation with mercury by Pavy in i860,1 considerable disagreement has developed as to the level of the nephron affected by this substance. In rats, Edwards,2 Moore, Goldstein and Canowitz,3 Mustakallio and Telkkia,4 and Wachstein 5 have localized the renal lesion to the proximal convoluted tubules, particularly the terminal segment. Similar localization has been reported by Edwards 2 in guinea pigs, rabbits and frogs; by Hunter 6 in rabbits; and by Simonds and Hepler7 in dogs. However, Oliver8 placed the mercury-induced lesions of rats within all cortical tubules. Both Lyons and Johnstone and Keith 10 described damage to the ascending limbs of Henle as well as the proximal convoluted tubules in dogs. Burmeister and McNally"1 implicated the subcapsular tubules in dogs. Harmon 12 and Oliver, MacDowell and Tracy 13 described cases of human mercury poisoning with degenerative changes in loops of Henle and convoluted tubules. The conflicting nature of these reports suggests an inherent failure to control variable experimental factors such as method of determination of nephron levels, detailed study of controls and possible variation in results with variations in dose of mercury. The majority of reports of mercury nephrotoxicity fail to state the basis on which the damaged segment of the nephron was identified. Without this information an accurate comparison of different experimental series is not possible. The papers describing the means of identifying tubular levels can be divided into 3 groups: microdissection of the nephron13; location of the segment within the kidney section 5; and histologic structure of the segment.14 Simonds and Hepler7 have questioned the reliability of nephron microdissection because of the difficulty in identifying segments once removed from the kidney. Although
This paper represents a portion of a Thesis accepted by the University of Manitoba towards a Master of Science Degree in Medicine. Accepted for publication, March 26, I962. * Present address: Department of Laboratory Medicine, Misericordia Hospital, Edmonton, Alberta, Canada. t Present address: Director of Laboratories, Misericordia Hospital, Winnipeg r, Manitoba, Canada.
297
298
RODIN AND CROWSON
Vol. 4r, No. 3
the use of histologic criteria is satisfactory in normal kidneys, it is of little use in identifying necrotic renal tubules. In considering these 3 methods, location of the tubular segments within the kidney appears to be the most reliable method for consistently identifying cross sections of the nephron in mercury-damaged kidneys. The investigations to be described in this paper constitute an attempt to delineate variable factors in order to establish a basis for experimental studies on mercury nephrotoxicity. Several possible causes of variability were eliminated by the use of only one species (the white Wistar rat), by the use of only one type of mercury compound (mercury bichloride), by the injection of solution into only one site in all animals (the thigh muscle), and by careful evaluation of nephron levels. In addition, many controls were examined in order to establish normal structural aberrations, and two different doses of mercury bichloride were compared for possible variations in location and extent of renal lesions.
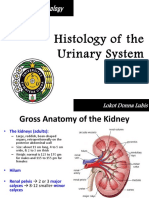
THE NORMAL RAT KIDNEY Any experimental study must be preceded by a careful evaluation of the normal range of variation. This is particularly true in histologic work where assessment is based on personal visual evaluation. The use of controls is the most satisfactory way to eliminate errors based on the misinterpretation of normal phenomena. An error originating from inadequate control studies is illustrated by two reports, one describing the presence of fat in renal tubules of dogs after ingestion of mercury bichloride,1' and the other describing its presence in normal dogs.7 As a preliminary to investigation of mercury nephrotoxicity in the rat kidney, a detailed assessment of the normal histologic structure of this organ was undertaken.
Material and Methods
One hundred adult albino Wistar rats of varying age and sex were killed by ether anesthesia and necropsied immediately. The kidneys were bisected and placed in a modified Heidenhain-Susa fixative for 24 hours, after which they were treated with Lugol's iodine solution for removal of mercurial salts from the fixative. Sections were prepared at 4 , from paraffin-embedded tissues and stained by a modification of Bencosme's hemalum-phloxine-saffron stain.15 This stain was chosen because of its easy reproducibility, its trichrome nature, and its ability to sharply differentiate both nuclear and cytoplasmic elements. With this stain, nuclei are violet, cytoplasm various shades of red, and collagen fibers and mucin orange to yellow. Nephron levels were identified on the basis of both histologic features and location. The proximal third of the proximal convoluted tubule was determined by its origin from a glomerulus, the middle third by its location in the outer two thirds of the cortex, and the terminal third by its location in the inner third of the cortex. The thin limb of Henle's loop was identified by its histologic characteristics and the thick limb by its location in the outer third of the medulla. Distal convoluted tubules were
Sept., I962
MERCURY NEPHROTOXICITY
299
determined from their location in the outer two thirds of the cortex and differentiated from proximal tubules on the basis of microscopic features.
Observations Inspection of stained slides with the naked eye clearly defined the division of the kidney parenchyma into two major and equal layers, an outer densely eosinophilic cortex and an inner medulla which was considerably paler staining. The junction between the cortex and medulla was prominent because of these differences in color intensity and because it was a relatively straight line (Fig. i). The cortex itself was divided into an inner and outer zone also, the outer one being slightly more eosinophilic than the inner but only indefinitely demarcated from the inner on gross inspection. The inner and outer cortical zones interdigitated rather strikingly with each other (Fig. 2). Under higher power the inner zone was seen to be free of glomeruli and composed largely of packed proximal convoluted tubules with eosinophilic cytoplasm and finely granular mitochondria (Fig. 3). A striking feature was disruption of epithelium in the distal portions of the proximal convoluted tubules. This was seen as an irregular shedding of from i to 3 epithelial cells into the lumen where they formed freely lying cellular masses. These detached cells retained their brush borders, and there was no change in eosinophilia, mitochondrial size or nuclear appearance (Fig. 3). Also present in the inner cortical zone were occasional loops of Henle which were seen as tubes with wider lumens than those of the proximal convoluted tubules and which tended to be cut more longitudinally in hemisections. The outer cortical zone resembled the human renal cortex in containing glomeruli, convoluted tubules, loops of Henle and the upper portions of collecting tubules. However, the immediate subcapsular zone was free of glomeruli and contained both proximal and distal convoluted tubules, the cytoplasm of which presented a rather striking irregularly vacuolated appearance giving a lacy pattern unlike that seen in other areas (Fig. 4). Fat stains showed that the vacuolated areas were free of lipid. The first i to 3 cells in the initial portion of the proximal convoluted tubule bulged into the glomerulus, forming a nipple-like protuberance. These "intraglomerular" tubular cells were histologically similar to the others. The cytoplasm of convoluted tubules was moderately eosinophilic and finely granular. However, in the distal convoluted tubular epithelium it was somewhat lighter staining and the nuclei were more hyperchromatic. An interesting finding in 6o per cent of the normal rats were focal collections of tubules having more eosinophilic and homogeneous cytoplasm
300
RODIN AND CROWSON
Vol. 4I, No. 3
with no mitochondrial detail. The nuclei were not altered. The tubules involved were both the proximal and distal convoluted portions situated in the outer cortical zone immediately adjacent to the inner zone. Occasionally the lumens of these tubules were more dilated than the rest and the free surfaces of the lining cells exhibited a more irregular appearance than elsewhere. The degree of prominence of these focal collections of tubules varied considerably from rat to rat (Fig. 5). Kidneys which were otherwise normal occasionally contained large hyaline casts in markedly dilated ascending limbs of Henle. These tubules had a considerably flattened epithelial lining and were sometimes associated with adjacent fibrosis and lymphocytic infiltration localized to the affected segments. The medulla was divided into two zones, but much more sharply than the cortex (Fig. i). The inner zone was much less eosinophilic than the outer and was associated with a single papilla extending into the renal pelvis. The staining pallor of this inner zone was due to a decreased concentration of tubules and to the pale, clear cytoplasm of the component collecting tubules. The outer zone of the medulla was composed largely of ascending and descending limbs of Henle and occasional collecting tubules which tended to run through the width of the zone in small straight bundles. These bundles were seen occasionally in cross section but more commonly in length. The nuclei of occasional epithelial cells in the loops of Henle in the outer medullary zone were unusually small, pyknotic and regular, having some resemblance to viral inclusions. This nuclear feature was seen only in an occasional cell in any one tubule, and the numbers varied from rat to rat (Fig. 6). Another characteristic of the rat kidney was the presence of rather abundant brown fat in the peripelvic regions. About i in ioo rats has hydronephrosis with dilatation of the pelvis to 2 or 3 times its normal size. However, of these, only 50 per cent show any definite evidence of pyelonephritis.
Discussion Our observations in the normal rat kidney were in general agreement with those of Wachstein,5 who identified mammalian nephron segments according to their location within the kidney. Although Wachstein stated that distal convoluted tubules predominated about glomeruli, Oliver 8 believed these were proximal convoluted tubules. Our findings differed. It was difficult to assess the periglomerular distribution of tubular types. However, in rats given o.oI mg. of mercury per gm. of body weight the proximal convoluted tubules became necrotic and the distal convoluted tubules remained intact at the end of I2 hours. With this method of dif-
Sept., z962
MERCURY NEPHROTOXICITY
301
ferentiating the tubules, neither type of convoluted segment had a tendency to localize about glomeruli. The rat kidney differed from the human kidney in having sharply delineated outer and inner cortical zones. The outer cortical zone resembled the human cortex, but the inner cortical zone contained no glomeruli and was composed almost entirely of terminal portions of proximal tubules; these were not present elsewhere. According to Peter16 and Suzuki,17 ascending loops of Henle are also a prominent component of the inner cortex. Thus, in the rat, there appears to be an excellent means of identifying the terminal portions of the proximal convoluted tubules. No reference has been found to the significance of vacuolation observed in tubular epithelium in the subcapsular region of the cortex. In our animals this was observed in all control and test animals. For practical purposes we have considered it to be an artifact; its significance lies in the possibility of its confusion with early nephrotoxic alterations. In fact, Burmeister and McNally 11 attributed an identical change to the effect of mercury bichloride in dogs. Imbibition of fluid from fixative solution by subcapsular tubular epithelium is the probable cause of the vacuolar appearance. The increased cytoplasmic eosinophilia in the epithelium of focal groups of tubules in the normal rat kidney was of interest. The possibility that this was induced by the use of ether as the killing agent was investigated by killing 4 rats instantaneously by fracturing their necks. The eosinophilia was also manifest in these animals. Its appearance resembled the increase in cytoplasmic chromophile substance and in the size and number of mitochondria associated with increased cellular function.18 It is suggested, therefore, that these focal alterations may have represented actively functioning units; the remaining tubules were probably "resting" at the time of killing. That only a certain percentage of nephrons are working at any one time is a well accepted physiologic fact. The existence of normal-appearing proximal convoluted tubular epithelium detached from the basement membrane must be kept in mind in the interpretation of an experimental series; this may be mistaken for early tubular injury by nephrotoxic agents. The cellular dislocation probably represents artifact induced by the microtome knife. Conclusions The following features in the normal rat kidney may be mistaken for alterations induced by nephrotoxic agents: i. Focal collections of hyaline casts in the loops of Henle situated in the outer medullary zone.
302
RODIN AND CROWSON
Vol. 4I, No. 3
2. Pyknotic nuclei in occasional epithelial cells of loops of Henle in the outer medullary zone. 3. Detachment of groups of epithelial cells from the basement membranes in the terminal third of the proximal convoluted tubule in the inner cortical zone. 4. Marked vacuolation of tubules situated in the subcapsular region. 5. Disruption of tubular epithelium seen along the tract of a knife nick. 6. Loss of mitochondrial detail with increased eosinophilia in the epithelium of groups of tubules situated near the junction of the inner and outer cortical zones.
DOSAGE OF MERCURY In order to achieve comparable results in experimental mercury nephrotoxicity, the level of mercury in the blood reaching the kidney must be consistent. However, the amount of mercury reaching the kidney does not depend on dose alone. Comparison of different experimental series is not possible if the type, concentration and route of injection of the mercury compound are not standardized, as these can influence the mercury content of the blood. Numerous mercury compounds have been employed experimentally, all varying in their mercury content. Pavy, in i860,1 used white precipitate which contains 79 per cent mercury. Many experimenters have used diuretics such as novasurol,10 which contains 34 per cent mercury, and salyrgan,'9 which has 3 7 per cent. One of the most popular mercury compounds used for nephrotoxicity has been mercury bichloride,20 which contains 74 per cent mercury. Kolmer and Lucke2' utilized numerous mercury compounds and concluded that the degree of injury caused by the different preparations was directly related to the amount of pure mercury absorbed, irrespective of the preparation. Mustakallio and Telkka22 regulated the dose of 6 different mercurial diuretics and mercury bichloride according to mercury content and found that changes were restricted to the proximal convoluted tubule except in the case of mercurophylline, which also affected the ascending limb of Henle's loop. It thus appears that within certain limits, reports of experiments utilizing different mercury compounds are comparable if mercury content is considered. The injection routes in experimental mercury poisoning have been subcutaneous,6 intramuscular,23 intraperitoneal,24 and intravenous.'9 The amount of mercury reaching the blood stream can vary significantly with these methods. Schamberg, Kolmer and Raiziss25 stated that 4 times more mercury could be given intramuscularly than intravenously.
Sept., I 962
MERCURY NEPHROTOXICITY
303
Any experimental study must consistently use the same route of in-
jection.
If the same mercury compound is injected by the same route, then comparative studies of the nephrotoxic effects of different doses of mercury would be valid. The experiment to be described below is an attempt to delineate any possible differences in the location of the renal lesions induced by different doses of mercury. Material and Methods
Ninety-six Wistar rats were divided into 2 groups of 48 rats each, i group being designated as "low dose" and the other as "high dose." The low dose group received mercury bichloride solution calculated at o.oos mg. of mercury per gm. of body weight, intramuscularly in the thigh. The high dose group received o.i mg. per gm. Four rats in each group were killed at hourly intervals following the injection, for a total of I2 hours. The kidneys were then processed as described in the previous section. Nephron segments were identified by a combination of cytologic features and location.
Observations In the group sacrificed at i hour no changes were noted in the low dose rats. However, in 3 of the 4 high dose rats, scattered groups of 2 or 3 tubules exhibited early nuclear pyknosis in their epithelial cells. These constituted the initial and middle segments of the proximal convoluted tubules and were located in the outer cortical zone (Fig. 7). In the group sacrificed at 3 hours, the low dose rats all exhibited cytologic changes similar to those seen at i hour in the high dose group. However, these were limited to the inner cortical zone and involved terminal segments of proximal convoluted tubules (Fig. 8). At 3 hours the high dose group showed involvement of additional portions of the initial and middle segments of proximal convoluted tubules. In the rats sacrificed at 4, 5, 6, 7 and 8 hours, both the low and high dose groups showed gradual increase in the extent of the degenerative alterations and in the size of the lesions. In addition, the tubules in high dose rats now exhibited scattered necrotic cells. However, in both dosage groups there continued to be selective localization of the degenerative lesions, the distal segment with low dosage and the more proximal segment of the proximal convoluted tubules with high dosage. By 9 hours in the low dose rats there was beginning confluence of degenerative lesions in the terminal segments of the proximal convoluted tubules, and an occasional necrotic cell was apparent. The high dose rats exhibited similar but more extensive alterations in the more proximal portions of the convoluted tubules. In addition, a few groups of terminal segments exhibited early degeneration. At I 2 hours all of the terminal segments of the proximal convoluted
304
RODIN AND CROWSON
Vol. 4zI, No. 3
tubules showed degeneration and necrosis in the low dosage group (Fig. 9). The pattern was characterized by a band of alteration across the kidney, formed by the necrotic inner cortical zone. In addition, an occasional cell in the more proximal portions of the proximal convoluted tubules had undergone necrosis. In the high dose rats all proximal portions of proximal convoluted tubules in the outer cortical zone were the seat of degenerative changes and contained numerous necrotic cells (Fig. Io). In addition, occasional cells in the terminal portions of proximal convoluted tubules had undergone degenerative changes. A careful search of the nephron distal to the proximal convoluted portion revealed only a few degenerated cells in the distal convoluted tubules in rats of the high dose group at I2 hours. Except in the proximal convoluted tubules there was thus a striking scarcity of lesions.
Discussion It was apparent that definite alterations in tubular epithelium appeared by one hour in the high dose group but not until 3 hours with the lower dosage. The observation that a larger amount of mercury produced lesions earlier was not surprising although this point has not been emphasized by previous workers. In fact, Edwards2 stated that the extent of the necrotizing action of mercury was not dependent on dosage or the size of the animal. However, he used only 2 doses (o.6 and o.8 mg. per rat). Quantitatively these do not differ significantly. Our observations indicated that the administration of a large amount of mercury resulted in a higher concentration in the glomerular filtrate so that tubular absorption of water resulted in a toxic concentration sooner than with the lower dosage. Only a few workers have investigated the early phase of the nephrotoxic lesion produced in rats by an amount of mercury equivalent to that in our experiments (0.005 mg. of mercury per gm. of rat weight). Mustakallio and Telkkii 22 noted initial alterations at 4 hours. The later onset of their lesions may have been due to differences in absorption since they introduced the mercury solution subcutaneously and we, intramuscularly. Wachstein and Meisel,26 using a high mercuhydrin dosage (0.4 mg. of mercury per gm. of rat weight), found degenerative changes throughout the entire proximal convoluted tubule within an hour. Their dosage was much higher than our "high dose" and this may well be the explanation for the greater extent of the early lesions in their animals. An unexpected observation was the predominant location of the low dose lesion in the terminal third of the proximal convoluted tubule and the involvement of the more proximal segments in the high dose group. This could be explained, as suggested by Simonds,27 by concentration of
Sept., zI962
MERCURY NEPHROTOXICITY
305
metallic poisons in the tubule lumen subsequent to the absorption of water. Thus, a toxic concentration of mercury in the glomerular filtrate is attained higher in the nephron with larger dosage; with the smaller dose, tubule reabsorption does not achieve toxic concentration until the terminal segment of the proximal convoluted tubule is reached. Another striking feature observed was the relative sparing of distal convoluted tubules. With a high dose only a few epithelial cells in this location exhibited degenerative phenomena; none were recorded in Wachstein and Meisel's report.26 Since the proximal segment is more active functionally than the distal convoluted tubule, a larger number of mitochondria containing sulfhydryl groups with protein and enzymes would probably be present. Mercury, being a heavy metal, combines with sulfhydryl groups 28 and thus would reach a higher concentration in cells located in the more proximal tubules. The focal inception of tubular lesions may also have a physiologic explanation. In the normal controls increased cytoplasmic eosinophilia was a focal feature of tubules at the junction of the inner and outer cortex. This was thought to indicate functional activity at the time of death. Mercury may only be toxic to actively functioning units and initially affect them alone. As other units became functional, they too would be affected until eventually all proximal convoluted tubules would become necrotic if the dose was large enough.
SUMMARY There exist differences of opinion as to the exact segment in the renal tubule primarily injured by mercury. Possible reasons for this disagreement were investigated by a study of normal histologic variations which might be subject to misinterpretation. Also investigated were the variations in lesions relating to differences in dosages of mercury. Normal rat kidneys exhibited several histologic features which might in some instances be mistaken for changes induced by nephrotoxic agents. These included hyaline casts and pyknotic nuclei in the loops of Henle, epithelial detachment in the terminal segments of proximal convoluted tubules, focal cytoplasmic eosinophilia in tubular epithelium, disruption of cells along the track of knife nicks, and epithelial vacuolation in subcapsular tubules. Mercury bichloride, with a dosage of o.oos mg. of mercury per gm. of body weight, given to rats intramuscularly, produced renal tubular lesions beginning in 3 hours and predominantly affecting the terminal segment of the proximal convoluted tubule. A dosage of O.I mg. per gm. of weight produced lesions which began in one hour and affected the more proximal portions of the convoluted tubules. The concentration of the
306
RODIN AND CROWSON
Vol. 4z, No. 3
mercuric ion in the glomerular filtrate would thus determine the level of cytotoxic activity within the nephron. The relationship of the amount of mercury to localization of tubular damage may explain many of the reported discrepancies related to mercury nephrotoxicity.
REFERENCES
I. PAVY, F. W. On the physiological effect of this substance on animals. Guy's Hosp. Rep., Ser. 3, i86o, 6, 505-5io. 2. EDWARDS, J. G. The renal tubule (nephron) as affected by mercury. Am. J. Path., 1942, i8, II0I-I027. 3. MOORE, R. A.; GOLDSTEIN, S., and CANOWITZ, A. The mitochondria in acute experimental nephrosis due to mercuric chloride. Arch. Path., I929, 8, 9309374. MUSTAKALLIO, K. K., and TELKKX, A. Histochemical localization of the mercurial inhibition of succinic dehydrogenase in rat kidney. Science, I953, iI8,
and abnormal kidney. J. Histochem., I955, 3, 246-270. 6. HUNTER, W. C. Experimental study of acquired resistance of the rabbit's renal epithelium to mercuric chloride. Ann. Int. Med., I929, 2, 796-806. 7. SIMONDS, J. P., and HEPLER, 0. E. Experimental nephropathies. I. A method of producing controlled selective injury of renal units by means of chemical agents. Arch. Path., I945, 39, I03-Io8. 8. OLIVER, J. Experimental nephritis in the frog. IV. Significance of the functional response to vascular and to parenchymal disturbances in the kidney. J. Exper. Med., I932, 55, 295-305. 9. LYON, G. Inflammatory changes in the kidney: an experimental study of the action of some toxins and poisons upon the kidney and also the spleen. J. Path. & Bact., I903-I904, 9, 400-455. IO. JOHNSTONE, B. I., and KEITH, H. M. Toxicity of novasurol (merbaphen); its action on the kidney of the rabbit. Arch. Int. Med., I928, 42, I89-2I6. II. BURMEISTER, W. H., and McNALLY, W. D. Acute mercury poisoning. A parallel histological and chemical study of the renal and hepatic tissue changes as compared with the rapidity of absorption and the amount of mercury present in the circulating blood at the time such changes occur. J. Med. Res., I9I7,
320-321. 5. WACHSTEIN, M. Histochemical staining reactions of the normally functioning
36, 87-98.
I2. HARMON, E. L. Human mercuric chloride poisoning by intravenous injection. Am. J. Path., I928, 4, 32I-336. I3. OLIVER, J.; MAcDOWELL, M., and TRAcY, A. The pathogenesis of acute renal failure, associated with traumatic and toxic injury: renal ischemia, nephrotoxic damage and the ischemuric episode. J. Clin. Invest., I951, 30, I307I439-
14. LUCKE, B. Lower nephron nephrosis (renal lesions of crush syndrome, of bums, transfusions and other conditions affecting lower segments of nephrons). Mit. Surgeon, 1946, 99, 37I-396. i5. BENCOSME, S. A. A trichrome staining method for routine use. Am. J. Clin. Path., I954, 24, 1324-I328. i6. PETER K. Untersuchungen uiber Bau und Entwicklung der Nieren. G. Fischer, Jena, I909, 446 pp.
Sept., 1962
MERCURY NEPHROTOXICITY
307
I7. SUZUKI, T. Zur Morphologie der Nierensekretion unter physiologischen und pathologischen Bedingungen. G. Fischer, Jena, I9I2, 244 PP. I8. MAxIMow, A. A., and BLOOM, W. A Textbook of Histology. W. B. Saunders Co., Philadelphia, I957, ed. 7, 628 pp. I9. JOHNSTONE, B. The comparative toxicity of merbaphen and salyrgan. J. Pharmacol. & Exper. Therap., I93I, 42, I07-I2I. 20. MENTEN, M. L. Pathological lesions produced in the kidney by small doses of mercuric chloride. J. Med. Res., I922, 43, 3I5-32I. 2I. KOLMER, J. A., and LUCKE, B. A study of the histologic changes produced experimentally in rabbits by mercurial compounds. Arch. Dermat. & Syph.,
I92I, 3, 53I-570.
22. MUSTAKALLIO, K. K., and TELKKX, A. Selective inhibition patterns of succinic dehydrogenase and local necrobiosis in tubules of rat kidney induced by six mercurial diuretics. Ann. med. exper. et biol. Fenniae, I955, 33, Suppl. i, i-i6. 23. KEITH, H. M., and JOHNSTONE, B. I. The action of merbaphen (novasurol) on the kidney of the dog; combined functional and pathologic study. Arch. Int.
Med., I929, 44, 438-454.
24. LIPPMAN, R. W. Effect of proteinuria on toxicity of mercurial diuretics in the
rat. Proc. Soc. Exper. Biol. & Med., I949, 72, 682-687.
25. SCHAMBERG, J. F.; KOLMER, J. A., and RAIZISs, G. W. A study of the comparative toxicity of the various preparations of mercury with a histological study of experimental mercurial nephritis. J. Cutaneous Dis., I9I5, 33, 8I9-840. 26. WACHSTEIN, M., and MEISEL, E. A comparative study of enzymatic staining reactions in the rat kidney with necrobiosis induced by ischemia and nephrotoxic agents (mercuhydrin and DL-serine). J. Histochem., I957, 5, 204-220. 27. SIMONDS, J. P. Renal pathologic changes in hypertension and glomerulonephritis; clinical interpretation. J.A.M.A., I942, I20, 89-93. 28. BARRON, E. S. G., and KALNITSKY, G. The inhibition of succinoxidase by heavy metals and its reactivation with dithiols. Biochem. J., I947, 4I, 346-351.
[Illustrations follow]
308
RODIN AND CROWSON
VOI. 4z, No. 3
LEGENDS
FOR
FIGURES
Photographs were prepared from sections stained with hemalum-phloxine-saffron stain. FIG. i. Hemisection of the normal rat kidney. There are 4 zones exhibiting decreasing intensity of staining from the capsular surface to the pelvis: A. Outer cortex. B. Inner cortex. C. Outer medulla. D. Inner medulla. X 8. FIG. 2. Junctional zone between the inner and outer cortical zones in the normal rat kidney. There is striking interdigitation. X So. FIG. 3. Terminal thirds of the proximal convoluted tubules in the inner cortex of the normal rat kidney. Abundant mitochondria give the cytoplasm a finely granular appearance. There is shedding of cytoplasmic masses into the lumen of one tubule. These masses have retained their normal mitochondrial pattern and brush borders. X 400.
Sept., I962
MERCURY NEPHROTOXICITY
309
:"I-f;
3
2
3I O
RODIN AND CROWSON
Vol. 4I, No. 3
FIG. 4. Tubules in the subcapsular region of the outer cortex in the normal rat kidney. The cytoplasm of these tubules has a vacuolated, lacy appearance. X 250. FIG. 5. Junctional zone between the inner and outer cortical zones. A portion of a glomerulus is shown in the upper right hand corner. Three tubules contain cells with darker staining cytoplasm, some loss of mitochondrial pattern but normal brush borders. X 250. FIG. 6. Loops of Henle in the outer zone of the medulla of the normal rat kidney. Occasional pyknotic nuclei resemble inclusion bodies. X 400.
Sept., I962
MERCURY NEPHROTOXICITY
3II
3I2
RODIN AND CROWSON
Vol. 41, No. 3
FIG. 7. Outer cortical zone of a rat kidney I hour after the injection of a "high dose' of mercury bichloride (o.i mg. per gm.). A portion of a glomerulus is on the right. Cells in the proximal portions of the proximal convoluted tubules exhibit increased cytoplasmic density. X 400. FIG. 8. Inner cortical zone of a rat kidney 3 hours after the injection of a "low dose" of mercury bichloride (o.oos mg. per gm.). The terminal portions of the proximal convoluted tubules exhibit irregular mitochondrial enlargement and nuclear pyknosis. X 400. FIG. 9. Midzone in the cortex of a rat kidney I 2 hours after the injection of a "low dose" of mercury bichloride. The lower third is the inner cortical zone with cells of the terminal portions of proximal convoluted tubules. These exhibit nuclear pyknosis and cytoplasmic degeneration. The upper two thirds is the outer cortical zone; this contains the normal proximal portions of the proximal convoluted tubules. X 250. FIG. IO. Midzone of the cortex in a rat kidney I2 hours after the injection of a "high dose" of mercury bichloride. The upper half is the outer cortical zone and contains the necrotic proximal portions of the proximal convoluted tubules. The lower half is the inner cortical zone; terminal portions of the proximal convoluted tubules exhibit milder degenerative changes. X 250.
Sept., I962
MERCURY NEPHROTOXICITY
313
10
You might also like
- A350 ResetDocument45 pagesA350 Resettayfunozcn2No ratings yet
- Lab Exercise 2Document4 pagesLab Exercise 2Angela Robles0% (1)
- 2012 BRAKES VSA System Components - TL PDFDocument114 pages2012 BRAKES VSA System Components - TL PDFsoftallNo ratings yet
- Argelander Initial InterviewDocument13 pagesArgelander Initial InterviewTiborNo ratings yet
- Archery Kim Hyun TakDocument88 pagesArchery Kim Hyun TakKHAIRAN100% (3)
- BOQ & Methodology Elastoroof PU Waterproofing..Document5 pagesBOQ & Methodology Elastoroof PU Waterproofing..Guru100% (1)
- Scanning and Transmission Electron Microscopy of The Kidney'Document19 pagesScanning and Transmission Electron Microscopy of The Kidney'CLPHtheoryNo ratings yet
- PMC2480949Document8 pagesPMC2480949Nat C.No ratings yet
- Kidney Introduction PDF 508Document10 pagesKidney Introduction PDF 508Shailesh DixitNo ratings yet
- Bromadiolona en PerrosDocument9 pagesBromadiolona en Perrosantonio arteagaNo ratings yet
- PIIS0022202X15487925Document13 pagesPIIS0022202X15487925PbscNo ratings yet
- Endosomal Reticulum PaperDocument5 pagesEndosomal Reticulum PaperMelina SelentNo ratings yet
- Urinary SystemDocument20 pagesUrinary SystemWadabiNo ratings yet
- Options For Histological Study of The Structure and Ultrastructure of Human Urinary Bladder EpitheliumDocument8 pagesOptions For Histological Study of The Structure and Ultrastructure of Human Urinary Bladder EpitheliumSergio CerveraNo ratings yet
- ArticleDocument6 pagesArticleحمزہ محبNo ratings yet
- Ultrastructural Changes in Alveolar Epithelium in Response To Freund's Adjuvant (Faulkner & Esterly 1971)Document16 pagesUltrastructural Changes in Alveolar Epithelium in Response To Freund's Adjuvant (Faulkner & Esterly 1971)gd_hbarNo ratings yet
- HAEMANGIOSARCOMA in DOGSDocument11 pagesHAEMANGIOSARCOMA in DOGSPdea CanineNo ratings yet
- Anatomical Relationship Between Urethra and Clitoris: The Journal of Urology July 1998Document7 pagesAnatomical Relationship Between Urethra and Clitoris: The Journal of Urology July 1998FaePapoulesNo ratings yet
- Renal Module Histology 2023-24Document28 pagesRenal Module Histology 2023-24ahmed saberNo ratings yet
- 01 CiliaDocument3 pages01 CiliaLaksh NrusimhanNo ratings yet
- Manx Cat StudiesDocument7 pagesManx Cat Studieslmary20074193No ratings yet
- Caecal Development in Kuttanad Duck (Anas Platyrhynchos: Domesticus)Document4 pagesCaecal Development in Kuttanad Duck (Anas Platyrhynchos: Domesticus)International Organization of Scientific Research (IOSR)No ratings yet
- Congenital Urachal Diverticulum in Dogs: A Case Report: Ojszczyk-Szczepaniak A., Miech A., Wojnowski TDocument4 pagesCongenital Urachal Diverticulum in Dogs: A Case Report: Ojszczyk-Szczepaniak A., Miech A., Wojnowski TMarina MagalhãesNo ratings yet
- Primo Vessel Inside A Lymph Vessel Emerging From A Cancer TissueDocument4 pagesPrimo Vessel Inside A Lymph Vessel Emerging From A Cancer TissueMaxNo ratings yet
- Chondro-Osseous Respiratory Epithelial Adenomatoid Hamartomas in 3 DogsDocument4 pagesChondro-Osseous Respiratory Epithelial Adenomatoid Hamartomas in 3 DogsfisheirNo ratings yet
- JZWM Jan 2010Document7 pagesJZWM Jan 2010llcoffeeNo ratings yet
- Janat00434 0049 PDFDocument17 pagesJanat00434 0049 PDFCristián Mora SeguraNo ratings yet
- 10 24880-Maeuvfd 495969-614239Document6 pages10 24880-Maeuvfd 495969-614239Francis Mary RosaNo ratings yet
- Intestinal Villi in The Dog and The Effect of Ank Ylostoma Caninum InfestationDocument5 pagesIntestinal Villi in The Dog and The Effect of Ank Ylostoma Caninum Infestationmusic cisumNo ratings yet
- StructuralDocument13 pagesStructuralClaudia Pérez LuisNo ratings yet
- Jilciphaga) AND CAVE SWIFTLET (Collocalia Linchi) : Kidney, Hematoxylen-EosinDocument8 pagesJilciphaga) AND CAVE SWIFTLET (Collocalia Linchi) : Kidney, Hematoxylen-EosinTan DuaNo ratings yet
- Histology of The Urinary System 2022Document68 pagesHistology of The Urinary System 2022My Putra Dr Luqman HakimNo ratings yet
- Pancreas L6 12 PDFDocument26 pagesPancreas L6 12 PDFVishwanath Sinduvadi100% (1)
- Splina Functii PDFDocument11 pagesSplina Functii PDFTotolici StefanNo ratings yet
- JurnalDocument6 pagesJurnalYazid Eriansyah PradantaNo ratings yet
- Ooriginal ContributionDocument14 pagesOoriginal ContributionAnna PawłowskaNo ratings yet
- Rare Form of Splenomegaly On A Bovine A Case Study. K.E. MasanganiseDocument4 pagesRare Form of Splenomegaly On A Bovine A Case Study. K.E. MasanganiseMony ElbazNo ratings yet
- Study On Clinical Profile, Management & Outcome of Gastrointestinal Duplication in ChildrenDocument35 pagesStudy On Clinical Profile, Management & Outcome of Gastrointestinal Duplication in ChildrenIJAR JOURNALNo ratings yet
- Raviteja1 PDFDocument6 pagesRaviteja1 PDFMekala LakshmanNo ratings yet
- Oval Corneal Opacities in Beagles: III. Histochemical Demonstration of Stromal Lipids Without HyperlipidemiaDocument12 pagesOval Corneal Opacities in Beagles: III. Histochemical Demonstration of Stromal Lipids Without HyperlipidemiaArif K BashaNo ratings yet
- 2003 Cce Citoesqueleto e CalcioDocument3 pages2003 Cce Citoesqueleto e CalcioCaio LeônidasNo ratings yet
- Cole 1926Document39 pagesCole 1926J.D. NobleNo ratings yet
- The Prevalence of Tonsilloliths and Other Oral and Maxillofacial Soft Tissue Calcifications On PanoramicDocument6 pagesThe Prevalence of Tonsilloliths and Other Oral and Maxillofacial Soft Tissue Calcifications On PanoramicInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Torneck Part IDocument9 pagesTorneck Part IEfren FloresNo ratings yet
- Morphology and Function of Malpighian Tubules and Associated Structures in The Cockroach, Periplaneta AmericanaDocument42 pagesMorphology and Function of Malpighian Tubules and Associated Structures in The Cockroach, Periplaneta AmericanaManojit ChatterjeeNo ratings yet
- 47 Pathology in PracticeDocument3 pages47 Pathology in PracticeCarlos Alberto Chaves VelasquezNo ratings yet
- Some Histological and Physiological Features of Avian KidneyDocument4 pagesSome Histological and Physiological Features of Avian KidneydrmohalkaramolNo ratings yet
- Post-Natal Development of Prescapular Lymph Node in One-Humped Camel (Camelus Dromedarius) - A Histological StudyDocument12 pagesPost-Natal Development of Prescapular Lymph Node in One-Humped Camel (Camelus Dromedarius) - A Histological StudyFrancisco SantosNo ratings yet
- Labiomandibular Paresthesia Caused by Endodontic Treatment - An Anatomic and Clinical Study PDFDocument13 pagesLabiomandibular Paresthesia Caused by Endodontic Treatment - An Anatomic and Clinical Study PDFAlexandra DumitracheNo ratings yet
- Lar Sell 1947Document51 pagesLar Sell 1947J.D. NobleNo ratings yet
- Lar Sell 1947Document51 pagesLar Sell 1947J.D. NobleNo ratings yet
- Congenital Cystic Disease of The Liver, Pancreas, and Kidney in A Nubian GoatDocument3 pagesCongenital Cystic Disease of The Liver, Pancreas, and Kidney in A Nubian GoatEma SomantriNo ratings yet
- SX ApertDocument7 pagesSX ApertMelissa SanchezNo ratings yet
- Kelompok 2 - PatmawatiDocument11 pagesKelompok 2 - PatmawatifatmawatiNo ratings yet
- Exercise 13 Urinary SystemDocument7 pagesExercise 13 Urinary SystemJovelou MihangosNo ratings yet
- Javma-Javma 20 04 0230Document3 pagesJavma-Javma 20 04 0230Fiorella YavarNo ratings yet
- G4 Articulo Globulos RojosDocument11 pagesG4 Articulo Globulos RojosAlejandra ParraNo ratings yet
- 1 s2.0 S2214854X20300029 MainDocument11 pages1 s2.0 S2214854X20300029 MainFuad Fajar AkhlisNo ratings yet
- The Distribution and Behaviour of Intravenously inDocument10 pagesThe Distribution and Behaviour of Intravenously inBruce RoserNo ratings yet
- Histology Lecture: Minerva Diana A. Dichoso, RMTDocument108 pagesHistology Lecture: Minerva Diana A. Dichoso, RMTVernadel ApolloNo ratings yet
- 2 NucleusDocument4 pages2 NucleusNur IslamNo ratings yet
- Bone BY OF: Grom7Th and Osteoclastic Activity As Ikdicated Radio 4utograyhic PlutoniumDocument51 pagesBone BY OF: Grom7Th and Osteoclastic Activity As Ikdicated Radio 4utograyhic PlutoniumMateus PinheiroNo ratings yet
- Primary Ciliary Dyskinesia/Kartagener Syndrome - Clinical and Genetic AspectsDocument13 pagesPrimary Ciliary Dyskinesia/Kartagener Syndrome - Clinical and Genetic AspectsvividNo ratings yet
- CloudEngine 6863 Data Center Switch DatasheetDocument13 pagesCloudEngine 6863 Data Center Switch DatasheetRiqo SetyoNo ratings yet
- CMP002Document98 pagesCMP002Black kosmosNo ratings yet
- Deck Design - Slab Bridge Jan 11 - 2017Document11 pagesDeck Design - Slab Bridge Jan 11 - 2017eph100% (1)
- Hatchet by Gary Paulsen Expectations For This Google DocumentDocument10 pagesHatchet by Gary Paulsen Expectations For This Google Documentapi-328359401No ratings yet
- TypesDocument3 pagesTypesProser Faith TabaculdeNo ratings yet
- The Magisterial Son (Monjoronson) : GLOBAL SUSTAINABILITY AND PLANETARY MANAGEMENTDocument149 pagesThe Magisterial Son (Monjoronson) : GLOBAL SUSTAINABILITY AND PLANETARY MANAGEMENTella..100% (3)
- Foundation Engineering in The Face of Uncertainty - ASCEDocument6 pagesFoundation Engineering in The Face of Uncertainty - ASCEbarouniamine100% (1)
- Astm G32 - 10Document19 pagesAstm G32 - 10ABINASH BEHERANo ratings yet
- 12-Efka - FA 4644 PDFDocument2 pages12-Efka - FA 4644 PDFAniket PatelNo ratings yet
- NCLEX RN Practice Test 5Document6 pagesNCLEX RN Practice Test 5Xiao Wei Ka HardiansyahNo ratings yet
- Cleaning, Sortation and GradingDocument33 pagesCleaning, Sortation and GradingRaisa100% (1)
- Reliable and Cost-Effective Sump Pumping With Sulzer's EjectorDocument2 pagesReliable and Cost-Effective Sump Pumping With Sulzer's EjectorDavid Bottassi PariserNo ratings yet
- Lab Report 4.Document7 pagesLab Report 4.Usman Ali Usman AliNo ratings yet
- The Total Test Time Is 1 Hour. Please Beqin by Writinq Your Name BelowDocument13 pagesThe Total Test Time Is 1 Hour. Please Beqin by Writinq Your Name BelowSaci Louis NaniNo ratings yet
- Advanced Power Electronics Corp.: AP4835GMDocument5 pagesAdvanced Power Electronics Corp.: AP4835GMSun TNT FlexNo ratings yet
- CDIL TransistorsDocument4 pagesCDIL Transistorsjjtrivedi8717No ratings yet
- Cytojournal: Time For Evidence-Based CytologyDocument10 pagesCytojournal: Time For Evidence-Based CytologyAtikah RahmadhaniNo ratings yet
- Structural Theory 1 (Double Integration Method)Document22 pagesStructural Theory 1 (Double Integration Method)acurvz2005No ratings yet
- TechnicalProposal 20130710B Rev0Document3 pagesTechnicalProposal 20130710B Rev0JomaargNo ratings yet
- Business Analysis of "Syafa Farm" Water SpinachDocument5 pagesBusiness Analysis of "Syafa Farm" Water SpinachhildaNo ratings yet
- Sodexho - Hotels in PuneDocument1,428 pagesSodexho - Hotels in PuneNiranjan100% (3)
- AutomobileDocument98 pagesAutomobilesrp188No ratings yet
- Ecological Relationships 1Document12 pagesEcological Relationships 1api-512405061No ratings yet
- jENS - 2017Document97 pagesjENS - 2017Manita RanzaNo ratings yet
- Shock AmbossDocument40 pagesShock AmbossAshraf AlbhlaNo ratings yet