Professional Documents
Culture Documents
General Properties: Symbol Number
General Properties: Symbol Number
Uploaded by
Sapari VelCopyright:
Available Formats
You might also like
- Materials Data for Cyclic Loading: Aluminium and Titanium AlloysFrom EverandMaterials Data for Cyclic Loading: Aluminium and Titanium AlloysRating: 1 out of 5 stars1/5 (1)
- Chemistry Reference TablesDocument8 pagesChemistry Reference Tablescauten2100% (1)
- Titanium: Titanium Is A Chemical Element With The Symbol TiDocument21 pagesTitanium: Titanium Is A Chemical Element With The Symbol TiVysakh VasudevanNo ratings yet
- The Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic ChemistryFrom EverandThe Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic ChemistryNo ratings yet
- Quality Control of Rebar Couplers in Splicing of Reinforcement BarsDocument12 pagesQuality Control of Rebar Couplers in Splicing of Reinforcement BarsDong-Yong KimNo ratings yet
- Tabel - CT PDFDocument3 pagesTabel - CT PDFFahri AtalahNo ratings yet
- CDS InformationDocument3 pagesCDS Informationanon-829132No ratings yet
- KryptonDocument4 pagesKryptonJarky SparcsNo ratings yet
- Comparative Properties of MetalsDocument2 pagesComparative Properties of MetalsAjay AgrawalNo ratings yet
- Details of The Chemical ElementDocument64 pagesDetails of The Chemical ElementRASHEN SAMARAWICKRAMANo ratings yet
- Iodine - Wikipedia PDFDocument131 pagesIodine - Wikipedia PDFsachin patilNo ratings yet
- Webelements Table 5sf 2012-06-07Document0 pagesWebelements Table 5sf 2012-06-07api-239300177No ratings yet
- With The - It Is An of K and NO, and Is Therefore An .: Potassium Nitrate Is ADocument6 pagesWith The - It Is An of K and NO, and Is Therefore An .: Potassium Nitrate Is ALeoNo ratings yet
- Element ProjectDocument11 pagesElement Projectapi-286120928No ratings yet
- Ciri-Ciri Umum, ,: / En-Uk-Aluminium1.ogg Æ L J Ʉ M Ɪ N I ƏMDocument2 pagesCiri-Ciri Umum, ,: / En-Uk-Aluminium1.ogg Æ L J Ʉ M Ɪ N I ƏMRointo Firnandus BerutuNo ratings yet
- List of The Elements With Their Atomic Symbols and Atomic MassesDocument4 pagesList of The Elements With Their Atomic Symbols and Atomic MasseshandsomenormalmaleNo ratings yet
- PalladiumDocument19 pagesPalladiumjosevitorromualdoNo ratings yet
- Silver: Silver Is A Chemical Element With The Symbol AgDocument25 pagesSilver: Silver Is A Chemical Element With The Symbol AgHunNo ratings yet
- HydrogenDocument4 pagesHydrogenChiun Er AngNo ratings yet
- Wikipedia Heat CapacityDocument3 pagesWikipedia Heat CapacitygabbyveliNo ratings yet
- Raw Materials For Manufacturing Lead-Acid BatteriesDocument36 pagesRaw Materials For Manufacturing Lead-Acid BatteriesBalakrishnan Pedda GovindierNo ratings yet
- Methane: Jump To Navigation Jump To Search Ethane CH4 (Disambiguation)Document14 pagesMethane: Jump To Navigation Jump To Search Ethane CH4 (Disambiguation)GeorgeNo ratings yet
- Chemistry Data SheetDocument2 pagesChemistry Data SheetAbre Groenewald0% (1)
- MethaneDocument6 pagesMethanevijay kumar honnaliNo ratings yet
- Stable Isotopes of Rhodium: Isotope Z (P) N (N) Atomic Mass Natural Abundance Nuclear SpinDocument2 pagesStable Isotopes of Rhodium: Isotope Z (P) N (N) Atomic Mass Natural Abundance Nuclear SpinKathleen ZulimNo ratings yet
- CBC Databook 1Document36 pagesCBC Databook 1anees19oct50% (2)
- Density of The ElementsDocument3 pagesDensity of The ElementsNileshhkNo ratings yet
- Noble Gas (Data Page) - WikipediaDocument7 pagesNoble Gas (Data Page) - WikipediaDwiki JuliansyahNo ratings yet
- AiCHe Student Pocket Handbook 85Document63 pagesAiCHe Student Pocket Handbook 85DigitalMastersTXNo ratings yet
- The D - Block ElementsDocument30 pagesThe D - Block ElementsNandya AristaNo ratings yet
- MolybdenumDocument128 pagesMolybdenumKishore KumarNo ratings yet
- Atomic WeightsDocument8 pagesAtomic WeightsSeamus AlaricNo ratings yet
- Combustion Basics: Joseph Colannino, P.EDocument20 pagesCombustion Basics: Joseph Colannino, P.ErezaimamNo ratings yet
- Titanium - WikipediaDocument21 pagesTitanium - WikipediaTiziana LylieNo ratings yet
- CH 19Document36 pagesCH 19SylviaNo ratings yet
- Tungsten, or WolframDocument19 pagesTungsten, or WolframVysakh VasudevanNo ratings yet
- Silver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeDocument4 pagesSilver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeEllaineNo ratings yet
- Nitrogen - WikipediaDocument30 pagesNitrogen - WikipediaPye PhyoNo ratings yet
- Per TableDocument12 pagesPer TableSreekumar C PillaiNo ratings yet
- Benzena: Nama IupacDocument16 pagesBenzena: Nama IupacSaya Adalah TsaniaNo ratings yet
- Chemistry 12 Data BookletDocument12 pagesChemistry 12 Data BookletEtoileCamelliaNo ratings yet
- High Temperature PEM Fuel Cells Work at Temperatures Above 100Document6 pagesHigh Temperature PEM Fuel Cells Work at Temperatures Above 100Carlos Ulises GonzalezNo ratings yet
- Transition Elements (B.sc-Ii) Inorganic Chemistry Paper-IDocument32 pagesTransition Elements (B.sc-Ii) Inorganic Chemistry Paper-IPinky SinghNo ratings yet
- Interactive Powerpoint Lanthanides-ActinidesDocument50 pagesInteractive Powerpoint Lanthanides-Actinidesapi-295463484No ratings yet
- Student Pocket HandbookDocument64 pagesStudent Pocket Handbookadarsh_mrNo ratings yet
- Palladium - Wikipedia, The Free EncyclopediaDocument12 pagesPalladium - Wikipedia, The Free EncyclopediaSellam PannerNo ratings yet
- Nitrogen: Jump To Navigationjump To SearchDocument5 pagesNitrogen: Jump To Navigationjump To Searchelika.alfonsoNo ratings yet
- Reference Tables For Physical Setting/CHEMISTRY: 2002 EditionDocument7 pagesReference Tables For Physical Setting/CHEMISTRY: 2002 EditionJustin LiangNo ratings yet
- Sulfur - WikipediaDocument119 pagesSulfur - Wikipediarock2903No ratings yet
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Chem II AP PacketDocument4 pagesChem II AP PacketAmanda Rose DalyNo ratings yet
- Chemistry Data Booklet Standard Grade and Intermediate 2: © Scottish Qualifications Authority 2007Document12 pagesChemistry Data Booklet Standard Grade and Intermediate 2: © Scottish Qualifications Authority 2007anilkumarappapurapuNo ratings yet
- PoloniumDocument20 pagesPoloniumKosuke IchinichiNo ratings yet
- Group IIADocument42 pagesGroup IIARyan D. GloryNo ratings yet
- Explosion Safety in Ethoxylation Reactors: M Braithwaite & A PekalskiDocument19 pagesExplosion Safety in Ethoxylation Reactors: M Braithwaite & A Pekalskikhali54No ratings yet
- Advanced Materials '93: Ceramics, Powders, Corrosion and Advanced ProcessingFrom EverandAdvanced Materials '93: Ceramics, Powders, Corrosion and Advanced ProcessingShigeyuki SomiyaNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- High Temperature Corrosion: Fundamentals and EngineeringFrom EverandHigh Temperature Corrosion: Fundamentals and EngineeringNo ratings yet
- Heterogeneous Catalysis at Nanoscale for Energy ApplicationsFrom EverandHeterogeneous Catalysis at Nanoscale for Energy ApplicationsNo ratings yet
- 2&3 Qus BankDocument6 pages2&3 Qus BankSapari VelNo ratings yet
- Ae2201 - III SemDocument3 pagesAe2201 - III SemSapari VelNo ratings yet
- Horse Power ConversionDocument1 pageHorse Power ConversionSapari VelNo ratings yet
- 2 and 3Document18 pages2 and 3Sapari VelNo ratings yet
- Geo Char ChartDocument1 pageGeo Char ChartSapari VelNo ratings yet
- Design and Analysis of Knuckle JointDocument25 pagesDesign and Analysis of Knuckle JointSapari Vel33% (3)
- 2 and 3Document18 pages2 and 3Sapari VelNo ratings yet
- St. Peter'S University: Design and Analysis of Knuckle JointDocument2 pagesSt. Peter'S University: Design and Analysis of Knuckle JointSapari VelNo ratings yet
- DFMAS-Unit 4 NotesDocument19 pagesDFMAS-Unit 4 NotesSapari VelNo ratings yet
- Mild SteelDocument1 pageMild SteelSapari Vel100% (1)
- DFMAS-Unit 4 NotesDocument19 pagesDFMAS-Unit 4 NotesSapari VelNo ratings yet
- Combustion Equation of Gasoline and DieselDocument4 pagesCombustion Equation of Gasoline and DieselSapari VelNo ratings yet
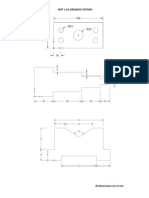
- Expt 1.co Ordinate SystemsDocument13 pagesExpt 1.co Ordinate SystemsSapari VelNo ratings yet
- Material Used in Engine Block CastingDocument1 pageMaterial Used in Engine Block CastingSapari VelNo ratings yet
- Laminated Object Manufacturing MethodDocument8 pagesLaminated Object Manufacturing MethodSapari VelNo ratings yet
- Calculation of Power Draw of Tumbling MillsDocument10 pagesCalculation of Power Draw of Tumbling MillsLevent ErgunNo ratings yet
- Question Bank-Unit 3 SujaiDocument2 pagesQuestion Bank-Unit 3 Sujaissujai83No ratings yet
- Guidelines For B.Tech Project Report Preparation: Order of The ContentsDocument10 pagesGuidelines For B.Tech Project Report Preparation: Order of The ContentsbhushansoniNo ratings yet
- Acid Base CementsDocument420 pagesAcid Base Cementskka2000100% (1)
- Kronig Penney ModelDocument16 pagesKronig Penney ModelPo Kai100% (1)
- Lecture 8 Disturbance Rejection: AME 455 Control Systems DesignDocument15 pagesLecture 8 Disturbance Rejection: AME 455 Control Systems DesignJason ChiangNo ratings yet
- Code: S7Lt-Iiih-I-12 Performance Standard: Content Standard: How Heat Is Transferred? I.ObjectivesDocument5 pagesCode: S7Lt-Iiih-I-12 Performance Standard: Content Standard: How Heat Is Transferred? I.ObjectivesEricka Mae Tizon100% (1)
- IBO 2009 Theory AnswersDocument9 pagesIBO 2009 Theory AnswersPei JingNo ratings yet
- CE486 Preliminary Design Report of Hospital Building in MenemenDocument13 pagesCE486 Preliminary Design Report of Hospital Building in MenemenAli OrhanNo ratings yet
- AxleDocument8 pagesAxlevaniyahareshNo ratings yet
- 13 - Fea PDFDocument121 pages13 - Fea PDFChetan The game changerNo ratings yet
- Capacitive Compensation at Nonsinsoidal Buses Based On IEEE STD 18-1992Document2 pagesCapacitive Compensation at Nonsinsoidal Buses Based On IEEE STD 18-1992tatacpsNo ratings yet
- Lateral Spring ConstantsDocument5 pagesLateral Spring ConstantsFrank SciallaNo ratings yet
- Shot in The Dark WorksheetDocument2 pagesShot in The Dark Worksheetapi-245364807No ratings yet
- Galileo's RampDocument8 pagesGalileo's RampMegan Hayes-GoldingNo ratings yet
- Thermodynamics SummaryDocument1 pageThermodynamics SummaryHumberto GilmerNo ratings yet
- Chapter 3 - Fall2015 PDFDocument41 pagesChapter 3 - Fall2015 PDFPhạm Ngọc ThạchNo ratings yet
- Core Mathematics C3 For Edexcel Advanced Level: Paper K Time: 1 Hour 30 MinutesDocument4 pagesCore Mathematics C3 For Edexcel Advanced Level: Paper K Time: 1 Hour 30 MinutesNashra19No ratings yet
- RRB ALP Syllabus 2017 PDF - RRB Assistant Loco Pilot SyllabusDocument5 pagesRRB ALP Syllabus 2017 PDF - RRB Assistant Loco Pilot SyllabusABHILASHNo ratings yet
- Transformer Design and Optimization A Literature Survey PDFDocument26 pagesTransformer Design and Optimization A Literature Survey PDFRushikesh MaliNo ratings yet
- Well PlanDocument168 pagesWell PlanNikhil BarshettiwarNo ratings yet
- Asme Sec V A-2 RT PDFDocument44 pagesAsme Sec V A-2 RT PDFmsalinasaguilar71% (7)
- Flowing WellsDocument18 pagesFlowing WellsJorge RidezNo ratings yet
- Physics Math Models Human IntuitionDocument4 pagesPhysics Math Models Human IntuitionAbha SrivastavaNo ratings yet
- Quick Testtrigo&SeDocument2 pagesQuick Testtrigo&Se丽娜钱霙No ratings yet
- Johnson Magnets PDFDocument7 pagesJohnson Magnets PDFYawarNo ratings yet
General Properties: Symbol Number
General Properties: Symbol Number
Uploaded by
Sapari VelOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
General Properties: Symbol Number
General Properties: Symbol Number
Uploaded by
Sapari VelCopyright:
Available Formats
General properties Name, symbol,number platinum, Pt, 78 Pronunciation /plt.n.m/ or /plt.
nm/
Element category Group, period, block
transition metal 10, 6, d
Standard atomic weight 195.084 Electron configuration [Xe] 4f14 5d9 6s1 Electrons per shell 2, 8, 18, 32, 17, 1 (Image)
Physical properties Phase Density (near r.t.) Liquid density atm.p. Melting point solid 21.45 gcm3 19.77 gcm3 2041.4 K3214.9 F 1768.3 C, , Boiling point 6917 F 3825 C,
4098 K, Heat of fusion Heat of vaporization Molar heat capacity 22.17 kJmol1 469 kJmol1 25.86 Jmol1K1
Vapor pressure
P (Pa)
10
100
1k
10 k
100 k
at T (K) 2330 (2550) 2815 3143 3556
4094
Atomic properties Oxidation states 6, 5, 4, 3 , 2, 1, -1, -2 (mildly basic oxide) Electronegativity Ionization energies 2.28 (Pauling scale) 1st: 870 kJmol1 2nd: 1791 kJmol1 Atomic radius Covalent radius Van der Waals radius 139 pm 1365 pm 175 pm
Miscellanea Crystal structure Magnetic ordering face-centered cubic paramagnetic
Electrical resistivity Thermal conductivity Thermal expansion Tensile strength Young's modulus Shear modulus Bulk modulus Poisson ratio Mohs hardness Vickers hardness Brinell hardness CAS registry number
(20 C) 105 nm 71.6 Wm1K1 (25 C) 8.8 mm1K1 125-240 MPa 168 GPa 61 GPa 230 GPa 0.38 44.5 549 MPa 392 MPa 7440-06-4
Most stable isotopes Main article: Isotopes of platinum
iso
NA
half-life
DM DE (MeV)
DP
190
Pt
0.014%
6.51011 y
3.18
186
Os
192
Pt
0.782%
192
Pt is stable with 114 neutrons
193
Pt
syn
50 y
193
Ir
194
Pt 32.967%
194
Pt is stable with 116 neutrons
195
Pt 33.832%
195
Pt is stable with 117 neutrons
196
Pt 25.242%
196
Pt is stable with 118 neutrons
198
Pt
7.163%
198
Pt is stable with 120 neutrons
Platinum (
/pltnm/ or /pltnm/) is a chemical
General properties
Name, symbol,number Pronunciation
palladium, Pd, 46 /pledim/
p-LAY-dee-m
Element category Group, period, block transition metal 10, 5, d
Standard atomic weight 106.42 Electron configuration Electrons per shell [Kr] 4d10 2, 8, 18, 18 (Image)
Physical properties
Phase Density (near r.t.) Liquid density atm.p. Melting point
solid 12.023 gcm3 10.38 gcm3 1828.05 K2830.82 F 1554.9 C, ,
Boiling point
5365 F 2963 C, 3236 K,
Heat of fusion Heat of vaporization Molar heat capacity
16.74 kJmol1 362 kJmol1 25.98 Jmol1K1
Vapor pressure
P (Pa)
10
100
1k
10 k
100 k
at T (K)
1721 1897 2117 2395 2753
3234
Atomic properties Oxidation states 0, +1, +2, +4, +6 (mildly basic oxide) Electronegativity Ionization energies 2.20 (Pauling scale) 1st: 804.4 kJmol1 2nd: 1870 kJmol1 3rd: 3177 kJmol1
Atomic radius Covalent radius Van der Waals radius
137 pm 1396 pm 163 pm
Miscellanea Crystal structure Magnetic ordering Electrical resistivity Thermal conductivity Thermal expansion face-centered cubic paramagnetic[1] (20 C) 105.4 nm 71.8 Wm1K1 (25 C) 11.8 mm1K1
Speed of sound(thin rod) (20 C) 3070 ms1 Young's modulus Shear modulus Bulk modulus Poisson ratio Mohs hardness Vickers hardness Brinell hardness CAS registry number 121 GPa 44 GPa 180 GPa 0.39 4.75 461 MPa 310 MPa 7440-05-3
Most stable isotopes
Main article: Isotopes of palladium
iso
NA
half-life DM
DE (MeV)
DP
100
Pd
syn
3.63 d
100
Rh
0.084, 0.074, 0.126
102
Pd 1.02%
102
Pd is stable with 56 neutrons
103
Pd
syn
16.991 d
103
Rh
104
Pd 11.14%
104
Pd is stable with 58 neutrons
105
Pd 22.33%
105
Pd is stable with 59 neutrons
106
Pd 27.33%
106
Pd is stable with 60 neutrons
107
Pd
trace
6.5106y
0.033
107
Ag
108
Pd 26.46%
108
Pd is stable with 62 neutrons
110
Pd 11.72%
110
Pd is stable with 64 neutrons
r Appearance
silvery white metallic
General properties
Name, symbol, number rhodium, Rh, 45 Pronunciation Element category Group, period, block /rodim/ ROH-dee-m
transition metal 9, 5, d
Standard atomic weight 102.90550 Electron configuration Electrons per shell [Kr] 5s1 4d8 2, 8, 18, 16, 1 (Image)
Physical properties Phase Density (near r.t.) Liquid density at m.p. Melting point solid 12.41 gcm3 10.7 gcm3 2237 K3567 F 1964 C, , Boiling point 6683 F 3695 C, 3968 K, Heat of fusion 26.59 kJmol1
Heat of vaporization Molar heat capacity
494 kJmol1 24.98 Jmol1K1
Vapor pressure
P (Pa)
10
100
1k
10 k
100 k
at T (K)
2288 2496 2749 3063 3405
3997
Atomic properties Oxidation states 6, 5, 4, 3, 2, 1[1], -1 (amphoteric oxide) Electronegativity Ionization energies 2.28 (Pauling scale) 1st: 719.7 kJmol1 2nd: 1740 kJmol1 3rd: 2997 kJmol1 Atomic radius Covalent radius 134 pm 1427 pm
Miscellanea Crystal structure Magnetic ordering Electrical resistivity Thermal conductivity face-centered cubic paramagnetic[2] (0 C) 43.3 nm 150 Wm1K1
Thermal expansion Speed of sound (thin rod) Young's modulus Shear modulus Bulk modulus Poisson ratio Mohs hardness Vickers hardness Brinell hardness CAS registry number
(25 C) 8.2 mm1K1 (20 C) 4700 ms1
380 GPa 150 GPa 275 GPa 0.26 6.0 1246 MPa 1100 MPa 7440-16-6
Most stable isotopes Main article: Isotopes of rhodium
iso
NA
half-life DM
DE (MeV)
DP
99
Rh
syn
16.1 d
99
Ru
0.089, 0.353, 0.528
101m
Rh
syn
4.34 d
101
Ru
IT
0.157
101
Rh
0.306, 0.545
101
Rh
syn
3.3 y
101
Ru
0.127, 0.198, 0.325
102m
Rh
syn
2.9 y
102
Ru
0.475, 0.631, 0.697, 1.046
102
Rh
syn
207 d
102
Ru
0.826, 1.301
102
Ru
1.151
102
Pd
0.475, 0.628
103
Rh
100%
103
Rh is stable with 58 neutrons
105
Rh
syn
35.36 h
0.247, 0.260, 0.566
105
Pd
0.306, 0.318
You might also like
- Materials Data for Cyclic Loading: Aluminium and Titanium AlloysFrom EverandMaterials Data for Cyclic Loading: Aluminium and Titanium AlloysRating: 1 out of 5 stars1/5 (1)
- Chemistry Reference TablesDocument8 pagesChemistry Reference Tablescauten2100% (1)
- Titanium: Titanium Is A Chemical Element With The Symbol TiDocument21 pagesTitanium: Titanium Is A Chemical Element With The Symbol TiVysakh VasudevanNo ratings yet
- The Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic ChemistryFrom EverandThe Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic ChemistryNo ratings yet
- Quality Control of Rebar Couplers in Splicing of Reinforcement BarsDocument12 pagesQuality Control of Rebar Couplers in Splicing of Reinforcement BarsDong-Yong KimNo ratings yet
- Tabel - CT PDFDocument3 pagesTabel - CT PDFFahri AtalahNo ratings yet
- CDS InformationDocument3 pagesCDS Informationanon-829132No ratings yet
- KryptonDocument4 pagesKryptonJarky SparcsNo ratings yet
- Comparative Properties of MetalsDocument2 pagesComparative Properties of MetalsAjay AgrawalNo ratings yet
- Details of The Chemical ElementDocument64 pagesDetails of The Chemical ElementRASHEN SAMARAWICKRAMANo ratings yet
- Iodine - Wikipedia PDFDocument131 pagesIodine - Wikipedia PDFsachin patilNo ratings yet
- Webelements Table 5sf 2012-06-07Document0 pagesWebelements Table 5sf 2012-06-07api-239300177No ratings yet
- With The - It Is An of K and NO, and Is Therefore An .: Potassium Nitrate Is ADocument6 pagesWith The - It Is An of K and NO, and Is Therefore An .: Potassium Nitrate Is ALeoNo ratings yet
- Element ProjectDocument11 pagesElement Projectapi-286120928No ratings yet
- Ciri-Ciri Umum, ,: / En-Uk-Aluminium1.ogg Æ L J Ʉ M Ɪ N I ƏMDocument2 pagesCiri-Ciri Umum, ,: / En-Uk-Aluminium1.ogg Æ L J Ʉ M Ɪ N I ƏMRointo Firnandus BerutuNo ratings yet
- List of The Elements With Their Atomic Symbols and Atomic MassesDocument4 pagesList of The Elements With Their Atomic Symbols and Atomic MasseshandsomenormalmaleNo ratings yet
- PalladiumDocument19 pagesPalladiumjosevitorromualdoNo ratings yet
- Silver: Silver Is A Chemical Element With The Symbol AgDocument25 pagesSilver: Silver Is A Chemical Element With The Symbol AgHunNo ratings yet
- HydrogenDocument4 pagesHydrogenChiun Er AngNo ratings yet
- Wikipedia Heat CapacityDocument3 pagesWikipedia Heat CapacitygabbyveliNo ratings yet
- Raw Materials For Manufacturing Lead-Acid BatteriesDocument36 pagesRaw Materials For Manufacturing Lead-Acid BatteriesBalakrishnan Pedda GovindierNo ratings yet
- Methane: Jump To Navigation Jump To Search Ethane CH4 (Disambiguation)Document14 pagesMethane: Jump To Navigation Jump To Search Ethane CH4 (Disambiguation)GeorgeNo ratings yet
- Chemistry Data SheetDocument2 pagesChemistry Data SheetAbre Groenewald0% (1)
- MethaneDocument6 pagesMethanevijay kumar honnaliNo ratings yet
- Stable Isotopes of Rhodium: Isotope Z (P) N (N) Atomic Mass Natural Abundance Nuclear SpinDocument2 pagesStable Isotopes of Rhodium: Isotope Z (P) N (N) Atomic Mass Natural Abundance Nuclear SpinKathleen ZulimNo ratings yet
- CBC Databook 1Document36 pagesCBC Databook 1anees19oct50% (2)
- Density of The ElementsDocument3 pagesDensity of The ElementsNileshhkNo ratings yet
- Noble Gas (Data Page) - WikipediaDocument7 pagesNoble Gas (Data Page) - WikipediaDwiki JuliansyahNo ratings yet
- AiCHe Student Pocket Handbook 85Document63 pagesAiCHe Student Pocket Handbook 85DigitalMastersTXNo ratings yet
- The D - Block ElementsDocument30 pagesThe D - Block ElementsNandya AristaNo ratings yet
- MolybdenumDocument128 pagesMolybdenumKishore KumarNo ratings yet
- Atomic WeightsDocument8 pagesAtomic WeightsSeamus AlaricNo ratings yet
- Combustion Basics: Joseph Colannino, P.EDocument20 pagesCombustion Basics: Joseph Colannino, P.ErezaimamNo ratings yet
- Titanium - WikipediaDocument21 pagesTitanium - WikipediaTiziana LylieNo ratings yet
- CH 19Document36 pagesCH 19SylviaNo ratings yet
- Tungsten, or WolframDocument19 pagesTungsten, or WolframVysakh VasudevanNo ratings yet
- Silver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeDocument4 pagesSilver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeEllaineNo ratings yet
- Nitrogen - WikipediaDocument30 pagesNitrogen - WikipediaPye PhyoNo ratings yet
- Per TableDocument12 pagesPer TableSreekumar C PillaiNo ratings yet
- Benzena: Nama IupacDocument16 pagesBenzena: Nama IupacSaya Adalah TsaniaNo ratings yet
- Chemistry 12 Data BookletDocument12 pagesChemistry 12 Data BookletEtoileCamelliaNo ratings yet
- High Temperature PEM Fuel Cells Work at Temperatures Above 100Document6 pagesHigh Temperature PEM Fuel Cells Work at Temperatures Above 100Carlos Ulises GonzalezNo ratings yet
- Transition Elements (B.sc-Ii) Inorganic Chemistry Paper-IDocument32 pagesTransition Elements (B.sc-Ii) Inorganic Chemistry Paper-IPinky SinghNo ratings yet
- Interactive Powerpoint Lanthanides-ActinidesDocument50 pagesInteractive Powerpoint Lanthanides-Actinidesapi-295463484No ratings yet
- Student Pocket HandbookDocument64 pagesStudent Pocket Handbookadarsh_mrNo ratings yet
- Palladium - Wikipedia, The Free EncyclopediaDocument12 pagesPalladium - Wikipedia, The Free EncyclopediaSellam PannerNo ratings yet
- Nitrogen: Jump To Navigationjump To SearchDocument5 pagesNitrogen: Jump To Navigationjump To Searchelika.alfonsoNo ratings yet
- Reference Tables For Physical Setting/CHEMISTRY: 2002 EditionDocument7 pagesReference Tables For Physical Setting/CHEMISTRY: 2002 EditionJustin LiangNo ratings yet
- Sulfur - WikipediaDocument119 pagesSulfur - Wikipediarock2903No ratings yet
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Chem II AP PacketDocument4 pagesChem II AP PacketAmanda Rose DalyNo ratings yet
- Chemistry Data Booklet Standard Grade and Intermediate 2: © Scottish Qualifications Authority 2007Document12 pagesChemistry Data Booklet Standard Grade and Intermediate 2: © Scottish Qualifications Authority 2007anilkumarappapurapuNo ratings yet
- PoloniumDocument20 pagesPoloniumKosuke IchinichiNo ratings yet
- Group IIADocument42 pagesGroup IIARyan D. GloryNo ratings yet
- Explosion Safety in Ethoxylation Reactors: M Braithwaite & A PekalskiDocument19 pagesExplosion Safety in Ethoxylation Reactors: M Braithwaite & A Pekalskikhali54No ratings yet
- Advanced Materials '93: Ceramics, Powders, Corrosion and Advanced ProcessingFrom EverandAdvanced Materials '93: Ceramics, Powders, Corrosion and Advanced ProcessingShigeyuki SomiyaNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- High Temperature Corrosion: Fundamentals and EngineeringFrom EverandHigh Temperature Corrosion: Fundamentals and EngineeringNo ratings yet
- Heterogeneous Catalysis at Nanoscale for Energy ApplicationsFrom EverandHeterogeneous Catalysis at Nanoscale for Energy ApplicationsNo ratings yet
- 2&3 Qus BankDocument6 pages2&3 Qus BankSapari VelNo ratings yet
- Ae2201 - III SemDocument3 pagesAe2201 - III SemSapari VelNo ratings yet
- Horse Power ConversionDocument1 pageHorse Power ConversionSapari VelNo ratings yet
- 2 and 3Document18 pages2 and 3Sapari VelNo ratings yet
- Geo Char ChartDocument1 pageGeo Char ChartSapari VelNo ratings yet
- Design and Analysis of Knuckle JointDocument25 pagesDesign and Analysis of Knuckle JointSapari Vel33% (3)
- 2 and 3Document18 pages2 and 3Sapari VelNo ratings yet
- St. Peter'S University: Design and Analysis of Knuckle JointDocument2 pagesSt. Peter'S University: Design and Analysis of Knuckle JointSapari VelNo ratings yet
- DFMAS-Unit 4 NotesDocument19 pagesDFMAS-Unit 4 NotesSapari VelNo ratings yet
- Mild SteelDocument1 pageMild SteelSapari Vel100% (1)
- DFMAS-Unit 4 NotesDocument19 pagesDFMAS-Unit 4 NotesSapari VelNo ratings yet
- Combustion Equation of Gasoline and DieselDocument4 pagesCombustion Equation of Gasoline and DieselSapari VelNo ratings yet
- Expt 1.co Ordinate SystemsDocument13 pagesExpt 1.co Ordinate SystemsSapari VelNo ratings yet
- Material Used in Engine Block CastingDocument1 pageMaterial Used in Engine Block CastingSapari VelNo ratings yet
- Laminated Object Manufacturing MethodDocument8 pagesLaminated Object Manufacturing MethodSapari VelNo ratings yet
- Calculation of Power Draw of Tumbling MillsDocument10 pagesCalculation of Power Draw of Tumbling MillsLevent ErgunNo ratings yet
- Question Bank-Unit 3 SujaiDocument2 pagesQuestion Bank-Unit 3 Sujaissujai83No ratings yet
- Guidelines For B.Tech Project Report Preparation: Order of The ContentsDocument10 pagesGuidelines For B.Tech Project Report Preparation: Order of The ContentsbhushansoniNo ratings yet
- Acid Base CementsDocument420 pagesAcid Base Cementskka2000100% (1)
- Kronig Penney ModelDocument16 pagesKronig Penney ModelPo Kai100% (1)
- Lecture 8 Disturbance Rejection: AME 455 Control Systems DesignDocument15 pagesLecture 8 Disturbance Rejection: AME 455 Control Systems DesignJason ChiangNo ratings yet
- Code: S7Lt-Iiih-I-12 Performance Standard: Content Standard: How Heat Is Transferred? I.ObjectivesDocument5 pagesCode: S7Lt-Iiih-I-12 Performance Standard: Content Standard: How Heat Is Transferred? I.ObjectivesEricka Mae Tizon100% (1)
- IBO 2009 Theory AnswersDocument9 pagesIBO 2009 Theory AnswersPei JingNo ratings yet
- CE486 Preliminary Design Report of Hospital Building in MenemenDocument13 pagesCE486 Preliminary Design Report of Hospital Building in MenemenAli OrhanNo ratings yet
- AxleDocument8 pagesAxlevaniyahareshNo ratings yet
- 13 - Fea PDFDocument121 pages13 - Fea PDFChetan The game changerNo ratings yet
- Capacitive Compensation at Nonsinsoidal Buses Based On IEEE STD 18-1992Document2 pagesCapacitive Compensation at Nonsinsoidal Buses Based On IEEE STD 18-1992tatacpsNo ratings yet
- Lateral Spring ConstantsDocument5 pagesLateral Spring ConstantsFrank SciallaNo ratings yet
- Shot in The Dark WorksheetDocument2 pagesShot in The Dark Worksheetapi-245364807No ratings yet
- Galileo's RampDocument8 pagesGalileo's RampMegan Hayes-GoldingNo ratings yet
- Thermodynamics SummaryDocument1 pageThermodynamics SummaryHumberto GilmerNo ratings yet
- Chapter 3 - Fall2015 PDFDocument41 pagesChapter 3 - Fall2015 PDFPhạm Ngọc ThạchNo ratings yet
- Core Mathematics C3 For Edexcel Advanced Level: Paper K Time: 1 Hour 30 MinutesDocument4 pagesCore Mathematics C3 For Edexcel Advanced Level: Paper K Time: 1 Hour 30 MinutesNashra19No ratings yet
- RRB ALP Syllabus 2017 PDF - RRB Assistant Loco Pilot SyllabusDocument5 pagesRRB ALP Syllabus 2017 PDF - RRB Assistant Loco Pilot SyllabusABHILASHNo ratings yet
- Transformer Design and Optimization A Literature Survey PDFDocument26 pagesTransformer Design and Optimization A Literature Survey PDFRushikesh MaliNo ratings yet
- Well PlanDocument168 pagesWell PlanNikhil BarshettiwarNo ratings yet
- Asme Sec V A-2 RT PDFDocument44 pagesAsme Sec V A-2 RT PDFmsalinasaguilar71% (7)
- Flowing WellsDocument18 pagesFlowing WellsJorge RidezNo ratings yet
- Physics Math Models Human IntuitionDocument4 pagesPhysics Math Models Human IntuitionAbha SrivastavaNo ratings yet
- Quick Testtrigo&SeDocument2 pagesQuick Testtrigo&Se丽娜钱霙No ratings yet
- Johnson Magnets PDFDocument7 pagesJohnson Magnets PDFYawarNo ratings yet