Professional Documents
Culture Documents
Half Life Introduction Lab
Half Life Introduction Lab
Uploaded by
api-251355123Copyright:
Available Formats
You might also like
- Gizmo Half-LifeDocument5 pagesGizmo Half-Lifeskyler29% (7)
- Electron Cloud LabDocument4 pagesElectron Cloud Labspecops4733% (6)
- Student Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Document5 pagesStudent Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Kristina33% (3)
- Period MonologuesDocument9 pagesPeriod Monologueslaurelbeth100% (1)
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDocument5 pagesStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationJahel HerculesNo ratings yet
- Report Sheet Half Life 2Document6 pagesReport Sheet Half Life 2Ayhan AbdulAzizNo ratings yet
- Radiometric Dating Lab: ProblemDocument5 pagesRadiometric Dating Lab: ProblemApril Mae ArcayaNo ratings yet
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDocument5 pagesStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationSai83% (6)
- Earth Science Midterm Review - 14-15Document2 pagesEarth Science Midterm Review - 14-15api-251355123No ratings yet
- Mapping The Ring of FireDocument4 pagesMapping The Ring of Fireapi-251355123No ratings yet
- Relative Humidity and Dew Point LabDocument6 pagesRelative Humidity and Dew Point Labapi-251355123No ratings yet
- Intermediate Steps To Understanding PDFDocument36 pagesIntermediate Steps To Understanding PDFVal Ribeiro79% (34)
- 4 SkittliumDocument4 pages4 SkittliumThe Life Of LGNo ratings yet
- 7.5 Halflife SEDocument5 pages7.5 Halflife SEWyatt KesterNo ratings yet
- 2014-10-20 - Lab - Radiometric Dating and Half LivesDocument4 pages2014-10-20 - Lab - Radiometric Dating and Half LivesMA. HAZEL TEOLOGONo ratings yet
- M2 Unit 3 G8Document8 pagesM2 Unit 3 G8Julia Geonzon LabajoNo ratings yet
- Half lifeSEDocument4 pagesHalf lifeSERusty WynderNo ratings yet
- 08.01 Half-Life and Radioactive DecayDocument3 pages08.01 Half-Life and Radioactive DecayAnonymous 8VJhV1eI2yNo ratings yet
- Teacher Guide: Half-Life: Learning ObjectivesDocument4 pagesTeacher Guide: Half-Life: Learning ObjectivesPeyton100% (1)
- Half LifeDocument2 pagesHalf LifeYasmen MendezNo ratings yet
- edTPA Instructional MaterialsDocument8 pagesedTPA Instructional MaterialsDanielle WilsonNo ratings yet
- Half Life Simulation With PenniesDocument3 pagesHalf Life Simulation With PenniesAbigail ShipeNo ratings yet
- Half-Life Student Lab Sheet-REVDocument6 pagesHalf-Life Student Lab Sheet-REVElPerritoSapeeNo ratings yet
- The 'Radioactive Dice' Experiment - Why Is The 'Half-Life' Slightly Wrong?Document6 pagesThe 'Radioactive Dice' Experiment - Why Is The 'Half-Life' Slightly Wrong?Pablo AlbinoNo ratings yet
- Atoms: Half Life Questions and AnswersDocument6 pagesAtoms: Half Life Questions and AnswersBubuNo ratings yet
- Half-Life 5centium InvDocument5 pagesHalf-Life 5centium InvJohn OsborneNo ratings yet
- Half-Life MM LabDocument5 pagesHalf-Life MM Labapi-1107897020% (1)
- 4.5.1 Half-LifeDocument4 pages4.5.1 Half-LifeMalik Rashid Ali LangrialNo ratings yet
- Nuclear Chemistry: ObjectivesDocument4 pagesNuclear Chemistry: ObjectivesKent Lewis GornezNo ratings yet
- Half LifeDocument11 pagesHalf LifeMarshell JonesNo ratings yet
- Models of The AtomDocument39 pagesModels of The AtomJames Excommunicado WickNo ratings yet
- 21 Radioactivity and Nuclear PhysicsDocument8 pages21 Radioactivity and Nuclear PhysicsAman BhuttaNo ratings yet
- Maths Exploration ProposalDocument2 pagesMaths Exploration Proposalazam96100% (1)
- Half-lifeSEDocument6 pagesHalf-lifeSEJBE legends100% (1)
- Physics Labs and PDsDocument33 pagesPhysics Labs and PDsBrihanna MontaqueNo ratings yet
- Zaundre Pusey - Half-lifeSEDocument8 pagesZaundre Pusey - Half-lifeSEJBE legendsNo ratings yet
- Earth Science Week 4 - Q2Document4 pagesEarth Science Week 4 - Q2Giane MadrigalNo ratings yet
- Radioactivity Physics Lab AnswersDocument3 pagesRadioactivity Physics Lab AnswersAaliyah Senior100% (1)
- PEKA RadioactiveDocument2 pagesPEKA Radioactivebrenwong@ymail.comNo ratings yet
- Atom A Closer LookDocument34 pagesAtom A Closer LookJohn Nash100% (1)
- Over-Populated or Under-Developed? The Real Story of Population GrowthDocument7 pagesOver-Populated or Under-Developed? The Real Story of Population GrowthMark Anthony BoloNo ratings yet
- Oxo AQA16 P7uu T801 CsaannDocument10 pagesOxo AQA16 P7uu T801 CsaannemailusageNo ratings yet
- Modeling Radioactive Decay With A DiceDocument4 pagesModeling Radioactive Decay With A DicePablo AlbinoNo ratings yet
- Aqa Gcse Science Command WordsDocument15 pagesAqa Gcse Science Command WordsChatur BatorNo ratings yet
- Absolute Dating Reading AssignmentDocument5 pagesAbsolute Dating Reading Assignmentapi-251355123No ratings yet
- Abs EnglishDocument17 pagesAbs EnglishshahabNo ratings yet
- Half Life and Radioactive DatingDocument13 pagesHalf Life and Radioactive DatingFaique HassanNo ratings yet
- (Chemistry) Lab ReviewerDocument3 pages(Chemistry) Lab ReviewerIris Kaye AbelleraNo ratings yet
- Simulating Half Life MakeupDocument2 pagesSimulating Half Life MakeupKris GribecNo ratings yet
- Chem Lab 2Document2 pagesChem Lab 2Samuel ArzadonNo ratings yet
- Number of Original Nuclei) To Drop To Half of Its Initial ValueDocument8 pagesNumber of Original Nuclei) To Drop To Half of Its Initial ValuesalmaNo ratings yet
- Beamline 2024Document7 pagesBeamline 2024Ermi TafiliNo ratings yet
- A Self-Guided Computer ActivityDocument23 pagesA Self-Guided Computer ActivityKang AmaNo ratings yet
- Candy AtomsDocument3 pagesCandy AtomsSrikarNo ratings yet
- Ejercios n4 TectonofisicaDocument4 pagesEjercios n4 TectonofisicaIsmael Mamani ChNo ratings yet
- Radiation and Half-LifeDocument6 pagesRadiation and Half-LifeAbdul NoorNo ratings yet
- Half-Life: Words To KnowDocument10 pagesHalf-Life: Words To KnowEthan RajakumarNo ratings yet
- EarthSci PetaaaDocument4 pagesEarthSci PetaaaJay GarciaNo ratings yet
- What Is The Centralized Region Inside The Atom Where The Protons and Neutrons Are Located and Holds Most of The Atom's Mass?Document35 pagesWhat Is The Centralized Region Inside The Atom Where The Protons and Neutrons Are Located and Holds Most of The Atom's Mass?JAMES RYAN EDMANo ratings yet
- The MAPDocument4 pagesThe MAPNidhi1106No ratings yet
- Let's Get Tiny!: Jumping into the Science of the Smallest Part of Matter with Quantum PhysicsFrom EverandLet's Get Tiny!: Jumping into the Science of the Smallest Part of Matter with Quantum PhysicsRating: 5 out of 5 stars5/5 (1)
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksFrom EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNo ratings yet
- Instead of the ITER project and the TOKAMAK principle: – a new type of fusion machineFrom EverandInstead of the ITER project and the TOKAMAK principle: – a new type of fusion machineRating: 5 out of 5 stars5/5 (1)
- Physics for Kids | Atoms, Electricity and States of Matter Quiz Book for Kids | Children's Questions & Answer Game BooksFrom EverandPhysics for Kids | Atoms, Electricity and States of Matter Quiz Book for Kids | Children's Questions & Answer Game BooksNo ratings yet
- Earth Science Midterm Diagram ReviewDocument4 pagesEarth Science Midterm Diagram Reviewapi-251355123No ratings yet
- 27 Solar Activity HomeworkDocument2 pages27 Solar Activity Homeworkapi-251355123No ratings yet
- Absolute Dating Reading AssignmentDocument5 pagesAbsolute Dating Reading Assignmentapi-251355123No ratings yet
- Volcano ReadingsDocument16 pagesVolcano Readingsapi-251355123No ratings yet
- Relative Dating Reading AssignmentDocument5 pagesRelative Dating Reading Assignmentapi-251355123No ratings yet
- 11 Tsunami Notes - TeacherDocument3 pages11 Tsunami Notes - Teacherapi-251355123No ratings yet
- Earthquake ReadingsDocument16 pagesEarthquake Readingsapi-251355123No ratings yet
- Fault Block Modeling LabDocument12 pagesFault Block Modeling Labapi-251355123100% (1)
- Graphing Sunspots LabDocument3 pagesGraphing Sunspots Labapi-2513551230% (1)
- 24 The Sun Notes - TeacherDocument2 pages24 The Sun Notes - Teacherapi-251355123No ratings yet
- 26 Structure of The Sun HomeworkDocument2 pages26 Structure of The Sun Homeworkapi-251355123No ratings yet
- Cloud Reference SheetDocument3 pagesCloud Reference Sheetapi-251355123No ratings yet
- Moon WebquestDocument5 pagesMoon Webquestapi-2513551230% (2)
- 21 Models of The Solar System HomeworkDocument2 pages21 Models of The Solar System Homeworkapi-251355123No ratings yet
- Weather Front and Air Mass WebquestDocument3 pagesWeather Front and Air Mass Webquestapi-251355123No ratings yet
- Pluto As A Planet Argumentative WritingDocument5 pagesPluto As A Planet Argumentative Writingapi-251355123No ratings yet
- 22 The Inner Planets HomeworkDocument2 pages22 The Inner Planets Homeworkapi-251355123No ratings yet
- Ellipse LabDocument4 pagesEllipse Labapi-251355123No ratings yet
- 25 Movements of The Moon HomeworkDocument2 pages25 Movements of The Moon Homeworkapi-251355123No ratings yet
- 20 Formation of The Solar System HomeworkDocument2 pages20 Formation of The Solar System Homeworkapi-251355123No ratings yet
- 18 Fronts HomeworkDocument2 pages18 Fronts Homeworkapi-251355123No ratings yet
- 17 Air MassesDocument2 pages17 Air Massesapi-251355123No ratings yet
- Weather Front LabDocument2 pagesWeather Front Labapi-251355123No ratings yet
- 19 Movements of Earth HomeworkDocument2 pages19 Movements of Earth Homeworkapi-251355123No ratings yet
- 18 Air Masses and Fronts Notes - TeacherDocument4 pages18 Air Masses and Fronts Notes - Teacherapi-251355123No ratings yet
- IWA City Stories SingaporeDocument2 pagesIWA City Stories SingaporeThang LongNo ratings yet
- Relatorio Mano JulioDocument7 pagesRelatorio Mano JulioProGeo Projetos AmbientaisNo ratings yet
- No Overlord Rulebook For Descent: Journeys in The Dark (2nd Edition)Document21 pagesNo Overlord Rulebook For Descent: Journeys in The Dark (2nd Edition)Jeremy ForoiNo ratings yet
- VDRLDocument4 pagesVDRLfarazhussainkhanNo ratings yet
- Teaching Language-Learning StrategiesDocument6 pagesTeaching Language-Learning StrategiesviviNo ratings yet
- GenMath Module 5 Week 5Document15 pagesGenMath Module 5 Week 5Immanuel CañecaNo ratings yet
- Unit 1 - Task 3 - Reader Guru Adventure! - Evaluation Quiz - Revisión Del IntentoDocument7 pagesUnit 1 - Task 3 - Reader Guru Adventure! - Evaluation Quiz - Revisión Del IntentoNelson AbrilNo ratings yet
- APA-AP - Activity 3-Emerging Ethical and Policy Issues of STSDocument36 pagesAPA-AP - Activity 3-Emerging Ethical and Policy Issues of STSJOHN MARTIN APA-APNo ratings yet
- Federal University OtuokeDocument5 pagesFederal University OtuokeeteleruthNo ratings yet
- Statement of Purpose Name: Krishna Teja SwarnaDocument2 pagesStatement of Purpose Name: Krishna Teja SwarnaMohammadRahemanNo ratings yet
- The Ezekiel Cycle of 500 YearsDocument1 pageThe Ezekiel Cycle of 500 YearsOtis Arms100% (1)
- Software Engineering. Specification, Implementation, VerificationDocument186 pagesSoftware Engineering. Specification, Implementation, Verificationazariel.rodrigo100% (1)
- Dialogic DSI Signaling Servers: SIU Mode User ManualDocument304 pagesDialogic DSI Signaling Servers: SIU Mode User ManualAdiansyah Rama67% (3)
- ME-458 Turbomachinery: Muhammad Shaban Lecturer Department of Mechanical EngineeringDocument113 pagesME-458 Turbomachinery: Muhammad Shaban Lecturer Department of Mechanical EngineeringAneeq Raheem50% (2)
- DSF Course Curriculum 1305231045Document8 pagesDSF Course Curriculum 1305231045Gaurav BhadaneNo ratings yet
- SPE 163723 Pressure Transient Analysis of Data From Permanent Downhole GaugesDocument24 pagesSPE 163723 Pressure Transient Analysis of Data From Permanent Downhole GaugesLulut Fitra FalaNo ratings yet
- Writing Home, Painting Home: 17th Century Dutch Genre Painting and The "Sailing Letters"Document17 pagesWriting Home, Painting Home: 17th Century Dutch Genre Painting and The "Sailing Letters"María MazzantiNo ratings yet
- Neuro TR Brochure - EN CompressedDocument8 pagesNeuro TR Brochure - EN CompressedJanam KuNo ratings yet
- Occupational Safety and Health Aspects of Voice and Speech ProfessionsDocument34 pagesOccupational Safety and Health Aspects of Voice and Speech ProfessionskaaanyuNo ratings yet
- MalappuramDocument20 pagesMalappuramFayizNo ratings yet
- Optimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsDocument7 pagesOptimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsNelson Andres Entralgo MaldonadoNo ratings yet
- Synchronous Alternators: Three-Phase BrushlessDocument5 pagesSynchronous Alternators: Three-Phase BrushlessĐại DươngNo ratings yet
- Chroma Bidirectional DC Power Supply Model 62000D SeriesDocument12 pagesChroma Bidirectional DC Power Supply Model 62000D SeriesaboofazilNo ratings yet
- Chinese Vocabulary BodyDocument3 pagesChinese Vocabulary BodyWei LeeNo ratings yet
- Safety Data Sheet Carbon Cathode SolutionDocument11 pagesSafety Data Sheet Carbon Cathode SolutionJeff BanasekNo ratings yet
- Getting The Most From Lube Oil AnalysisDocument16 pagesGetting The Most From Lube Oil AnalysisGuru Raja Ragavendran Nagarajan100% (2)
- Poster FinalDocument1 pagePoster FinalKrish vasisthaNo ratings yet
- Miller PreviewDocument252 pagesMiller PreviewcqpresscustomNo ratings yet
Half Life Introduction Lab
Half Life Introduction Lab
Uploaded by
api-251355123Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Half Life Introduction Lab
Half Life Introduction Lab
Uploaded by
api-251355123Copyright:
Available Formats
Half Life Introduction Lab:
Name: Background Activities: In relative dating, we are simply trying to determine whether one rock layer is older than another or gathering an idea of the geological history of a certain area. In these cases, we are using observations and clues to determine a relative age. However, sometimes it is necessary to be more accurate and determine actual years or an absolute age. One of the way scientists determine absolute age is through radioactive dating, which uses the half-life of radioactive materials to figure out an approximate age. Rocks generally have small amounts of radioactive material that can be used to figure out the age of a substance. Remember to earlier in the year when we discussed how atoms of the same element, which have different number of neutrons are called isotopes. These radioactive isotopes will emit particles and energy, while in the process breaking down into stable elements. Each of these radioactive isotopes will decay at a constant rate which can be used to calculate the age of the rocks. The time it takes for half of a sample of a radioactive isotope (parent isotope) to break down into more stable isotopes (daughter isotopes) is called a half-life. Thus after one half-life, one half of the parent isotope has broken down into the daughter isotope. After two half-lives, half of the remaining one half of the parent isotope breaks down into the daughter isotope, leaving only of the original amount of the parent isotope. To better understand the concept, imagine you have a million dollars and you have to spend of what you have everyday. How many days do you think it would take to get to zero? Give it a try below and graph your results: Days 0 1 Remaining 1,000,000 500,000 Date: Period:
Another way to look at it, is you have a sample that was initially comprised 100% of the radioactive parent isotope Carbon 14. Carbon 14 has a half life of 5,730 years, which means, that after 5,730 years, of the Carbon 14 has decayed into its daughter isotope of Nitrogen 14. Use the models below to fill in the chart below:
Life Carbon 14 Nitrogen 14 Years Passed
0 16 0 0
Lab Activity: 1. Count your nuclei (candy). Write that number in the data table under the heading "Number of Radioactive Nuclei, Toss 0." In the column marked "Prediction for Next Toss" write the number of radioactive nuclei you think you will have with your next toss. (Radioactive nuclei will be those candies with the marked side down, while stable nuclei will have an S side up .) 2. Place your "nuclei" in a paper cup, cover and gently shake the cup. Pour the "nuclei" onto your paper towel. Separate the "nuclei" into two piles, one with the marked side up and the other with the marked side down. Count the number of "nuclei" in each pile. On your data table, record the number of "radioactive nuclei" candies with the marked side down. Predict how many radioactive "nuclei" you will have after the next toss. 3. Return only the radioactive "nuclei" to your paper cup. Keep the decayed nuclei in a separate pile. Only after you have completed the activity, copied your data to the overhead and you get my permission can you consume your nuclei. 4. Continue this process until there are no radioactive "nuclei" left. Add more rows to your data table, if needed. Put your data on the table on the document camera and copy other groups.
Toss # of Radioactive Prediction of next toss
10
11
12
13
14
15
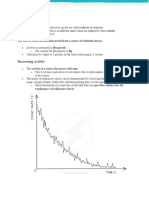
Class data: Number of Radioactive Nuclei Toss 0 1 2 3 4 5 Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Class Totals Graph:
10
11
12
13
14
15
Using the pooled data, prepare a graph by plotting the number of radioactive "nuclei" and the number of tosses, which we will call half-lives. Be sure your axes are labeled, have units and that you have an appropriate title. Be sure to use an appropriate scale for your graph so that you use most of the graph paper. Connect your points with a smooth curve.
Analysis: Please answer all in complete sentences! 1. How good is our assumption that half of our radioactive "nuclei" decay in each half-life? Explain.
2. If you started with a sample of 600 radioactive nuclei, how many should remain undecayed after three half-lives?
3. If 175 undecayed nuclei remained from a sample of 2800 nuclei, how many half-lives have passed? 4. Why did we pool the class data?
5. Is there any way to predict when a specific piece of candy will land marked side up or "decayed?" If you could follow the fate of an individual atom in a sample of radioactive material, could you predict when it would decay? Explain.
6. Strontium-90 has a half-life of 28.8 years. If you start with a 10-gram sample of strontium-90, how much will be left after 115.2 years? Show your work.
*Bonus Challenge (show your work): Selenium-83 has a half-life of 25 minutes. How many minutes would it take for a 10mg sample to decay and only have 1.25mg of it remain?
You might also like
- Gizmo Half-LifeDocument5 pagesGizmo Half-Lifeskyler29% (7)
- Electron Cloud LabDocument4 pagesElectron Cloud Labspecops4733% (6)
- Student Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Document5 pagesStudent Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Kristina33% (3)
- Period MonologuesDocument9 pagesPeriod Monologueslaurelbeth100% (1)
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDocument5 pagesStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationJahel HerculesNo ratings yet
- Report Sheet Half Life 2Document6 pagesReport Sheet Half Life 2Ayhan AbdulAzizNo ratings yet
- Radiometric Dating Lab: ProblemDocument5 pagesRadiometric Dating Lab: ProblemApril Mae ArcayaNo ratings yet
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDocument5 pagesStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationSai83% (6)
- Earth Science Midterm Review - 14-15Document2 pagesEarth Science Midterm Review - 14-15api-251355123No ratings yet
- Mapping The Ring of FireDocument4 pagesMapping The Ring of Fireapi-251355123No ratings yet
- Relative Humidity and Dew Point LabDocument6 pagesRelative Humidity and Dew Point Labapi-251355123No ratings yet
- Intermediate Steps To Understanding PDFDocument36 pagesIntermediate Steps To Understanding PDFVal Ribeiro79% (34)
- 4 SkittliumDocument4 pages4 SkittliumThe Life Of LGNo ratings yet
- 7.5 Halflife SEDocument5 pages7.5 Halflife SEWyatt KesterNo ratings yet
- 2014-10-20 - Lab - Radiometric Dating and Half LivesDocument4 pages2014-10-20 - Lab - Radiometric Dating and Half LivesMA. HAZEL TEOLOGONo ratings yet
- M2 Unit 3 G8Document8 pagesM2 Unit 3 G8Julia Geonzon LabajoNo ratings yet
- Half lifeSEDocument4 pagesHalf lifeSERusty WynderNo ratings yet
- 08.01 Half-Life and Radioactive DecayDocument3 pages08.01 Half-Life and Radioactive DecayAnonymous 8VJhV1eI2yNo ratings yet
- Teacher Guide: Half-Life: Learning ObjectivesDocument4 pagesTeacher Guide: Half-Life: Learning ObjectivesPeyton100% (1)
- Half LifeDocument2 pagesHalf LifeYasmen MendezNo ratings yet
- edTPA Instructional MaterialsDocument8 pagesedTPA Instructional MaterialsDanielle WilsonNo ratings yet
- Half Life Simulation With PenniesDocument3 pagesHalf Life Simulation With PenniesAbigail ShipeNo ratings yet
- Half-Life Student Lab Sheet-REVDocument6 pagesHalf-Life Student Lab Sheet-REVElPerritoSapeeNo ratings yet
- The 'Radioactive Dice' Experiment - Why Is The 'Half-Life' Slightly Wrong?Document6 pagesThe 'Radioactive Dice' Experiment - Why Is The 'Half-Life' Slightly Wrong?Pablo AlbinoNo ratings yet
- Atoms: Half Life Questions and AnswersDocument6 pagesAtoms: Half Life Questions and AnswersBubuNo ratings yet
- Half-Life 5centium InvDocument5 pagesHalf-Life 5centium InvJohn OsborneNo ratings yet
- Half-Life MM LabDocument5 pagesHalf-Life MM Labapi-1107897020% (1)
- 4.5.1 Half-LifeDocument4 pages4.5.1 Half-LifeMalik Rashid Ali LangrialNo ratings yet
- Nuclear Chemistry: ObjectivesDocument4 pagesNuclear Chemistry: ObjectivesKent Lewis GornezNo ratings yet
- Half LifeDocument11 pagesHalf LifeMarshell JonesNo ratings yet
- Models of The AtomDocument39 pagesModels of The AtomJames Excommunicado WickNo ratings yet
- 21 Radioactivity and Nuclear PhysicsDocument8 pages21 Radioactivity and Nuclear PhysicsAman BhuttaNo ratings yet
- Maths Exploration ProposalDocument2 pagesMaths Exploration Proposalazam96100% (1)
- Half-lifeSEDocument6 pagesHalf-lifeSEJBE legends100% (1)
- Physics Labs and PDsDocument33 pagesPhysics Labs and PDsBrihanna MontaqueNo ratings yet
- Zaundre Pusey - Half-lifeSEDocument8 pagesZaundre Pusey - Half-lifeSEJBE legendsNo ratings yet
- Earth Science Week 4 - Q2Document4 pagesEarth Science Week 4 - Q2Giane MadrigalNo ratings yet
- Radioactivity Physics Lab AnswersDocument3 pagesRadioactivity Physics Lab AnswersAaliyah Senior100% (1)
- PEKA RadioactiveDocument2 pagesPEKA Radioactivebrenwong@ymail.comNo ratings yet
- Atom A Closer LookDocument34 pagesAtom A Closer LookJohn Nash100% (1)
- Over-Populated or Under-Developed? The Real Story of Population GrowthDocument7 pagesOver-Populated or Under-Developed? The Real Story of Population GrowthMark Anthony BoloNo ratings yet
- Oxo AQA16 P7uu T801 CsaannDocument10 pagesOxo AQA16 P7uu T801 CsaannemailusageNo ratings yet
- Modeling Radioactive Decay With A DiceDocument4 pagesModeling Radioactive Decay With A DicePablo AlbinoNo ratings yet
- Aqa Gcse Science Command WordsDocument15 pagesAqa Gcse Science Command WordsChatur BatorNo ratings yet
- Absolute Dating Reading AssignmentDocument5 pagesAbsolute Dating Reading Assignmentapi-251355123No ratings yet
- Abs EnglishDocument17 pagesAbs EnglishshahabNo ratings yet
- Half Life and Radioactive DatingDocument13 pagesHalf Life and Radioactive DatingFaique HassanNo ratings yet
- (Chemistry) Lab ReviewerDocument3 pages(Chemistry) Lab ReviewerIris Kaye AbelleraNo ratings yet
- Simulating Half Life MakeupDocument2 pagesSimulating Half Life MakeupKris GribecNo ratings yet
- Chem Lab 2Document2 pagesChem Lab 2Samuel ArzadonNo ratings yet
- Number of Original Nuclei) To Drop To Half of Its Initial ValueDocument8 pagesNumber of Original Nuclei) To Drop To Half of Its Initial ValuesalmaNo ratings yet
- Beamline 2024Document7 pagesBeamline 2024Ermi TafiliNo ratings yet
- A Self-Guided Computer ActivityDocument23 pagesA Self-Guided Computer ActivityKang AmaNo ratings yet
- Candy AtomsDocument3 pagesCandy AtomsSrikarNo ratings yet
- Ejercios n4 TectonofisicaDocument4 pagesEjercios n4 TectonofisicaIsmael Mamani ChNo ratings yet
- Radiation and Half-LifeDocument6 pagesRadiation and Half-LifeAbdul NoorNo ratings yet
- Half-Life: Words To KnowDocument10 pagesHalf-Life: Words To KnowEthan RajakumarNo ratings yet
- EarthSci PetaaaDocument4 pagesEarthSci PetaaaJay GarciaNo ratings yet
- What Is The Centralized Region Inside The Atom Where The Protons and Neutrons Are Located and Holds Most of The Atom's Mass?Document35 pagesWhat Is The Centralized Region Inside The Atom Where The Protons and Neutrons Are Located and Holds Most of The Atom's Mass?JAMES RYAN EDMANo ratings yet
- The MAPDocument4 pagesThe MAPNidhi1106No ratings yet
- Let's Get Tiny!: Jumping into the Science of the Smallest Part of Matter with Quantum PhysicsFrom EverandLet's Get Tiny!: Jumping into the Science of the Smallest Part of Matter with Quantum PhysicsRating: 5 out of 5 stars5/5 (1)
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksFrom EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNo ratings yet
- Instead of the ITER project and the TOKAMAK principle: – a new type of fusion machineFrom EverandInstead of the ITER project and the TOKAMAK principle: – a new type of fusion machineRating: 5 out of 5 stars5/5 (1)
- Physics for Kids | Atoms, Electricity and States of Matter Quiz Book for Kids | Children's Questions & Answer Game BooksFrom EverandPhysics for Kids | Atoms, Electricity and States of Matter Quiz Book for Kids | Children's Questions & Answer Game BooksNo ratings yet
- Earth Science Midterm Diagram ReviewDocument4 pagesEarth Science Midterm Diagram Reviewapi-251355123No ratings yet
- 27 Solar Activity HomeworkDocument2 pages27 Solar Activity Homeworkapi-251355123No ratings yet
- Absolute Dating Reading AssignmentDocument5 pagesAbsolute Dating Reading Assignmentapi-251355123No ratings yet
- Volcano ReadingsDocument16 pagesVolcano Readingsapi-251355123No ratings yet
- Relative Dating Reading AssignmentDocument5 pagesRelative Dating Reading Assignmentapi-251355123No ratings yet
- 11 Tsunami Notes - TeacherDocument3 pages11 Tsunami Notes - Teacherapi-251355123No ratings yet
- Earthquake ReadingsDocument16 pagesEarthquake Readingsapi-251355123No ratings yet
- Fault Block Modeling LabDocument12 pagesFault Block Modeling Labapi-251355123100% (1)
- Graphing Sunspots LabDocument3 pagesGraphing Sunspots Labapi-2513551230% (1)
- 24 The Sun Notes - TeacherDocument2 pages24 The Sun Notes - Teacherapi-251355123No ratings yet
- 26 Structure of The Sun HomeworkDocument2 pages26 Structure of The Sun Homeworkapi-251355123No ratings yet
- Cloud Reference SheetDocument3 pagesCloud Reference Sheetapi-251355123No ratings yet
- Moon WebquestDocument5 pagesMoon Webquestapi-2513551230% (2)
- 21 Models of The Solar System HomeworkDocument2 pages21 Models of The Solar System Homeworkapi-251355123No ratings yet
- Weather Front and Air Mass WebquestDocument3 pagesWeather Front and Air Mass Webquestapi-251355123No ratings yet
- Pluto As A Planet Argumentative WritingDocument5 pagesPluto As A Planet Argumentative Writingapi-251355123No ratings yet
- 22 The Inner Planets HomeworkDocument2 pages22 The Inner Planets Homeworkapi-251355123No ratings yet
- Ellipse LabDocument4 pagesEllipse Labapi-251355123No ratings yet
- 25 Movements of The Moon HomeworkDocument2 pages25 Movements of The Moon Homeworkapi-251355123No ratings yet
- 20 Formation of The Solar System HomeworkDocument2 pages20 Formation of The Solar System Homeworkapi-251355123No ratings yet
- 18 Fronts HomeworkDocument2 pages18 Fronts Homeworkapi-251355123No ratings yet
- 17 Air MassesDocument2 pages17 Air Massesapi-251355123No ratings yet
- Weather Front LabDocument2 pagesWeather Front Labapi-251355123No ratings yet
- 19 Movements of Earth HomeworkDocument2 pages19 Movements of Earth Homeworkapi-251355123No ratings yet
- 18 Air Masses and Fronts Notes - TeacherDocument4 pages18 Air Masses and Fronts Notes - Teacherapi-251355123No ratings yet
- IWA City Stories SingaporeDocument2 pagesIWA City Stories SingaporeThang LongNo ratings yet
- Relatorio Mano JulioDocument7 pagesRelatorio Mano JulioProGeo Projetos AmbientaisNo ratings yet
- No Overlord Rulebook For Descent: Journeys in The Dark (2nd Edition)Document21 pagesNo Overlord Rulebook For Descent: Journeys in The Dark (2nd Edition)Jeremy ForoiNo ratings yet
- VDRLDocument4 pagesVDRLfarazhussainkhanNo ratings yet
- Teaching Language-Learning StrategiesDocument6 pagesTeaching Language-Learning StrategiesviviNo ratings yet
- GenMath Module 5 Week 5Document15 pagesGenMath Module 5 Week 5Immanuel CañecaNo ratings yet
- Unit 1 - Task 3 - Reader Guru Adventure! - Evaluation Quiz - Revisión Del IntentoDocument7 pagesUnit 1 - Task 3 - Reader Guru Adventure! - Evaluation Quiz - Revisión Del IntentoNelson AbrilNo ratings yet
- APA-AP - Activity 3-Emerging Ethical and Policy Issues of STSDocument36 pagesAPA-AP - Activity 3-Emerging Ethical and Policy Issues of STSJOHN MARTIN APA-APNo ratings yet
- Federal University OtuokeDocument5 pagesFederal University OtuokeeteleruthNo ratings yet
- Statement of Purpose Name: Krishna Teja SwarnaDocument2 pagesStatement of Purpose Name: Krishna Teja SwarnaMohammadRahemanNo ratings yet
- The Ezekiel Cycle of 500 YearsDocument1 pageThe Ezekiel Cycle of 500 YearsOtis Arms100% (1)
- Software Engineering. Specification, Implementation, VerificationDocument186 pagesSoftware Engineering. Specification, Implementation, Verificationazariel.rodrigo100% (1)
- Dialogic DSI Signaling Servers: SIU Mode User ManualDocument304 pagesDialogic DSI Signaling Servers: SIU Mode User ManualAdiansyah Rama67% (3)
- ME-458 Turbomachinery: Muhammad Shaban Lecturer Department of Mechanical EngineeringDocument113 pagesME-458 Turbomachinery: Muhammad Shaban Lecturer Department of Mechanical EngineeringAneeq Raheem50% (2)
- DSF Course Curriculum 1305231045Document8 pagesDSF Course Curriculum 1305231045Gaurav BhadaneNo ratings yet
- SPE 163723 Pressure Transient Analysis of Data From Permanent Downhole GaugesDocument24 pagesSPE 163723 Pressure Transient Analysis of Data From Permanent Downhole GaugesLulut Fitra FalaNo ratings yet
- Writing Home, Painting Home: 17th Century Dutch Genre Painting and The "Sailing Letters"Document17 pagesWriting Home, Painting Home: 17th Century Dutch Genre Painting and The "Sailing Letters"María MazzantiNo ratings yet
- Neuro TR Brochure - EN CompressedDocument8 pagesNeuro TR Brochure - EN CompressedJanam KuNo ratings yet
- Occupational Safety and Health Aspects of Voice and Speech ProfessionsDocument34 pagesOccupational Safety and Health Aspects of Voice and Speech ProfessionskaaanyuNo ratings yet
- MalappuramDocument20 pagesMalappuramFayizNo ratings yet
- Optimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsDocument7 pagesOptimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsNelson Andres Entralgo MaldonadoNo ratings yet
- Synchronous Alternators: Three-Phase BrushlessDocument5 pagesSynchronous Alternators: Three-Phase BrushlessĐại DươngNo ratings yet
- Chroma Bidirectional DC Power Supply Model 62000D SeriesDocument12 pagesChroma Bidirectional DC Power Supply Model 62000D SeriesaboofazilNo ratings yet
- Chinese Vocabulary BodyDocument3 pagesChinese Vocabulary BodyWei LeeNo ratings yet
- Safety Data Sheet Carbon Cathode SolutionDocument11 pagesSafety Data Sheet Carbon Cathode SolutionJeff BanasekNo ratings yet
- Getting The Most From Lube Oil AnalysisDocument16 pagesGetting The Most From Lube Oil AnalysisGuru Raja Ragavendran Nagarajan100% (2)
- Poster FinalDocument1 pagePoster FinalKrish vasisthaNo ratings yet
- Miller PreviewDocument252 pagesMiller PreviewcqpresscustomNo ratings yet