Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
91 viewsArias Stella Reaction 40
Arias Stella Reaction 40
Uploaded by
Febry FirmansyahThe document discusses the Arias-Stella Reaction (ASR), an atypical endometrial change associated with chorionic tissue effect. It summarizes new data on the histologic and immunohistochemical characteristics of ASR since its initial description over 40 years ago. The author clarifies common misconceptions about ASR and discusses its five histologic variants, pathogenesis, and distinguishing features from endometrial cancer. The response of the initial describing pathologist and subsequent confirmation of ASR in literature are also reviewed.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Umbilical Cord Accidents and Legal Implications Edit 14Document6 pagesUmbilical Cord Accidents and Legal Implications Edit 14DanNo ratings yet
- 10 1016@j Ajog 2019 07 010 PDFDocument13 pages10 1016@j Ajog 2019 07 010 PDFDaniel GamarraNo ratings yet
- Ectopic Pregnancy With Special Reference To Tubal Pregnancy and IDocument52 pagesEctopic Pregnancy With Special Reference To Tubal Pregnancy and IAbishaNo ratings yet
- Life at The Cell Below Cell LevelDocument3 pagesLife at The Cell Below Cell Levelquick0No ratings yet
- Neonatal White Matter Damage and the Fetal InflammDocument6 pagesNeonatal White Matter Damage and the Fetal InflammMerlin MaelissaNo ratings yet
- Diagnosing Congenital Malformations in The FetusDocument34 pagesDiagnosing Congenital Malformations in The FetusMonica SurduNo ratings yet
- Nódulos Tiroideos FolicularesDocument5 pagesNódulos Tiroideos FolicularesAide GuzmanNo ratings yet
- The New Cell Physiology - Gilbert LingDocument83 pagesThe New Cell Physiology - Gilbert Lingroan2No ratings yet
- ArticleDocument6 pagesArticleحمزہ محبNo ratings yet
- Arias StellaDocument5 pagesArias StellawitaNo ratings yet
- Eye 198865Document15 pagesEye 198865Apps OmNo ratings yet
- Tinjauan Pustaka: DefinisiDocument19 pagesTinjauan Pustaka: DefinisiKhandar YosuaNo ratings yet
- Hypertrophic Pyloric Stenosis in The Adult: Johannesburg AetiologyDocument3 pagesHypertrophic Pyloric Stenosis in The Adult: Johannesburg AetiologyzapomannNo ratings yet
- Soft Tissue SarcomasDocument10 pagesSoft Tissue SarcomasTejal ParulkarNo ratings yet
- NIH Public Access: Author ManuscriptDocument20 pagesNIH Public Access: Author ManuscriptJes LopNo ratings yet
- The Preterm Parturition Syndrome: Review ArticleDocument26 pagesThe Preterm Parturition Syndrome: Review ArticleRubiahSheBiachNo ratings yet
- Em Pathology em 50 Years On - 2017 - PatholDocument2 pagesEm Pathology em 50 Years On - 2017 - PatholHamed AhmedNo ratings yet
- University Journal of Surgery and Surgical Specialities: Dhivya Lakshmi S JDocument4 pagesUniversity Journal of Surgery and Surgical Specialities: Dhivya Lakshmi S JYonathanWaisendiNo ratings yet
- Annsurg00522 0075Document11 pagesAnnsurg00522 0075SIUSANTO HadiNo ratings yet
- Demystifying Endometrial Hyperplasia: Mini-Symposium: Endometrial PathologyDocument8 pagesDemystifying Endometrial Hyperplasia: Mini-Symposium: Endometrial PathologyNi Wayan Ana PsNo ratings yet
- Infant Development The Embryology of Early Human Behavior 1952Document1 pageInfant Development The Embryology of Early Human Behavior 1952blueberrkapNo ratings yet
- Acino in BreastDocument2 pagesAcino in BreastDima PathNo ratings yet
- Penile Anomalies in AdolescenceDocument10 pagesPenile Anomalies in AdolescenceIoannis ValioulisNo ratings yet
- Sample (1) Penis PDFDocument28 pagesSample (1) Penis PDFcristian ionut finaruNo ratings yet
- Abnormal Uterine Bleeding: Understanding, Diagnosis, and TreatmentFrom EverandAbnormal Uterine Bleeding: Understanding, Diagnosis, and TreatmentNo ratings yet
- 1 s2.0 S0015028211027579 MainDocument6 pages1 s2.0 S0015028211027579 Mainvantrung_liNo ratings yet
- Debate: What Is Polycystic Ovary Syndrome?Document9 pagesDebate: What Is Polycystic Ovary Syndrome?Laiq AhmadNo ratings yet
- EtiologyDocument2 pagesEtiologyJan JeweyNo ratings yet
- Biomarkers in Abnormal Uterine Bleeding: Precision Medicine in Assisted Reproductive Technologies Special IssueDocument12 pagesBiomarkers in Abnormal Uterine Bleeding: Precision Medicine in Assisted Reproductive Technologies Special IssueWahyuning PutriNo ratings yet
- Vestibular DisorderDocument417 pagesVestibular DisorderDEEKSHA ABROLNo ratings yet
- Management Fam 1998Document5 pagesManagement Fam 1998Envhy WinaNo ratings yet
- Research Paper About CellsDocument7 pagesResearch Paper About Cellsfvhvmqqj100% (1)
- Lobulated Spleen With A Fissure On Diaphragmatic Surface: RD RDDocument7 pagesLobulated Spleen With A Fissure On Diaphragmatic Surface: RD RDShantu ShirurmathNo ratings yet
- Medical NotesDocument421 pagesMedical NotesDanielle100% (7)
- Etiology of OtosclerosisDocument65 pagesEtiology of OtosclerosisANKIT PAREKHNo ratings yet
- Ultrasound Assessment of The Polycystic Ovary: International Consensus De®nitionsDocument10 pagesUltrasound Assessment of The Polycystic Ovary: International Consensus De®nitionsArkhan HanafiNo ratings yet
- The Perinatal Paradigm - Klaus y KennellDocument13 pagesThe Perinatal Paradigm - Klaus y KennellAna CorderoNo ratings yet
- 1098 2353 (200103) 14:2 158::aid Ca1025 3.0.co 2 QDocument6 pages1098 2353 (200103) 14:2 158::aid Ca1025 3.0.co 2 QAlejandro González MedinaNo ratings yet
- Pathology and Classification of Ovarian TumorsDocument12 pagesPathology and Classification of Ovarian Tumorsmohamaed abbasNo ratings yet
- Gastroschisis Omphalocele PDFDocument5 pagesGastroschisis Omphalocele PDFFariz Eka SetiawanNo ratings yet
- Disproporsi Kepala PanggulDocument14 pagesDisproporsi Kepala PanggulIntan PermataNo ratings yet
- Cervical DysplasiaDocument17 pagesCervical DysplasialilithsnakesNo ratings yet
- Discussion: Meigs' Syndrome and Pseudo-Meigs' SyndromeDocument3 pagesDiscussion: Meigs' Syndrome and Pseudo-Meigs' SyndromeFlapianne SimenceriauNo ratings yet
- Medical Cell Biology: Xiaoying LiuDocument44 pagesMedical Cell Biology: Xiaoying LiuSayan KonarNo ratings yet
- Obstetrics and Gynecology ClinicsDocument247 pagesObstetrics and Gynecology ClinicsAlexandr TrotskyNo ratings yet
- Glandular Lesions of the Uterine Cervix: Cytopathology with Histologic CorrelatesFrom EverandGlandular Lesions of the Uterine Cervix: Cytopathology with Histologic CorrelatesNo ratings yet
- Role of Laparoscopic Surgery in Endometriosis Associated Infertility - Literature ReviewDocument7 pagesRole of Laparoscopic Surgery in Endometriosis Associated Infertility - Literature ReviewFaza KahfiNo ratings yet
- Republic of The PhilippinesDocument5 pagesRepublic of The Philippineshatdog adadaNo ratings yet
- Placenta As Witness 07Document15 pagesPlacenta As Witness 07Vetési OrsolyaNo ratings yet
- Evolution of The Human Pelvis and Obstructed Labor - 2019Document14 pagesEvolution of The Human Pelvis and Obstructed Labor - 2019residentesmihgz112023.2024No ratings yet
- Rare Congenital Genitourinary AnomaliesDocument27 pagesRare Congenital Genitourinary Anomaliesد. محمد عبد الباقي فهميNo ratings yet
- Interdisciplinary Neurosurgery: Jimmy Ntimbani, Adrian Kelly, Patrick LekgwaraDocument4 pagesInterdisciplinary Neurosurgery: Jimmy Ntimbani, Adrian Kelly, Patrick LekgwaraAndres Eduardo GranadosNo ratings yet
- Kista OvariumDocument5 pagesKista OvariumAde Gustina SiahaanNo ratings yet
- Lecture Guide: Introduction To EmbryologyDocument23 pagesLecture Guide: Introduction To EmbryologyAldrin Hardy PabloNo ratings yet
- 1 s2.0 S0140673610603558 MainDocument11 pages1 s2.0 S0140673610603558 MainDr. Eser AĞARNo ratings yet
- Tumors in InvertebratesDocument19 pagesTumors in InvertebrateshamzaNo ratings yet
- The Botanical Review 1945 v.11. 87-107Document21 pagesThe Botanical Review 1945 v.11. 87-107albrewimi1No ratings yet
- Fuller Albright-Primul Articol de HPPDocument2 pagesFuller Albright-Primul Articol de HPPadelinaNo ratings yet
- Adenomatoid Tumors of The Uterus: An Analysis of 60 CasesDocument7 pagesAdenomatoid Tumors of The Uterus: An Analysis of 60 CasesGabriela OliveiraNo ratings yet
- ZalatnaiDocument9 pagesZalatnaiPatricia BezneaNo ratings yet
- Wind Runner 2Document1 pageWind Runner 2Febry FirmansyahNo ratings yet
- Hta 06 11 PDFDocument9 pagesHta 06 11 PDFFebry FirmansyahNo ratings yet
- Windrunner 4Document1 pageWindrunner 4Febry FirmansyahNo ratings yet
- For 2 ExDocument1 pageFor 2 ExFebry FirmansyahNo ratings yet
- Effects of Physical Exercise On Quality of Life, Exercise Capacity and Pulmonary Function in Children With AsthmaDocument6 pagesEffects of Physical Exercise On Quality of Life, Exercise Capacity and Pulmonary Function in Children With AsthmaFebry FirmansyahNo ratings yet
- Stroke Rehabilitation - An Overview of The Journey: InsightDocument2 pagesStroke Rehabilitation - An Overview of The Journey: InsightFebry FirmansyahNo ratings yet
- Optimal Stimulation Frequency of Transcutaneous Electrical Nerve Stimulation On People With Knee OsteoarthritisDocument6 pagesOptimal Stimulation Frequency of Transcutaneous Electrical Nerve Stimulation On People With Knee OsteoarthritisFebry FirmansyahNo ratings yet
- BCR 3462Document13 pagesBCR 3462Febry FirmansyahNo ratings yet
- Laporan Kasus 3: Genito Urinary SystemDocument2 pagesLaporan Kasus 3: Genito Urinary SystemFebry FirmansyahNo ratings yet
- UntitledDocument2 pagesUntitledelleNo ratings yet
- EmpiricalBasis GottmanDocument10 pagesEmpiricalBasis GottmanMundoSinViolenciaNo ratings yet
- Basion Horizontal CobenDocument3 pagesBasion Horizontal CobenJegan KumarNo ratings yet
- Acute Gynaecological Emergencies-1Document14 pagesAcute Gynaecological Emergencies-1Anivasa Kabir100% (1)
- Getting The Most From Lube Oil AnalysisDocument16 pagesGetting The Most From Lube Oil AnalysisGuru Raja Ragavendran Nagarajan100% (2)
- Consumer Protection Act - SeminarDocument16 pagesConsumer Protection Act - SeminarAbdul KhadeerNo ratings yet
- Teachers' Interview PDFDocument38 pagesTeachers' Interview PDFlalitNo ratings yet
- Summary 2Document2 pagesSummary 2Admin OfficeNo ratings yet
- Capitalisation AssignmentDocument5 pagesCapitalisation AssignmentFayis FYSNo ratings yet
- SBT Sekolah Berprestasi Tinggi (HPS) High Performing SchoolsDocument14 pagesSBT Sekolah Berprestasi Tinggi (HPS) High Performing SchoolsAminNo ratings yet
- MC&OB Unit 4Document17 pagesMC&OB Unit 4Tanya MalviyaNo ratings yet
- Ai-Ai ResumeDocument3 pagesAi-Ai ResumeNeon True BeldiaNo ratings yet
- Offer For C Check On NT-495-MG Harbour Generator Engine Against Customer Job No. E20006Document1 pageOffer For C Check On NT-495-MG Harbour Generator Engine Against Customer Job No. E20006bkrNo ratings yet
- Inactive Volcanoes in The Philippine SDocument6 pagesInactive Volcanoes in The Philippine SChristian ParadoNo ratings yet
- Emmanuel Levinas - God, Death, and TimeDocument308 pagesEmmanuel Levinas - God, Death, and Timeissamagician100% (3)
- Academic Journal Guide 2021-MethodologyDocument22 pagesAcademic Journal Guide 2021-MethodologySyedNo ratings yet
- Rear Drive Halfshafts: 2016.0 RANGE ROVER (LG), 205-05Document46 pagesRear Drive Halfshafts: 2016.0 RANGE ROVER (LG), 205-05SergeyNo ratings yet
- Chapter 1 Peanut Growing and HarvestingDocument18 pagesChapter 1 Peanut Growing and HarvestingKapil BhattNo ratings yet
- pdfGenerateNewCustomer SAOAO1030380906Reciept SAOAO1030380906ResidentAccountOpenFormDocument11 pagespdfGenerateNewCustomer SAOAO1030380906Reciept SAOAO1030380906ResidentAccountOpenFormajsingh0702No ratings yet
- Act 1 Almeyda JTLDocument2 pagesAct 1 Almeyda JTLAltairNo ratings yet
- Informe Sobre El Manejo de CostasDocument88 pagesInforme Sobre El Manejo de CostasMetro Puerto RicoNo ratings yet
- Gershwin George Rhapsody in Blue For Sax Quartet 64734Document113 pagesGershwin George Rhapsody in Blue For Sax Quartet 64734Jessica HowardNo ratings yet
- Tle 7-1st Periodic TestDocument2 pagesTle 7-1st Periodic TestReymart TumanguilNo ratings yet
- Eastron Electronic Co., LTDDocument2 pagesEastron Electronic Co., LTDasd qweNo ratings yet
- Optimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsDocument7 pagesOptimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsNelson Andres Entralgo MaldonadoNo ratings yet
- Reflection Paper On "Legal Research, Legal Writing, and Legal Analysis: Putting Law School Into Practice" by Suzanne RoweDocument2 pagesReflection Paper On "Legal Research, Legal Writing, and Legal Analysis: Putting Law School Into Practice" by Suzanne RoweRobert Jay Regz Pastrana IINo ratings yet
- HSC 11 Scalars and Vectors Ch2Document5 pagesHSC 11 Scalars and Vectors Ch2Snehal PanchalNo ratings yet
- Exploring Music in ContextDocument3 pagesExploring Music in ContextpkutinNo ratings yet
- Telegram DocumeDocument21 pagesTelegram Documemilli birhanuNo ratings yet
- Whittington 22e Solutions Manual Ch14Document14 pagesWhittington 22e Solutions Manual Ch14潘妍伶No ratings yet
Arias Stella Reaction 40
Arias Stella Reaction 40
Uploaded by
Febry Firmansyah0 ratings0% found this document useful (0 votes)
91 views16 pagesThe document discusses the Arias-Stella Reaction (ASR), an atypical endometrial change associated with chorionic tissue effect. It summarizes new data on the histologic and immunohistochemical characteristics of ASR since its initial description over 40 years ago. The author clarifies common misconceptions about ASR and discusses its five histologic variants, pathogenesis, and distinguishing features from endometrial cancer. The response of the initial describing pathologist and subsequent confirmation of ASR in literature are also reviewed.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses the Arias-Stella Reaction (ASR), an atypical endometrial change associated with chorionic tissue effect. It summarizes new data on the histologic and immunohistochemical characteristics of ASR since its initial description over 40 years ago. The author clarifies common misconceptions about ASR and discusses its five histologic variants, pathogenesis, and distinguishing features from endometrial cancer. The response of the initial describing pathologist and subsequent confirmation of ASR in literature are also reviewed.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
91 views16 pagesArias Stella Reaction 40
Arias Stella Reaction 40
Uploaded by
Febry FirmansyahThe document discusses the Arias-Stella Reaction (ASR), an atypical endometrial change associated with chorionic tissue effect. It summarizes new data on the histologic and immunohistochemical characteristics of ASR since its initial description over 40 years ago. The author clarifies common misconceptions about ASR and discusses its five histologic variants, pathogenesis, and distinguishing features from endometrial cancer. The response of the initial describing pathologist and subsequent confirmation of ASR in literature are also reviewed.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 16
1
The Arias-Stella Reaction:
Facts and Fancies Four Decades After
Javier Arias-Stella
Instituto de Patologa y Biologa Molecular Arias Stella, Lima, Per
Summary: Since its first description more than four decades ago, the atypical endometrial
change associated with chorionic tissue effect has been widely confirmed in the literature.
However, errors and inaccuracies in text books and other publications often occur. This
review clarifies some of these misconceptions and presents a summary of new data on the
histologic and immunohistochemical characteristics of the change. A brief discussion on
the pathogenesis and biologic significance of the alteration is included. Key Words:
Arias-Stella Reaction Atypical endometriumEndometrial change.
Forty-six years ago, I bravely knocked on the door of Dr. Fred W. Stewarts office.
He was the Chairman of the Pathology Department of Memorial Hospital for
Cancer in New York City. I had been a fellow in Pathology for 2 months and finally
I was reaching my main goal at this famous hospital: to consult with the Pathologist
then considered one of the foremost in tumors diagnosis in the United States on two
cases that I had seen while a medical student back home in Per and that had been
diagnosed by my seniors as forms of early endometrial cancer. However, given the
uniqueness of the changes, and because one case was associated with an intramural
chorioadenoma destruens and the other with an ectopic pregnancy, I thought that
this was some form of an endometrial reaction resulting from the chorionic
hormonal stimulation.
2
I expected that Dr. Stewart would send me to the bibliographic references, which I
had searched in vain for more than 2 years. His answer, left me dumbfounded: I
dont know! Javier -you have something to study. The rest of the story is not for
this occasion, but I have recreated the background, development and immediate
corollary in the form of a story for medical students, which will soon published (1).
The initial publication appeared in the Archives of Pathology in 1954 (2) and it is
interesting today to recall the very first reaction in the literature. Dr. Emil Novak,
the author of the textbook Gynecological and Obstetric Pathology, which during
my youth was considered to be the Bible, was among other things the editor of the
Survey of Obstetrics and Gynecology. He made the following comments when my
article was published (1,3):
This is an interesting study, but one upon which it would be difficult to comment
unless one had made similar studies, which no one appears to have done
While I have examined many thousands of endometria including many containing
trophoblastic rest, after miscarriage hydatidiform mole, chorioadenoma destruens,
or choriocarcinoma, I cannot say that I have noted the particular cellular changes
which Arias-Stella has described.
To show how foolish an objection this last statement is, I may say also that I
examined many ovaries before 1921, and that not a few of them showed
endometrium, but I did not appreciate the importance and the frequency of
endometriosis until after Sampsons first publication in 1921, nor did anyone else.
Similar confessions could be made about other pathologic, or for that matter,
clinical entities, for which we are all now on the alert.
3
Dr. Novak was not a formal pathologist, as we understand it today. He practiced
clinical gynecology in Baltimore and because he had helped Dr. Cullen, who had
organized the gynecologic pathology laboratory at Johns Hopkins, he was now its
director -a common situation in the early years of surgical pathology. It is therefore
not surprising that pathologists of his time viewed him with reservations and that
his gynecology peers did not let him admit patients to Johns Hopkins (4).
Looking back, his sincere, humble, and wise comment, as well as the magnitude and
quality of his contributions to science, leave no doubt that he knew very well the
trade of pathology. He deserves the recognition that is owed to him by our
community of pathologists.
Since my first report, the original description has been widely confirmed and
mentioned in the literature (references in [5]), although errors and inaccuracies
occur in some textbooks and publications. Common misconceptions are to consider
the change, exclusively, a hypersecretory phenomenon, or a nonspecific regressive
alteration due to fetal death, trophoblastic disturbance, or decreased hormonal levels
(6,7). Some have, equivocally, stated that the occurrence of mitosis in carcinoma
and its absence in the Arias-Stella reaction is important for the differential
diagnosis (8). This article summarizes the most recent contributions to the
literature, both mine and those of other investigators, and attempts to clarify the
pathogenesis.
DEFINITION
The main characteristic of the reaction is cellular enlargement, mainly of the
nucleus, to double or many times normal size. Without the presence of nuclear
4
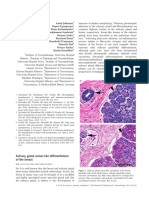
enlargement, the phenomenon cannot be diagnosed. Hypertrophied nuclei can show
an ovoid or round shape with granular or vesicular viable chromatin, an irregular
outline and a hyperchromatic appearance or a compact, pyknotic pattern (Fig. 1).
Mazur, et al. (9) have used the term optically clear nuclei to describe an aspect of
the alteration, which is characterized by centronuclear vacuolization, resulting from
the replacement of the chromatin by a net of fine filaments. Another morphological
variant is the occurrence of intranuclear cytoplasmic invaginations
pseudoinclusions. One or another of these alterations of the macronuclei has been
confused with herpetic endometritis (10) (Fig. 2).
Usually the alterations are focal; they involve a group of glands or only some part of
them. Occasionally, they can be quite extensive or florid. It is important to
emphasize that the presence of hypersecretory glands alone does not constitute the
phenomenon. The presence of hypersecretory foci in the gestational endometrium
was described by Opitz at the beginning of the last century (11).
HISTOLOGIC VARIANTS
Even though at the beginning we described two forms of the alteration (2), and later
on we recognized a third form (12), accumulated experience allows us to distinguish
the five histologic variants: 1) minimal atypia; 2) early secretory pattern; 3)
secretory or hypersecretory pattern; 4) regenerative, proliferative, or nonsecretory
pattern; 5) monstrous cell pattern. The variants are a function of comparison with
the phases of normal endometrium and degree of atypicality.
5
Minimal Atypia
Minimal atypia is the pattern usually seen at the beginning of gestation. The
nuclear enlargement is minimal and occurs in limited foci (Fig. 3). Because the
decidual reaction is absent or is not conspicuous in the early stages of gestation, the
diagnosis of minimal atypia may have a special practical meaning. This is what we
have found in some women who have been evaluated for infertility (Table 1).
Early Secretory Pattern
The early secretory pattern resembles normal early secretory endometrium at day
17-18 of the menstrual cycle. In these cases, nuclear enlargement is marked and the
endometrial cells show subnuclear or subnuclear and supranuclear vacuoles (Fig. 4).
The nuclei are centrally located. The cells are frequently arranged in a palisading
fashion, with the nuclei at the same level. The affected glands can show
intraluminal papillary projections.
Since the original description, my colleagues and I pointed out the occurrence of
mitosis in the phenomenon and, more recently, that they can be normal and
abnormal (Tables 2 and 3) (13). It is precisely in this model of presentation that
they are more frequent and abnormal (Fig. 5). It is understandable why this
histologic variant is the one most likely to be confused with adenocarcinoma. In my
experience this model has been common in cases of uterine abortion and ectopic
pregnancy.
Secretory or Hypersecretory Pattern
Secretory or hypersecretory pattern is the classically recognized form of reaction.
The glandular cells display intense and diffuse cytoplasmic vacuolization, which
6
predominates as a distinct morphologic feature. The enlarged and hyperchromatic
nuclei are usually pyknotic (Fig. 6). Focally, one can distinguish cells that are less
vacuolated and show a dense cytoplasm (Fig. 7), which is due to their different
histochemical composition.
The comparative epithelial immunohistochemical profile of the normal functional
endometrium with the atypical change has demonstrated that the epithelial
membrane antigen (EMA) and the CK7 give an intense and homogenous reaction in
both normal endometrium and the Arias-Stella Reaction (ASR) (Table 4). The
vacuolization that characterizes the hipersecretory pattern of the atypical change
highlights the increased membranous localization of staining with these markers,
creating a chicken-wire pattern (Fig. 8).
Ultrastructural studies done by de Brux (14), Thasher and Richart (15), and Salazar
and Burgess (16) have shown few organelles and sparse particles that resemble
granules of ribonucleic acid in the clear cells, and a conspicuous Golgi apparatus,
vesicles of endoplasmic reticulum, numerous mitochondrial crests, and Palade
grains in the dark cells. One detail that has been found repeatedly is the presence of
parallel rows of rough endoplasmic reticulum (Fig. 9), which is a characteristic
described in various tumors of Mullerian origin. This variant is found mainly in
uterine abortions.
Regenerative, Proliferative or Nonsecretory Pattern
In the regenerative, proliferative or nonsecretory pattern there is usually no
evidence of secretory activity, or it is minimal in the glandular cells. The enlarged
nuclei display a vesicular configuration or a granular chromatin with a well-
7
delineated nuclear membrane. The glands are similar to those observed in the
proliferative or regenerative endometrium (Fig. 10). I have found this variant in
cases of hydatidiform mole, choriocarcinoma, ectopic pregnancy, and uterine
abortion.
Monstrous Cell Pattern
In rare cases, the monstrous cell pattern, which is usually focal, affects the whole
endometrium. The histologic section is dominated by the presence of giant and
bizarre nuclei, which involve all the cells in the glands. (Fig. 11). The atypical
nuclei show a dense, homogeneous chromatin and, frequently, pseudoinclusions.
This model of atypia originates problems of histologic interpretation. In our
experience, my colleagues and I have found it in 0.5% of florid alterations.
LOCATION OF THE CHANGE
Even though the alteration was originally described in the functional endometrium,
which is more sensitive to hormonal effects, today we have proved that when the
stimulation is intense, the change can also occur in less sensitive basal regions.
From the beginning my colleagues and I pointed out that not only the glandular but
also the covering epithelium could be affected and that the alteration occurred in
both the endocervix and the tubal epithelium (17,18).
In recent years our observations and those of many authors have demonstrated that
under the stimulation of chorionic tissue gestational condition or trophoblastic
proliferation the change can be observed in many territories and lesions, including
endometriosis (peritoneum, subcutaneous, umbilical) (19); endocervical polyps
8
(20,21); vaginal adenosis (22,23); germinal inclusion cysts (ovary) (24); para-
ovarian and para-tubaric cysts (25); and mucinous cystoadenoma (26).
It is interesting that Albukerk and Berlin (27) have described nuclear changes that
resemble the Arias-Stella reaction in the luteal cyst of the gestation and that
Clement and Scully (28) described similar atypias in luteinized follicular cysts of
gestation and puerperium.
CHRONOLOGY BETWEEN THE PRESENCE OF
CHORIONIC TISSUE AND
ARIAS-STELLA REACTION
Two questions that must be answered is: How early in the gestation and how late
after delivery does the endometrial atypia occur? Fortunately, studies by Holmes
and Lyle (29) and Dahlerup and Jorgenson (30) and the review by Oertel (31) of
material that Hertig examined to elucidate the early stages of human embryogenesis
have answered these questions.
PRACTICAL VALUE OF THE
ARIAS-STELLA REACTION
The accumulated information shows that in certain clinical situations, finding the
Arias-Stella reaction can be specially significant, as an isolated histological sign, or
as part of a group of changes, in making a diagnosis. According to our experience
and that of other investigators, the change has been of histologic diagnostic
significance for recognizing early uterine pregnancy (12), ectopic pregnancy
(32,33), trophoblastic tumors (indirect evidence), and postabortion or postpartum
metropathies. Histologic differential diagnosis can be problematic in the following
9
conditions: metastatic Adenocarcinoma versus foci of endometriosis in pregnancy
(5); endometrial Adenocarcinoma versus florid Arias-Stella reaction (8,34);
Adenocarcinoma versus Arias-Stella reaction infallopian tube (35); and atypical
mucinous cystoadenomas, germinal inclusion cysts, and paratubal cysts in
pregnancy and puerperium (26).
It is interesting to note that in recent years there has been special emphasis in
finding the change in the endocervix and recognizing atypical cells in Papanicolau
smears.
Schneider (36) has found the alteration in the endocervix in 17 of 191 uteri, which
were removed during gestation, proving that the proximal segment of the cervical
canal was the most frequent location. Cove (37) and Rhatigan (38) have described
cases of endocervical Arias-Stella reaction that caused confusion with premalignant
or malignant conditions. The same problem was encountered by Cariani and
Guderian (39) and McCormick and Menaci-Williams (40) in endocervical polyps
with Arias-Stella reaction. McCormick and Menaci-Williams reported the
occurrence of tripolar mitoses, confirming our finding of abnormal mitosis in the
reaction.
In my experience the endocervical Arias-Stella reaction, whether in normal mucosa
or polyps, frequently shows the monstrous cell pattern (Fig. 12), and gives rise to
the differential diagnosis with neoplastic processes. The gestational state is often
ignored and it is the debate over the nature of the cervical lesion that reveals the
pregnancy conditions.
10
Hilrich and Hipke (41) were the first to show that atypical endometrial cells could
appear in Papanicolau smears. Since then, many observers have verified the same
finding (42,43). Albukerk and Gnecco (44) pointed out that the abnormal cells in
the Papanicolau smear could be confused with neoplastic cytology and that they are
found more frequently in cases of ectopic pregnancy, in which the lack of a uterine-
placental barrier makes it easier for desquamation and passage of the cells to the
vaginal fundus.
In recent years, Pisharodi and Jovanoska (45), Mulvany et al. (46), Yates et al. (47),
and Michael and Esfahani (48), have reported observations showing that the
abnormal cytology due to Arias-Stella reaction, of the endometrium or the
endocervix, in pregnant women, originates problems of cytologic interpretation.
We have verified this occurrence in Figure 13 illustrates one such cases.
Recently, because of similar experiences, Benoit and Kiny (49) recommended
including Arias-Stella reaction cells among the atypical glandular cells to be
considered in Papanicolau smears from women in pregnancy or puerperium.
PATHOGENESIS
An understanding of the mechanism of enlargement of the nucleus and the unique
histologic reaction is essential. Three basic facts must first be mentioned:
1. Nuclear hypertrophy is due to an increase in the contents of DNA. The finding
of double Barr corpuscle by Dahlerup and Jorgensen (30), the karyometric
studies of Chiara (50) and the Fulgen microspectrocytophotometric
11
determinations of Wagner and Richart (51) early established that nuclei enlarge
because of polyploidism and that aneuploidism does not exist.
2. The aspect of the histological reaction is evidence of occurrence of proliferative
and secretory stimuli, acting simultaneously.
3. The change can be present in normal physiologic conditions, including term
pregnancy (52), postpartum (30), therapeutic abortion (51,53), and early normal
pregnancy (12).
The glandular changes in the endometrium are a consequence of the action of
estrogens and progesterone. The former stimulate cellular proliferation and the latter
stimulate secretory activity (54,55).
With this background, since my first observations I postulated that estrogen and
progesterone are, in some way, involved in the pathogenesis of the change. Early
on, using human chorionic hormones and estrogens, I was able to obtain,
experimentally, proliferative and secretory activity simultaneously in normal rats,
and, using estrogens and progesterones in castrated rats to induce the same changes.
In these experiments I also observed focal nuclear enlargement (56,57). In 1966,
experimental studies by Dr. Dallenbach (58) confirmed these findings.
Since then I have performed some experiments on humans. Administering a
combination of estrogens and progesterone to postmenopausal women who were
going to have hysterectomies, I was able to induce, after 1 month of treatment in
some of them, endometrial changes that were focal and suggestive of the Arias-
Stella reaction (5).
12
In another experiment, due to the action of high doses of estradiol and
hydroxyprogesterone, I was able to produce focal changes that were similar to the
endometrial atypia (5).
Conversely, since the observations of Dockerty et al. (59) and Azzopardi and Zayid
(60) it has been recognized that therapy with synthetic progesterone-estrogen can
induce endometrial changes of the Arias-Stella reaction type. Recently, Huettner
and Gersell (61) have reported the induction of what they consider Arias-Stella
reaction in eight postmenopausal or perimenopausal women who received treatment
with progestational hormones.
Since the classic studies by Good and Moyer on the Macaca mulata, it has been
shown that by adjusting the doses of estrogens and progesterone, it is possible to
obtain, experimentally, secretory or proliferative endometrial responses (62).
All of the above supports, my proposition that the pathogenesis of the phenomenon
depends on a balance of these two hormonal effects.
How do we continue exploring along these lines of thought?
Today, we know more about the hormonal mechanisms. In the case of female
hormones, we know that there are cellular receptors, that estrogens stimulate the
synthesis of DNA, RNA and proteins, by a chain reaction mechanism, and that
progesterone reduces the synthesis of DNA and RNA (63-65). We know the cell
cycle and we can identify, in tissues, some of the factors of intermediation (Fig. 14).
Therefore, there is room for further investigation. We have investigated, using
immunohistochemical methods, the presence of estrogen and progesterone receptors
and markers of cellular proliferation, Ki-67 and proliferating cell nuclear antigen
13
(PCNA), in 20 cases of florid Arias Stella reaction, compared with findings in the
normal phases of the endometrium and in endometrial hyperplasia.
In the absence of an accepted standardized method for evaluation
immunohistochemical reactions, we have adopted the method recently proposed by
Allred et al. (66) for scoring the immunostaining signal for estrogen receptors and
progesterone receptors in breast cancer. In each case the best stained fields were
chosen for the evaluation. In the Arias-Stella reaction we found that the estrogen
receptors and the progesterone receptors (Table 5) had a total score less than in the
normal functional phases and in hyperplasia.
Score for the cellular proliferation markers (Table 6) were also less than in the
proliferative phase, but nevertheless, higher than those of the normal secretory
phase. To summarize, the results of the immunohistochemical study shows that:
1. Estrogen and progesterone receptors (Fig. 15) are present in the foci of the
endometrial atypia.
2. The positive reaction for the proliferative factors, such as antigen Ki-67 and
PCNA (Fig. 16), is also present in these foci. It should be stressed that estrogen
receptors, progesterone receptors, Ki67, and PCNA have been found present in
enlarged typical Arias-Stella reaction cells (Fig. 17).
These results are, in essence, in agreement with the immunohistochemical study
recently reported by Doss et al. (67).
14
Steroid hormones can act by either of two ways: the nongenomic mechanism or the
classical, or genomic, form (68). The nongenomic effects are fast -occurring in
seconds or minutes- are highly specific, do not require nuclear receptors, and do not
induce RNA or protein synthesis.
The nature of the Arias-Stella reaction corresponds to that of a long duration
process -days, weeks or months- and as we have demonstrated above, it is
accompanied by the involvement of receptors and growth factors that lead to DNA
synthesis. Therefore, the evidence supports the view that the steroid action in the
Arias-Stella reaction follows, basically, the classic genomic path.
All of these findings suggest that the pathogenesis is related to the effects of
estrogen and progesterone acting simultaneously, but as highlighted in the
immunohistochemical study, with a higher estrogen weighting than found in the
normal secretory phase. This imbalance would be a determining factor, through
intermediary mechanisms, to induce the increased synthesis of DNA that leads to
the occurrence of polyploidism.
It is interesting to note that recently, the late Mexican Professor Mrquez-Monter et
al. (69) compared the macronuclei of the Arias-Stella reaction to those seen in
megaloblastic anemia, suggesting that in some cases, they result from a change in
the cellular cycle by mutation of the check point bound to the mitogenic factor,
which determines the continuation of DNA synthesis. It cannot be ruled out if in
these hormonal interactions there is a role for factors directly derived from
chorionic tissue.
15
CELL BIOLOGY
How DO WE locate this alteration from the perspective of cell biology? The
characteristics that we have highlighted reveal and adaptive, controllable, and
reversible cellular reaction induced by hormonal actions that comprise proliferative
and antagonistic stimuli. Because the cells that modify are mature cells that are not
replaced, it is not a form of metaplasia.
If the variability in the shape and size of the cells, the pleomorphism and
hyperchromasia, and the presence of atypical mitosis could suggest a dysplasia, the
DNA polyploid contents, absence of aneuploidy, and absence of progression would
not correspond with this process (Fig. 18, Table 7) (70).
At this level of knowledge, the accumulated information leads us to think of a
singular form of transdifferentiation: conversion of a differentiated cell into another
differentiated cell. In this case it would be called atypical transdifferentiation (Fig.
18).
This atypical transdifferentiation, which is characterized by polyploidism, absence
of aneuploidism, and absence of progression of lesions, and which is found in
tissues that are sensitive to antagonistic hormonal factors, would also occur in other
areas, including atypical cells in seminal vesicles (71,72); monstrous cellsin
epididymis and duct deferens (73,74); atypical cells in lutein cysts in pregnancy
(27); atypical cells in luteinized follicle cyst of pregnancy and puerperium (28); and
bizarre, benign cells in tyroid (dyshormogenetic goiter) and other endocrine organs
(75).
16
CONCLUSION
All of the above information allows us to conclude that the description of
endometrial atypia, associated with the effect of the chorionic tissue, has had until
now, practical significance in gynecologic and obstetric pathology. Owing to its
peculiar relation to the synthesis of DNA, it is possible that elucidating the
pathogenesis of the changes will have even greater importance in the scope of cell
biology. This is the challenge for future generations.
You might also like
- Umbilical Cord Accidents and Legal Implications Edit 14Document6 pagesUmbilical Cord Accidents and Legal Implications Edit 14DanNo ratings yet
- 10 1016@j Ajog 2019 07 010 PDFDocument13 pages10 1016@j Ajog 2019 07 010 PDFDaniel GamarraNo ratings yet
- Ectopic Pregnancy With Special Reference To Tubal Pregnancy and IDocument52 pagesEctopic Pregnancy With Special Reference To Tubal Pregnancy and IAbishaNo ratings yet
- Life at The Cell Below Cell LevelDocument3 pagesLife at The Cell Below Cell Levelquick0No ratings yet
- Neonatal White Matter Damage and the Fetal InflammDocument6 pagesNeonatal White Matter Damage and the Fetal InflammMerlin MaelissaNo ratings yet
- Diagnosing Congenital Malformations in The FetusDocument34 pagesDiagnosing Congenital Malformations in The FetusMonica SurduNo ratings yet
- Nódulos Tiroideos FolicularesDocument5 pagesNódulos Tiroideos FolicularesAide GuzmanNo ratings yet
- The New Cell Physiology - Gilbert LingDocument83 pagesThe New Cell Physiology - Gilbert Lingroan2No ratings yet
- ArticleDocument6 pagesArticleحمزہ محبNo ratings yet
- Arias StellaDocument5 pagesArias StellawitaNo ratings yet
- Eye 198865Document15 pagesEye 198865Apps OmNo ratings yet
- Tinjauan Pustaka: DefinisiDocument19 pagesTinjauan Pustaka: DefinisiKhandar YosuaNo ratings yet
- Hypertrophic Pyloric Stenosis in The Adult: Johannesburg AetiologyDocument3 pagesHypertrophic Pyloric Stenosis in The Adult: Johannesburg AetiologyzapomannNo ratings yet
- Soft Tissue SarcomasDocument10 pagesSoft Tissue SarcomasTejal ParulkarNo ratings yet
- NIH Public Access: Author ManuscriptDocument20 pagesNIH Public Access: Author ManuscriptJes LopNo ratings yet
- The Preterm Parturition Syndrome: Review ArticleDocument26 pagesThe Preterm Parturition Syndrome: Review ArticleRubiahSheBiachNo ratings yet
- Em Pathology em 50 Years On - 2017 - PatholDocument2 pagesEm Pathology em 50 Years On - 2017 - PatholHamed AhmedNo ratings yet
- University Journal of Surgery and Surgical Specialities: Dhivya Lakshmi S JDocument4 pagesUniversity Journal of Surgery and Surgical Specialities: Dhivya Lakshmi S JYonathanWaisendiNo ratings yet
- Annsurg00522 0075Document11 pagesAnnsurg00522 0075SIUSANTO HadiNo ratings yet
- Demystifying Endometrial Hyperplasia: Mini-Symposium: Endometrial PathologyDocument8 pagesDemystifying Endometrial Hyperplasia: Mini-Symposium: Endometrial PathologyNi Wayan Ana PsNo ratings yet
- Infant Development The Embryology of Early Human Behavior 1952Document1 pageInfant Development The Embryology of Early Human Behavior 1952blueberrkapNo ratings yet
- Acino in BreastDocument2 pagesAcino in BreastDima PathNo ratings yet
- Penile Anomalies in AdolescenceDocument10 pagesPenile Anomalies in AdolescenceIoannis ValioulisNo ratings yet
- Sample (1) Penis PDFDocument28 pagesSample (1) Penis PDFcristian ionut finaruNo ratings yet
- Abnormal Uterine Bleeding: Understanding, Diagnosis, and TreatmentFrom EverandAbnormal Uterine Bleeding: Understanding, Diagnosis, and TreatmentNo ratings yet
- 1 s2.0 S0015028211027579 MainDocument6 pages1 s2.0 S0015028211027579 Mainvantrung_liNo ratings yet
- Debate: What Is Polycystic Ovary Syndrome?Document9 pagesDebate: What Is Polycystic Ovary Syndrome?Laiq AhmadNo ratings yet
- EtiologyDocument2 pagesEtiologyJan JeweyNo ratings yet
- Biomarkers in Abnormal Uterine Bleeding: Precision Medicine in Assisted Reproductive Technologies Special IssueDocument12 pagesBiomarkers in Abnormal Uterine Bleeding: Precision Medicine in Assisted Reproductive Technologies Special IssueWahyuning PutriNo ratings yet
- Vestibular DisorderDocument417 pagesVestibular DisorderDEEKSHA ABROLNo ratings yet
- Management Fam 1998Document5 pagesManagement Fam 1998Envhy WinaNo ratings yet
- Research Paper About CellsDocument7 pagesResearch Paper About Cellsfvhvmqqj100% (1)
- Lobulated Spleen With A Fissure On Diaphragmatic Surface: RD RDDocument7 pagesLobulated Spleen With A Fissure On Diaphragmatic Surface: RD RDShantu ShirurmathNo ratings yet
- Medical NotesDocument421 pagesMedical NotesDanielle100% (7)
- Etiology of OtosclerosisDocument65 pagesEtiology of OtosclerosisANKIT PAREKHNo ratings yet
- Ultrasound Assessment of The Polycystic Ovary: International Consensus De®nitionsDocument10 pagesUltrasound Assessment of The Polycystic Ovary: International Consensus De®nitionsArkhan HanafiNo ratings yet
- The Perinatal Paradigm - Klaus y KennellDocument13 pagesThe Perinatal Paradigm - Klaus y KennellAna CorderoNo ratings yet
- 1098 2353 (200103) 14:2 158::aid Ca1025 3.0.co 2 QDocument6 pages1098 2353 (200103) 14:2 158::aid Ca1025 3.0.co 2 QAlejandro González MedinaNo ratings yet
- Pathology and Classification of Ovarian TumorsDocument12 pagesPathology and Classification of Ovarian Tumorsmohamaed abbasNo ratings yet
- Gastroschisis Omphalocele PDFDocument5 pagesGastroschisis Omphalocele PDFFariz Eka SetiawanNo ratings yet
- Disproporsi Kepala PanggulDocument14 pagesDisproporsi Kepala PanggulIntan PermataNo ratings yet
- Cervical DysplasiaDocument17 pagesCervical DysplasialilithsnakesNo ratings yet
- Discussion: Meigs' Syndrome and Pseudo-Meigs' SyndromeDocument3 pagesDiscussion: Meigs' Syndrome and Pseudo-Meigs' SyndromeFlapianne SimenceriauNo ratings yet
- Medical Cell Biology: Xiaoying LiuDocument44 pagesMedical Cell Biology: Xiaoying LiuSayan KonarNo ratings yet
- Obstetrics and Gynecology ClinicsDocument247 pagesObstetrics and Gynecology ClinicsAlexandr TrotskyNo ratings yet
- Glandular Lesions of the Uterine Cervix: Cytopathology with Histologic CorrelatesFrom EverandGlandular Lesions of the Uterine Cervix: Cytopathology with Histologic CorrelatesNo ratings yet
- Role of Laparoscopic Surgery in Endometriosis Associated Infertility - Literature ReviewDocument7 pagesRole of Laparoscopic Surgery in Endometriosis Associated Infertility - Literature ReviewFaza KahfiNo ratings yet
- Republic of The PhilippinesDocument5 pagesRepublic of The Philippineshatdog adadaNo ratings yet
- Placenta As Witness 07Document15 pagesPlacenta As Witness 07Vetési OrsolyaNo ratings yet
- Evolution of The Human Pelvis and Obstructed Labor - 2019Document14 pagesEvolution of The Human Pelvis and Obstructed Labor - 2019residentesmihgz112023.2024No ratings yet
- Rare Congenital Genitourinary AnomaliesDocument27 pagesRare Congenital Genitourinary Anomaliesد. محمد عبد الباقي فهميNo ratings yet
- Interdisciplinary Neurosurgery: Jimmy Ntimbani, Adrian Kelly, Patrick LekgwaraDocument4 pagesInterdisciplinary Neurosurgery: Jimmy Ntimbani, Adrian Kelly, Patrick LekgwaraAndres Eduardo GranadosNo ratings yet
- Kista OvariumDocument5 pagesKista OvariumAde Gustina SiahaanNo ratings yet
- Lecture Guide: Introduction To EmbryologyDocument23 pagesLecture Guide: Introduction To EmbryologyAldrin Hardy PabloNo ratings yet
- 1 s2.0 S0140673610603558 MainDocument11 pages1 s2.0 S0140673610603558 MainDr. Eser AĞARNo ratings yet
- Tumors in InvertebratesDocument19 pagesTumors in InvertebrateshamzaNo ratings yet
- The Botanical Review 1945 v.11. 87-107Document21 pagesThe Botanical Review 1945 v.11. 87-107albrewimi1No ratings yet
- Fuller Albright-Primul Articol de HPPDocument2 pagesFuller Albright-Primul Articol de HPPadelinaNo ratings yet
- Adenomatoid Tumors of The Uterus: An Analysis of 60 CasesDocument7 pagesAdenomatoid Tumors of The Uterus: An Analysis of 60 CasesGabriela OliveiraNo ratings yet
- ZalatnaiDocument9 pagesZalatnaiPatricia BezneaNo ratings yet
- Wind Runner 2Document1 pageWind Runner 2Febry FirmansyahNo ratings yet
- Hta 06 11 PDFDocument9 pagesHta 06 11 PDFFebry FirmansyahNo ratings yet
- Windrunner 4Document1 pageWindrunner 4Febry FirmansyahNo ratings yet
- For 2 ExDocument1 pageFor 2 ExFebry FirmansyahNo ratings yet
- Effects of Physical Exercise On Quality of Life, Exercise Capacity and Pulmonary Function in Children With AsthmaDocument6 pagesEffects of Physical Exercise On Quality of Life, Exercise Capacity and Pulmonary Function in Children With AsthmaFebry FirmansyahNo ratings yet
- Stroke Rehabilitation - An Overview of The Journey: InsightDocument2 pagesStroke Rehabilitation - An Overview of The Journey: InsightFebry FirmansyahNo ratings yet
- Optimal Stimulation Frequency of Transcutaneous Electrical Nerve Stimulation On People With Knee OsteoarthritisDocument6 pagesOptimal Stimulation Frequency of Transcutaneous Electrical Nerve Stimulation On People With Knee OsteoarthritisFebry FirmansyahNo ratings yet
- BCR 3462Document13 pagesBCR 3462Febry FirmansyahNo ratings yet
- Laporan Kasus 3: Genito Urinary SystemDocument2 pagesLaporan Kasus 3: Genito Urinary SystemFebry FirmansyahNo ratings yet
- UntitledDocument2 pagesUntitledelleNo ratings yet
- EmpiricalBasis GottmanDocument10 pagesEmpiricalBasis GottmanMundoSinViolenciaNo ratings yet
- Basion Horizontal CobenDocument3 pagesBasion Horizontal CobenJegan KumarNo ratings yet
- Acute Gynaecological Emergencies-1Document14 pagesAcute Gynaecological Emergencies-1Anivasa Kabir100% (1)
- Getting The Most From Lube Oil AnalysisDocument16 pagesGetting The Most From Lube Oil AnalysisGuru Raja Ragavendran Nagarajan100% (2)
- Consumer Protection Act - SeminarDocument16 pagesConsumer Protection Act - SeminarAbdul KhadeerNo ratings yet
- Teachers' Interview PDFDocument38 pagesTeachers' Interview PDFlalitNo ratings yet
- Summary 2Document2 pagesSummary 2Admin OfficeNo ratings yet
- Capitalisation AssignmentDocument5 pagesCapitalisation AssignmentFayis FYSNo ratings yet
- SBT Sekolah Berprestasi Tinggi (HPS) High Performing SchoolsDocument14 pagesSBT Sekolah Berprestasi Tinggi (HPS) High Performing SchoolsAminNo ratings yet
- MC&OB Unit 4Document17 pagesMC&OB Unit 4Tanya MalviyaNo ratings yet
- Ai-Ai ResumeDocument3 pagesAi-Ai ResumeNeon True BeldiaNo ratings yet
- Offer For C Check On NT-495-MG Harbour Generator Engine Against Customer Job No. E20006Document1 pageOffer For C Check On NT-495-MG Harbour Generator Engine Against Customer Job No. E20006bkrNo ratings yet
- Inactive Volcanoes in The Philippine SDocument6 pagesInactive Volcanoes in The Philippine SChristian ParadoNo ratings yet
- Emmanuel Levinas - God, Death, and TimeDocument308 pagesEmmanuel Levinas - God, Death, and Timeissamagician100% (3)
- Academic Journal Guide 2021-MethodologyDocument22 pagesAcademic Journal Guide 2021-MethodologySyedNo ratings yet
- Rear Drive Halfshafts: 2016.0 RANGE ROVER (LG), 205-05Document46 pagesRear Drive Halfshafts: 2016.0 RANGE ROVER (LG), 205-05SergeyNo ratings yet
- Chapter 1 Peanut Growing and HarvestingDocument18 pagesChapter 1 Peanut Growing and HarvestingKapil BhattNo ratings yet
- pdfGenerateNewCustomer SAOAO1030380906Reciept SAOAO1030380906ResidentAccountOpenFormDocument11 pagespdfGenerateNewCustomer SAOAO1030380906Reciept SAOAO1030380906ResidentAccountOpenFormajsingh0702No ratings yet
- Act 1 Almeyda JTLDocument2 pagesAct 1 Almeyda JTLAltairNo ratings yet
- Informe Sobre El Manejo de CostasDocument88 pagesInforme Sobre El Manejo de CostasMetro Puerto RicoNo ratings yet
- Gershwin George Rhapsody in Blue For Sax Quartet 64734Document113 pagesGershwin George Rhapsody in Blue For Sax Quartet 64734Jessica HowardNo ratings yet
- Tle 7-1st Periodic TestDocument2 pagesTle 7-1st Periodic TestReymart TumanguilNo ratings yet
- Eastron Electronic Co., LTDDocument2 pagesEastron Electronic Co., LTDasd qweNo ratings yet
- Optimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsDocument7 pagesOptimal Design of Low-Cost and Reliable Hybrid Renewable Energy System Considering Grid BlackoutsNelson Andres Entralgo MaldonadoNo ratings yet
- Reflection Paper On "Legal Research, Legal Writing, and Legal Analysis: Putting Law School Into Practice" by Suzanne RoweDocument2 pagesReflection Paper On "Legal Research, Legal Writing, and Legal Analysis: Putting Law School Into Practice" by Suzanne RoweRobert Jay Regz Pastrana IINo ratings yet
- HSC 11 Scalars and Vectors Ch2Document5 pagesHSC 11 Scalars and Vectors Ch2Snehal PanchalNo ratings yet
- Exploring Music in ContextDocument3 pagesExploring Music in ContextpkutinNo ratings yet
- Telegram DocumeDocument21 pagesTelegram Documemilli birhanuNo ratings yet
- Whittington 22e Solutions Manual Ch14Document14 pagesWhittington 22e Solutions Manual Ch14潘妍伶No ratings yet