Professional Documents
Culture Documents
56 Koshiba Takeuchi Et Al
56 Koshiba Takeuchi Et Al
Uploaded by
JamesComeyJustaBitch0 ratings0% found this document useful (0 votes)
18 views5 pagesjustalittlebitch
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentjustalittlebitch
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
18 views5 pages56 Koshiba Takeuchi Et Al
56 Koshiba Takeuchi Et Al
Uploaded by
JamesComeyJustaBitchjustalittlebitch
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 5
LETTERS
Reptilian heart development and the molecular basis
of cardiac chamber evolution
Kazuko Koshiba-Takeuchi
1,2,3,4
*, Alessandro D. Mori
1,2,3,5,6
*, Bogac L. Kaynak
1,2,3
*, Judith Cebra-Thomas
7
,
Tatyana Sukonnik
1,2,3
, Romain O. Georges
8
, Stephany Latham
9
, Laural Beck
9
, R. Mark Henkelman
10,11
,
Brian L. Black
3,12
, Eric N. Olson
13
, Juli Wade
9
, Jun K. Takeuchi
4
, Mona Nemer
8,14
, Scott F. Gilbert
15
& Benoit G. Bruneau
1,2,3,5,6
The emergence of terrestrial life witnessed the need for more
sophisticated circulatory systems. This has evolved in birds,
mammals and crocodilians into complete septation of the heart
into left andright sides, allowing separate pulmonary and systemic
circulatory systems, a key requirement for the evolution of
endothermy
13
. However, the evolution of the amniote heart is
poorly understood. Reptilian hearts have been the subject of
debate in the context of the evolution of cardiac septation: do they
possess a single ventricular chamber or two incompletely septated
ventricles
47
? Here we examine heart development in the red-eared
slider turtle, Trachemys scripta elegans (a chelonian), and the
green anole, Anolis carolinensis (a squamate), focusing on gene
expressioninthe developing ventricles. Bothreptiles initially form
a ventricular chamber that homogenously expresses the T-box
transcription factor gene Tbx5. Incontrast, inbirds and mammals,
Tbx5 is restricted to left ventricle precursors
8,9
. In later stages,
Tbx5 expression in the turtle (but not anole) heart is gradually
restricted to a distinct left ventricle, forming a leftright gradient.
This suggests that Tbx5 expression was refined during evolution to
pattern the ventricles. In support of this hypothesis, we show that
loss of Tbx5 in the mouse ventricle results in a single chamber
lacking distinct identity, indicating a requirement for Tbx5 in
septation. Importantly, misexpression of Tbx5 throughout the
developing myocardiumto mimic the reptilian expression pattern
also results in a single mispatterned ventricular chamber lacking
septation. Thus ventricular septation is established by a steep and
correctly positioned Tbx5 gradient. Our findings provide a
molecular mechanism for the evolution of the amniote ventricle,
and support the concept that altered expression of developmental
regulators is a key mechanism of vertebrate evolution.
Amphibians have a three-chambered heart, whereas mammalian,
crocodilian and avian hearts have four chambers, two each for pulmo-
nary and systemic circulations. The acquisition of a fully septated vent-
ricle has evolved independently in birds, mammals and crocodilians
10
,
andis animportant example of convergent evolution. Non-crocodilian
reptiles (squamates, chelonians and rhynchocephalians) hold a unique
place in the evolution of the heart, as their ventricular chambers are
apparent intermediates between these forms
47
. In reptiles, shunting
can produce functional separations between left and right circulatory
systems, but only complete septation allows a dual pressure system
required for endothermy. Therefore, the evolutionary status of the
reptilian ventricles is controversial
7
. Is it a primitive arrangement
presaging the septated heart of crocodilians, birds and mammals? Or
is it anadaptationtoparticular circulatory requirements? Development
of reptilian hearts has not been addressed in over 100 years
11
, and thus
the developmental basis of reptilian heart formation is not known.
Furthermore, clear insight into the evolution of cardiac septation has
not emerged from molecular studies of heart development
3
.
Transcription factors of the T-box family are important regulators
of heart formation
12
. One T-box gene, Tbx5, has an expression pattern
that suggests a role in the evolution of cardiac septation (see
Supplementary Note 1). Inamphibians, Tbx5 is expressed throughout
the developing heart
13
. Inbirds and mammals, there is a steep gradient
of Tbx5 expression from high levels in the prospective left ventricle to
lowlevels in the prospective right ventricle
8,9
. Reduced dosage of Tbx5
in humans and mice leads to defects in interventricular septum (IVS)
formation andpatterning
1417
, suggesting that a steepgradient of Tbx5
is critical for IVSformation. The evolutionary role of Tbx5 inseptation
is unknown.
We examined cardiac embryology of the red-eared slider turtle,
T. scripta elegans (a chelonian), and the green anole, A. carolinensis
(a squamate), focusing on the ventricles. Although the phylogenetic
relationship of turtles to other reptiles is controversial based on ana-
tomical considerations
18,19
, molecular phylogenies consistently
group turtles with the archosaurs (birds and crocodiles)
20,21
. Anoles
are considered to be more basal than archosaurs
1921
. The post-
hatching anole heart has a thick muscular ridge (Fig. 1ad and
Supplementary Fig. 1) that separates a proximal outflow tract, or
cavum pulmonale
6,11
, from the main ventricular chamber. Turtles
have a smaller muscular ridge and are thought to have a primitive
IVS-like structure
4,6,11
, as we determined by three-dimensional
reconstructions revealing a dense coalescence of trabeculae spanning
the full depth of the heart (Fig. 1eh and Supplementary Fig. 1).
Initially, developing turtle and anole hearts showed no clear evidence
of ventricular septation (Fig. 1i and Supplementary Figs 24). In
contrast, the chick has a well-developed IVS at comparable early
stages (Fig. 1i and Supplementary Fig. 3). In the turtle, a structure
resembling an IVS appears only at stage 21 (Fig. 1i). Alligator
*These authors contributed equally to this work.
1
Gladstone Institute of Cardiovascular Disease, San Francisco, California 94158, USA.
2
Department of Pediatrics,
3
Cardiovascular Research Institute, University of California, San
Francisco, California 94158, USA.
4
Division of Cardiovascular Research, Global-Edge Institute, Tokyo Institute of Technology, Yokohama, Kanagawa 226-8503, Japan.
5
Program in
StemCell and Developmental Biology, The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada.
6
Department of Molecular Genetics, University of Toronto, Toronto, Ontario
M5S 1A8, Canada.
7
Biology Department, Millersville University, Millersville, Pennsylvania 17551, USA.
8
Institut de Recherches Cliniques de Montreal, Programme de Biologie
Moleculaire, Universite de Montreal, Montreal, Quebec H3C 3J7, Canada.
9
Department of Psychology and Program in Neuroscience, Michigan State University, East Lansing,
Michigan 48824, USA.
10
The Mouse Imaging Centre, The Hospital for Sick Children, Toronto, Ontario M5G 1X8, Canada.
11
Department of Medical Biophysics, University of Toronto,
Toronto, Ontario M5S 1A8, Canada.
12
Department of Biochemistry and Biophysics, University of California, San Francisco, California 94158, USA.
13
Department of Molecular Biology,
University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA.
14
Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, Ontario
K1H 8M5 Canada.
15
Department of Biology, Swarthmore College, Swarthmore, Pennsylvania 19081, USA.
Vol 461 | 3 September 2009| doi:10.1038/nature08324
95
Macmillan Publishers Limited. All rights reserved 2009
embryos (Fig. 1j and Supplementary Fig. 1) have a muscular ridge
and a distinct IVS. The muscular ridge has been interpreted as ana-
logous to the IVS, leading to the impression that reptiles have mul-
tiple septa
46
. We speculate that the development of the muscular
ridge in reptiles reflects persistent growth of the proximal outflow
tract
11
, as seen transiently in the chick heart (Fig. 1ik and
Supplementary Fig. 3).
To observe molecular patterning of reptile ventricles, we examined
expression of Tbx5. In mammals and birds, Tbx5 messenger RNA
(mRNA) and protein are highly enriched in the prospective left vent-
ricle (Fig. 2b, c and Supplementary Fig. 5)
8,9
. At looping heart tube
stages, Tbx5 was broadly expressed throughout the embryonic turtle
and anole hearts (Fig. 2a, d), similar to Xenopus Tbx5 (ref. 13), but
unlike its early restricted expression in chick and mouse (Fig. 2b, c).
In the anole, Tbx5 expression extended to the boundary of the vent-
ricle and outflow tract, where the muscular ridge forms. At later
stages, Tbx5 expression in the turtle (stage 15) and anole (stage 13)
remained homogeneous throughout the ventricle (Fig. 2e, h and data
not shown). In comparable stages in chick, it was sharply restricted to
left ventricle primordium. At stages 1718 in the turtle, Tbx5 mRNA
levels decreased in right ventricle primordium, remaining enriched
in left ventricle primordium, creating a steep leftright gradient,
although not as sharply defined as in chick (Fig. 2fi and
Supplementary Figs 6 and 7). This gradient was maintained at stage
21 (Fig. 2h, i). Tbx5 expression in Anolis was not restricted in the
ventricle (Fig. 2f, i and Supplementary Fig. 7). We examined expres-
sion of Tbx5 target genes expressed in trabeculae but excluded from
mammalian IVS myocardium
14,16,17,22
. Bmp10 was expressed
throughout the early turtle and anole trabeculae, but was excluded
in turtles at stages 1718 from an expansion of the compact myocar-
diumcorresponding to presumptive IVS precursors, correlating with
the boundary of Tbx5 expression (Fig. 2j and Supplementary Fig. 6).
This suggests a conserved molecular transition in the trabeculae that
form the IVS. Turtle Nppa (not found in anoles
23
) formed a gradient
Turtle
a
a
a
la
ot
ot
ot
ot
la
la
la
la la
ra
ra ra
ra
ra
ra
ra
v
v
v
v v
v
v
v
v
lv
lv
lv
rv
rv
rv
la
Anole
Early Late
Chick
14 12
6 9 13 16
32 28 23 18
17 21
mr
mr
mr
mr mr
mr
ot
ot ot
ot
lv
lv rv
rv
v
v
ra
ra ra ra
la
la
la
la
IVS?
IVS?
IVS?
ot
v
la ra
mr
ot
v
la
ra
mr
a
j
k
b
c
e
f
g d h
i
Alligator
21 21 Front Back
mr
IVS
rv
rv lv
lv
ot
ot
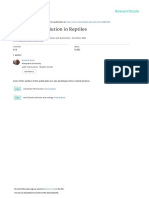
Figure 1 | Reptilian heart development. ah, OPT of post-hatching anole
(ad) and turtle (eh) hearts. a, e, External view; b, c, f, g, chamber fill;
d, h, histology. i, Histological analysis of heart development in turtle, anole
and chick embryos. Four representative stages shownare equivalent between
species (arrow, interventricular groove). j, Histology of stage 21 embryonic
alligator heart. k, OPT of stage 17 (left) and stage 21 (right) turtle hearts. In
all reptile embryos (ik), note close apposition of mr and ot. a, atrium; IVS?,
IVS-like structure; la, left atrium; lv, left ventricle; mr, muscular ridge; ot,
outflow tract; ra, right atrium; rv, right ventricle.
a b c
d e
f
g
h
i
j k
Stage 15
0
30
20
10
0
1
2
Stage 17 Stage 21
*
*
20 28 11 16 15 17 21
L
V
/
R
V
r
a
t
i
o
T
b
x
5
m
R
N
A
l
e
v
e
l
s
Chick Turtle
Stage
Turtle
Anolis
Stage 12
v
ot
a
Turtle Mouse
*
Stage 9
f
hl
he
dr
Stage 23
ot
a
rv lv
Chick
Stage 18
f
hl
he
dr
E9.5
ra
la
rv
avc
lv
Anolis stage 13 Turtle stage 15 Chick stage 28
as as
as la la
la
ra
ra
ra
rv
avc
avc
avc
lv
v
v
ra
ra la
la
la
lv
lv
rv
v
rv
vv
ra
Turtle stage 17 Anolis stage 16 Chick stage 30
la
Chick stage 20
as
ra
rv
lv
ot
Anolis stage 6
a
v
Tbx5
v
ra la
Bmp10,
Anolis stage 15
[
[
ra la
lv
rv
Bmp10,
Turtle stage 17
Bmp10,
Turtle stage18
rv
ra la
Nppa,
Turtle stage 17
lv
lv
rv
Front Back
rv
lv
ot
la
la
ra
ra
Turtle stage 17
Chick stage 28
rv
rv
Front Back
lv
lv
ot
la
la
ra
ra
*
*
Figure 2 | Gene expression in amniote embryos. ac, Tbx5 expression in
turtle, chick and mouse. a, b, mRNA expression. Left panels, whole-embryo
views. he, heart; fl, forelimbs; dr, dorsal retina; hl, hindlimbs. Right panels,
close-up ventral views of embryonic hearts. c, Tbx5 immunohistochemistry;
red arrowheads, right ventricle/left ventricle junction; purple arrowhead,
epicardium. la, left atrium; lv, left ventricle; ot, outflow tract; ra, right
atrium; rv, right ventricle. dg, Expression of Tbx5. as, atrial septum; avc,
atrioventricular cushion. Arrowheads mark the boundary between left
ventricle and right ventricle, or ventricle (v) and ot for anole in
d. h, Quantification of Tbx5 mRNA levels in turtle left ventricle and right
ventricle; data are mean6s.d. normalized to stage 15 right ventricle.
*P,0.005 by t-test. i, Ratio of Tbx5 mRNA levels between the left ventricle
and right ventricle. j, Bmp10 expression in turtle and anole hearts.
Arrowheads, interventricular groove and septum. Brackets, thickness of
Bmp10-negative area. k, Nppa expression in the turtle is in a leftright
gradient similar to Tbx5.
LETTERS NATURE| Vol 461 | 3 September 2009
96
Macmillan Publishers Limited. All rights reserved 2009
similar to Tbx5 (Fig. 2k). Thus turtle ventricles, but not those of
Anolis, acquire distinctions between left and right components late
in development.
A steep Tbx5 gradient in chick and mouse may have evolved to
pattern the ventricles. Reducing Tbx5 levels supports this
14,16,17
. To
address a potential role for Tbx5 in septation, we deleted Tbx5
from segments of developing mouse ventricles, using a conditionally
deletable Tbx5 allele (Tbx5
LDN
)
16
, and ventricular myocyte-specific
Nkx2.5::Cre mice
24
(Fig. 3a). These mice (Nkx2.5::Cre
tg/0
;Tbx5
LDN/LDN
mice, or Tbx5
V-del
mice) lacked morphological distinctions between the
left ventricle and right ventricle that were obvious in wild-type embryos
by embryonic day 9.5 (Fig. 3b). Embryos with this univentricular
phenotype persisted until embryonic day 11.5 (Fig. 3c, d). Expression
of Nppa and Bmp10, normally excluded from the interventricular
groove, was expanded throughout the single ventricle of Tbx5
V-del
embryos (Fig. 3e,f). Hand1 was expressed at lower levels, but in its
normal domains, the left ventricle and right ventricle primordia
(Fig. 3g). Thus loss of Tbx5 fromdeveloping ventricles results in a single
mispatterned ventricle.
To determine if a steep Tbx5 gradient at the interventricular mid-
point is critical for IVS formation, we deleted Tbx5 with
Mef2cAHF::Cre mice
25
(Fig. 3h). Because Mef2cAHF::Cre is active
in the right ventricle and IVS precursors, but not in the left ventricle
free wall, the Tbx5 expression boundary is shifted leftwards.
Tbx5
LDN/LDN
;Mef2cAHF::Cre (Tbx5
AHF-del
) mice lacked an IVS
(Fig. 3ik). Gene expression analysis showed that a distinction
between left ventricle and right ventricle was maintained (Fig. 3l, m);
however, a clear absence of IVS-enriched markers (Irx2, Dkk3) at the
ventricular midpoint, although maintained in the adjacent trabeculae,
emphasizes the absence of ventricular septation (Fig. 3n and
Supplementary Fig. 8). Thus a boundary of cells expressing high
Tbx5 levels is necessary within a segment of myocardium where IVS
outgrowth will occur. This implies a prepattern within which Tbx5
must function; the nature of this prepattern is unknown (see
Supplementary Note 2). Tbx5 expression and additional patterning
cues may have co-evolved, or the prepattern may exist in all amniotes.
Regardless, IVS formation requires a sharp Tbx5 boundary, which
indicates that Tbx5 patterning was a major factor in the evolution of
septation.
Our loss-of-function experiments demonstrate a requirement for
Tbx5 in IVS formation distinct from a more global role in differenti-
ation. These results do not address the evolutionary role of Tbx5
patterning; in particular, whether the broad expression of Tbx5
observed in anole and turtle would preclude IVS formation. Previous
misexpression attempts yielded variable results ranging from no effect
to severely malformed hearts (ref. 9 and J.K.T., unpublished observa-
tions). We misexpressed Tbx5 in the ventricles by crossing a mouse
line bearing a stable Cre-activatable transgene expressing moderate
WT WT Tbx5
vdel/vdel
Tbx5 Tbx5 Tbx5
AHF-del
Mef2cAHFCre Tbx5
v-del
Nkx2.5::Cre
Tbx5
vdel/vdel
Tbx5
AHFdel/AHFdel
Tbx5
AHFdel/AHFdel
Nppa, E9.5
ra ra
la
la
rv
rv
lv lv
WT WT
Nppa, E10.5
Bmp10, E10.5
Irx2, E10.5
Bmp10, E10.5
Hand1, E10.5
E11.5
lv
rv v
ra la
rv lv
ot
ra la
v
E9.5
a
b
c
d
e
f
g
h
i
j
k
l
m
n
Figure 3 | Ventricle-restricted deletion of mouse Tbx5. a, Strategy for
ventricular deletion of Tbx5. bd, OPT of wild-type (WT) and Tbx5
vdel/vdel
embryos andhearts at embryonic day(E) 9.5(b) andE11.5(c, d). Arrowheads
indicate position of the IVS. eg, Gene expression for indicated transcripts.
h, Strategy for Tbx5 deletion in anterior heart field derivatives. ik, OPT of
wild-type (WT) and Tbx5
AHFdel/AHFdel
hearts at E10.5. i, External view;
j, chamber fill; k, virtual sections. ln, Gene expression for indicated
transcripts.
WT
CAT-Tbx5;
Mef2cAHF::Cre
Mef2cAHF::Cre
or Nkx2.5::Cre
Tbx5
misexpression
Turtle Alligator
Chicken
Anole
Mouse
Human
Xenopus
d
c a
b
Nppa
Tbx5
Bmp10
[
[
[
IVS
IVS
IVS
lv
lv lv
rv rv rv
ra
ra
ra
la
la
la
WT
CAT-Tbx5
CAT-Tbx5; Nkx2.5::Cre
Nppa
Bmp10
[
Tbx5
rv
lv v
la
ra
ra
la
[
[
[
[
[
Figure 4 | Misexpression of Tbx5 results in loss of IVS patterning.
a, Strategy for ventricular misexpression of Tbx5. b, Morphology and gene
expression in CAT-Tbx5;Mef2cAHF::Cre embryos for indicated transcripts.
Brackets, IVS region, magnified in lower panels. Arrowheads, trabecular
Bmp10 expression. la, left atrium; lv, left ventricle; ra, right atrium; rv, right
ventricle; v, ventricle. c, Morphology and gene expression in CAT-
Tbx5;Nkx2.5::Cre embryos at embryonic day 11.5. Orange arrows,
interventricular septal region (IVS). Brackets showa rudimentary septumin
a mutant embryo. d, Representation of embryonic heart structures and
patterns of Tbx5 expression (blue) in vertebrate evolution.
NATURE| Vol 461 | 3 September 2009 LETTERS
97
Macmillan Publishers Limited. All rights reserved 2009
Tbx5 levels upon induction (CAT-Tbx5)
26
with Mef2cAHF::Cre or
Nkx2.5::Cre mice (Fig. 4). CAT-Tbx5;Mef2cAHF::Cre embryos survived
until embryonic day 11 and had a single ventricle at embryonic day
10.25. Molecular analysis revealed expanded expression of Tbx5,
Nppa and Bmp10 across the interventricular groove of CAT-
Tbx5;Mef2cAHF::Cre embryos (Fig. 4b). CAT-Tbx5;Nkx2.5::Cre
embryos survived longer (until embryonic day 12), presumably
because this manipulation avoided secondary effects of Tbx5 over-
expression in cardiac progenitors. CAT-Tbx5;Nkx2.5::Cre embryos at
embryonic day 11.5 also had defective ventricular septation and mis-
patterned gene expression (Fig. 4c and Supplementary Fig. 9).
Interestingly, owing to the mosaic expression of Tbx5 by Nkx2.5::Cre,
some embryos had no septumat all, whereas others with a more graded
expression of Tbx5 had a rudiment of a septum in which not all genes
were mispatterned(Fig. 4c andSupplementary Fig. 9). Thus misexpres-
sion of Tbx5 in a pattern reminiscent of the reptilian heart leads to loss
of IVS patterning and morphogenesis, further supporting a role for
Tbx5 patterning in the evolution of septation.
Our results provide evidence that the reptilian heart, although
evolved to function physiologically under conditions particular to
reptilian life
7
, is an evolutionary intermediate between amphibian
and avian/crocodilian hearts in its ventricular development. The
dynamic expression of Tbx5 and its leftward restriction suggest a
temporal refinement model in which early restriction of Tbx5 expres-
sion to left ventricle precursors, as seen inchick andmouse, provides a
robust patterning cue for ventricular septation. Inthis model (Fig. 4d),
a quantitative gradient of Tbx5 is essential for proper formation and
patterning of the IVS. Our mouse genetic analyses, including
decreased dosage
14,16
, are consistent with an important role for a steep
gradient of Tbx5 in chamber patterning and IVS formation. In the
reptilian heart, the delayed and less pronounced establishment of this
patterning may contribute to varying degrees of septation. Therefore
patterning of Tbx5, in the archosaurian and synapsid lineages, is
likely to be an important mechanism in the convergent evolution of
septation. Our findings generally support the concept that altered
expression of developmental regulators is an important aspect of
morphological evolution
27
.
METHODS SUMMARY
Embryos were isolated from T. scripta elegans eggs (Kliebert Turtle and Alligator
Farm). Green anole (A. carolinensis) embryos were collected in captivity. Mouse
strains were as previously described
16,2426
. Wholemount and section in situ
hybridizations were performed using standard protocols. Immunohisto-
chemistry and optical projection tomography (OPT) were performed as previ-
ously described
26,28
. For all mouse experiments, at least three embryos were
examined for each genotype at each stage, all with comparable results.
Full Methods and any associated references are available in the online version of
the paper at www.nature.com/nature.
Received 18 December 2008; accepted 27 July 2009.
1. Farmer, C. G. Evolution of the vertebrate cardio-pulmonary system. Annu. Rev.
Physiol. 61, 573592 (1999).
2. Hillenius, W. J. & Ruben, J. A. The evolution of endothermy in terrestrial
vertebrates: Who? When? Why? Physiol. Biochem. Zool. 77, 10191042 (2004).
3. Olson, E. N. Gene regulatory networks in the evolution and development of the
heart. Science 313, 19221927 (2006).
4. Holmes, E. B. Areconsideration of the phylogeny of the tetrapod heart. J. Morphol.
147, 209228 (1975).
5. Webb, G. J. W. Comparative cardiac anatomy of the Reptilia. III. The heart of
crocodilians and an hypothesis on the completion of the interventricular septum
of crocodilians and birds. J. Morphol. 161, 221240 (1979).
6. Farrell, A. P., Gamperl, A. K. & Francis, E. T. B. in Biology of the Reptilia Vol. 19 (eds
Gans, C. & Gaunt, A. S.) 375424 (Society for the Study of Amphibians and
Reptiles, 1998).
7. Hicks, J. W. The physiological and evolutionary significance of cardiovascular
shunting patterns in reptiles. News Physiol. Sci. 17, 241245 (2002).
8. Bruneau, B. G. et al. Chamber-specific cardiac expression of Tbx5 and heart
defects in HoltOram syndrome. Dev. Biol. 211, 100108 (1999).
9. Takeuchi, J. K. et al. Tbx5 specifies the left/right ventricles and ventricular septum
position during cardiogenesis. Development 130, 59535964 (2003).
10. Seymour, R. S. et al. Evidence for endothermic ancestors of crocodiles at the stem
of archosaur evolution. Physiol. Biochem. Zool. 77, 10511067 (2004).
11. Greil, A. Beitrage zur vergleichenden Anatomie und Entwicklungsgeschichte des
Herzens und des Truncus arteriosus der Wirbeltiere. Morphol. Jahrb. 31, 123310
(1903).
12. Stennard, F. A. & Harvey, R. P. T-box transcription factors and their roles in
regulatory hierarchies in the developing heart. Development 132, 48974910
(2005).
13. Horb, M. E. & Thomsen, G. H. Tbx5 is essential for heart development.
Development 126, 17391751 (1999).
14. Bruneau, B. G. et al. A murine model of HoltOram syndrome defines roles of the
T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106, 709721
(2001).
15. Bruneau, B. G. The developmental genetics of congenital heart disease. Nature
451, 943948 (2008).
16. Mori, A. D. et al. Tbx5-dependent rheostatic control of cardiac gene expression
and morphogenesis. Dev. Biol. 297, 566586 (2006).
17. Koshiba-Takeuchi, K. et al. Cooperative and antagonistic interactions between
Sall4 and Tbx5 pattern the mouse limb and heart. Nature Genet. 38, 175183
(2006).
18. Rieppel, O. Turtle origins. Science 283, 945946 (1999).
19. Lyson, T. & Gilbert, S. F. Turtles all the way down: loggerheads at the root of the
chelonian tree. Evol. Dev. 11, 133135 (2009).
20. Hedges, S. B. & Poling, L. L. A molecular phylogeny of reptiles. Science 283,
9981001 (1999).
21. Shedlock, A. M. et al. Phylogenomics of nonavian reptiles and the structure of the
ancestral amniote genome. Proc. Natl Acad. Sci. USA 104, 27672772 (2007).
22. Chen, H. et al. BMP10 is essential for maintaining cardiac growth during murine
cardiogenesis. Development 131, 22192231 (2004).
23. Trajanovska, S. & Donald, J. A. Molecular cloning of natriuretic peptides from the
heart of reptiles: loss of ANP in diapsid reptiles and birds. Gen. Comp. Endocrinol.
156, 339346 (2008).
24. McFadden, D. G. et al. The Hand1 and Hand2 transcription factors regulate
expansion of the embryonic cardiac ventricles in a gene dosage-dependent
manner. Development 132, 189201 (2004).
25. Verzi, M. P. et al. The right ventricle, outflow tract, and ventricular septum
comprise a restricted expression domain within the secondary/anterior heart
field. Dev. Biol. 287, 437449 (2005).
26. Georges, R. et al. Distinct expression and function of alternatively spliced Tbx5
isoforms in cell growth and differentiation. Mol. Cell. Biol. 28, 40524067 (2008).
27. Carroll, S. B. Evo-devo and an expanding evolutionary synthesis: a genetic theory
of morphological evolution. Cell 134, 2536 (2008).
28. Lickert, H. et al. Baf60c is essential for function of BAF chromatin remodelling
complexes in heart development. Nature 432, 107112 (2004).
Supplementary Information is linked to the online version of the paper at
www.nature.com/nature.
Acknowledgements We thank J. N. Wylie and L. Davidson for technical assistance,
M. Harris and J. Fallon for alligator embryos, T. Sanger and J. Gibson-Brown for
unpublished data on Anolis staging, T. Ogura for chick Tbx5 and Tbx20 probes, and
G. Howard and S. Ordway for editorial assistance. This work was funded in part by
the March of Dimes Birth Defects Foundation (B.G.B.), the J. David Gladstone
Institutes (B.G.B.), William H. Younger Jr (B.G.B.); a National Institutes of Health
program project grant (P01HL089707 to B.G.B., B.L.B.); scholarships from the
Natural Sciences and Engineering Research Council of Canada, the Heart and
Stroke Richard Lewar Centre for Excellence, University of Toronto, and Ontario
Graduate Scholarship (A.D.M.); the Fumi Yamamura Memorial Foundation for
Female Natural Scientists and Grants-in-Aid for Scientific Research (C) (K.K.-T.),
MEXTs program for young independent researchers (K.K.-T., J.K.T.), Sumitomo
Foundation and Nakajima Foundation (J.K.T.), a Canada Research Chair in Imaging
(R.M.H.), the Heart and Stroke foundation of Canada and the Canadian Institutes
for Health Research (M.N.), and grants from the National Science Foundation
(RUI-0748508 to S.F.G., J.C.-T. and IOS-0742833 to J.W.). Funding for the J. David
Gladstone Institutes from a National Institutes of Health/ National Center for
Research Resources grant (C06 RR018928) is also acknowledged.
Author Contributions K.K.-T. performed reptile histology and gene expression
studies; A.D.M., B.L.K., T.S. and B.G.B. performed mouse experiments; J.C.-T. and
S.F.G. obtained turtle specimens and isolated T. scripta Tbx5 complementary DNA
(cDNA); S.L. and L.B. isolated Anolis specimens under direction of J.W.; B.L.K.
acquired and reconstructed OPT images; R.O.G. performed Tbx5
immunohistochemistry under direction of M.N.; R.M.H. directed initial mouse
embryo OPT; J.K.T. obtained chick and mouse specimens; R.O.G., M.N., B.L.B. and
E.N.O. provided genetically modified mice before publication; B.G.B. conceived and
directed the project, and wrote the paper. All authors contributed to the written
manuscript.
Author Information Reprints and permissions information is available at
www.nature.com/reprints. Correspondence and requests for materials should be
addressed to B.G.B. (bbruneau@gladstone.ucsf.edu).
LETTERS NATURE| Vol 461 | 3 September 2009
98
Macmillan Publishers Limited. All rights reserved 2009
METHODS
Reptiles. Embryos from T. scripta elegans eggs (Kliebert Turtle and Alligator
Farm) were dissected free of extra-embryonic membranes and staged according
to Greenbaum & Carr
29
. Green anole (A. carolinensis) embryos were collected in
captivity during the spring and summer breeding season. The day the eggs were
discovered in the cage was considered embryonic day 0. Eggs were incubated at
27 uCuntil embryo collection at embryonic days 4, 8, 11, 12, 14, 18 and 19 and on
the day of hatching. Anole embryos were staged as previously described
30
. All
embryos were fixed in 4% paraformaldehyde, followed by stepwise dehydration
in methanol.
Mouse genetics. Mouse strains were as previously described
16,2426
.
Reptile cDNAs. Turtle total RNA was prepared from pooled day 14 T. scripta
embryos (stages 1215). PCR was performed from cDNA with 59-GTTTC
CCAGTTACAAAGTGAAGG (forward) and 59-GTCTCACTGTGCTCCTGGG
(reverse) primers designed against the chick Tbx5 sequence. PCR products
matching the estimated size (540 base pairs) were extracted from a 1% agarose
gel, ligated into pCRII (Invitrogen) and used to transform DH5a-competent
cells. Positive plasmids were identified by hybridization with digoxygenin-
labelled chick Tbx5 and by amplification using nested primers also designed
against chick Tbx5 and Tbx4 (59-TAYGTGCACCCGGAYTCYCCWGC and
59-TGGTASGARGTCACAGMGATRAA). Anolis cDNA probes were obtained
by PCRfromgenomic DNA, using sequences obtained at the National Center for
Biotechnology Information Trace Archive (http://www.ncbi.nlm.nih.gov/
Traces/trace.cgi?).
29. Greenbaum, E. & Carr, J. L. Staging criteria for embryos of the spiny softshell
turtle, Apalone spinifera (Testudines: Trionychidae). J. Morphol. 254, 272291
(2002).
30. Sanger, T. J., Losos, J. B. & Gibson-Brown, J. J. A developmental staging series for
the lizard genus Anolis: a new system for the integration of evolution,
development, and ecology. J. Morphol. 269, 129137 (2008).
doi:10.1038/nature08324
Macmillan Publishers Limited. All rights reserved 2009
You might also like
- Lab Exercise 2Document4 pagesLab Exercise 2Angela Robles0% (1)
- Risk Assessment (Snake Bites, Scorpion Sting, Reptiles Bites)Document3 pagesRisk Assessment (Snake Bites, Scorpion Sting, Reptiles Bites)Raza Muhammad Soomro100% (1)
- 96-FBI Criminal Report RequestDocument2 pages96-FBI Criminal Report RequestJamesComeyJustaBitch100% (1)
- Game Snakes Ladders With English Game Questions and InstructionsDocument10 pagesGame Snakes Ladders With English Game Questions and InstructionsquadromagicoNo ratings yet
- AnimalClassificationandAnimalCharacteristicsSortsFREEBIE PDFDocument8 pagesAnimalClassificationandAnimalCharacteristicsSortsFREEBIE PDFtanleeleanNo ratings yet
- Historical Perspective: A Decade of Discoveries in Cardiac BiologyDocument8 pagesHistorical Perspective: A Decade of Discoveries in Cardiac BiologyDiego AbrahamNo ratings yet
- Developmental Biology: The Epicardium in The Embryonic and Adult ZebrafishDocument16 pagesDevelopmental Biology: The Epicardium in The Embryonic and Adult ZebrafishplatusNo ratings yet
- Embryology of The Hind GutDocument10 pagesEmbryology of The Hind Gutproject-247758No ratings yet
- Nature 2014 Long JADocument13 pagesNature 2014 Long JAAlfredo PerettiNo ratings yet
- Cardiogenesis and The Regulation of Cardiac-Specific Gene ExpressionDocument14 pagesCardiogenesis and The Regulation of Cardiac-Specific Gene ExpressiontyasNo ratings yet
- Biodesarrollo Corazon ReptilesDocument12 pagesBiodesarrollo Corazon ReptilesAlex Ramos maqueraNo ratings yet
- 2011 BFnature09655 MOESM201 ESMDocument11 pages2011 BFnature09655 MOESM201 ESMliqinggreenNo ratings yet
- Identification Guide To Antarctic CopepodsDocument42 pagesIdentification Guide To Antarctic CopepodsmauricioNo ratings yet
- Metabolic Control of Progenitor Cell Propagation During Drosophila Tracheal RemodelingDocument17 pagesMetabolic Control of Progenitor Cell Propagation During Drosophila Tracheal Remodelingdede chenNo ratings yet
- Journal of Morphology - 2019 - Kroneman - Comparative Analysis of Avian Hearts Provides Little Evidence For Variation AmongDocument16 pagesJournal of Morphology - 2019 - Kroneman - Comparative Analysis of Avian Hearts Provides Little Evidence For Variation AmongJohanne May Capitulo LopezNo ratings yet
- Ectopic Thyroid: Etiology, Pathology and Management: ReviewDocument9 pagesEctopic Thyroid: Etiology, Pathology and Management: ReviewIonuț RusNo ratings yet
- tmp75B6 TMPDocument4 pagestmp75B6 TMPFrontiersNo ratings yet
- Structure and Function of The Developing Zebrafish HeartDocument10 pagesStructure and Function of The Developing Zebrafish HeartKarthik ShettyNo ratings yet
- tmp3FED TMPDocument3 pagestmp3FED TMPFrontiersNo ratings yet
- Case 7: Hydranencephaly: Alfred B. Kurtz, MD Pamela T. Johnson, MDDocument4 pagesCase 7: Hydranencephaly: Alfred B. Kurtz, MD Pamela T. Johnson, MDKenzo Adhi WiranataNo ratings yet
- Lobulated Spleen With A Fissure On Diaphragmatic Surface: RD RDDocument7 pagesLobulated Spleen With A Fissure On Diaphragmatic Surface: RD RDShantu ShirurmathNo ratings yet
- Frog EmbryosDocument43 pagesFrog EmbryosNics Martinez100% (7)
- 560 FTPDocument8 pages560 FTPSrinivas PrasadNo ratings yet
- Kleckowska Et Al Macroanatomical Investigation of ARGDocument5 pagesKleckowska Et Al Macroanatomical Investigation of ARGGNo ratings yet
- Embryology of Oesophageal Atresia: Adonis S. Ioannides, Andrew J. CoppDocument10 pagesEmbryology of Oesophageal Atresia: Adonis S. Ioannides, Andrew J. CoppXimenita GhilardiNo ratings yet
- Structure of The Reproductive Apparatus and Life Cycle of Milax Gagates Draparnaud Mollusca Gastropoda PulmonataDocument40 pagesStructure of The Reproductive Apparatus and Life Cycle of Milax Gagates Draparnaud Mollusca Gastropoda PulmonataRaihana Naifa ENo ratings yet
- Tetralogy of Fallot, Agarwala 2017Document5 pagesTetralogy of Fallot, Agarwala 2017rinayondaNo ratings yet
- Maduracion de La AmigadalaDocument15 pagesMaduracion de La AmigadalaAracelis Calzadilla NúñezNo ratings yet
- E7.5 E8.0 E8.5 E12.5Document11 pagesE7.5 E8.0 E8.5 E12.5خـޢޢـطـޢޢـ آحـޢޢـمـޢޢـرNo ratings yet
- Ancient Animal MicroRNAs and The Evolution of Tissue IdentityDocument10 pagesAncient Animal MicroRNAs and The Evolution of Tissue IdentityRebecca PotterNo ratings yet
- Comisuritis Posterior 2Document8 pagesComisuritis Posterior 2VORTIZNo ratings yet
- 2 PDFDocument8 pages2 PDFanon_243369186No ratings yet
- AVIAN-The Avıan Spleen Anatomy, Physiology and DiagnosticsDocument6 pagesAVIAN-The Avıan Spleen Anatomy, Physiology and Diagnosticstaner_soysuren100% (1)
- Developmental Dynamics - 2006 - Campione - Cardiovascular Development Toward Biomedical ApplicabilityDocument3 pagesDevelopmental Dynamics - 2006 - Campione - Cardiovascular Development Toward Biomedical Applicabilitystevenburrow06No ratings yet
- Congenital LaryngomalaciaDocument8 pagesCongenital LaryngomalaciaRettha SigiroNo ratings yet
- Tiroida Ectopica 1Document8 pagesTiroida Ectopica 1Ionuț RusNo ratings yet
- Sall1 Maintains Nephron Progenitors and NascentDocument12 pagesSall1 Maintains Nephron Progenitors and NascentWilan KrisnaNo ratings yet
- 2012cooper Et Al Wires Development of Bat FlightDocument7 pages2012cooper Et Al Wires Development of Bat Flightapi-237346249No ratings yet
- Principles of DevelopmentDocument9 pagesPrinciples of Developmentsoccerrocksj18No ratings yet
- JHG 201710Document5 pagesJHG 201710maow2No ratings yet
- Hendler 1986Document8 pagesHendler 1986byhz7wwdm4No ratings yet
- Journal of Morphology SpermwarteryDocument14 pagesJournal of Morphology SpermwarteryJustin RamonNo ratings yet
- Baf60mice Takeuchi2007Document7 pagesBaf60mice Takeuchi2007vweakeNo ratings yet
- Zool Lab 4Document17 pagesZool Lab 4pascualfrancejosephNo ratings yet
- Establishment of Vertebrate Left-Right Asymmetry: Hiroshi Hamada, Chikara Meno, Daisuke Watanabe and Yukio SaijohDocument11 pagesEstablishment of Vertebrate Left-Right Asymmetry: Hiroshi Hamada, Chikara Meno, Daisuke Watanabe and Yukio SaijohDianne AcobaNo ratings yet
- Anani Et Al 2014Document12 pagesAnani Et Al 2014nugrahoneyNo ratings yet
- Vanderputte 2011Document8 pagesVanderputte 2011adamNo ratings yet
- Anomalous Development of Thyroid Gland A Cadaveric Study in Coastal Population of Andhra PradeshDocument4 pagesAnomalous Development of Thyroid Gland A Cadaveric Study in Coastal Population of Andhra Pradeshتقوى اللهNo ratings yet
- Description of Reproductive System of Indian Water Scorpion, Laccotrephes Maculatus Fabr. (Hemiptera, Heteroptera: Nepidae)Document13 pagesDescription of Reproductive System of Indian Water Scorpion, Laccotrephes Maculatus Fabr. (Hemiptera, Heteroptera: Nepidae)Kanhiya MahourNo ratings yet
- Tetralogía en Hurón - CASODocument6 pagesTetralogía en Hurón - CASOLAURA AILED LOPEZ LOZANo ratings yet
- The Transient Cortical Zone in The Adrenal Gland: The Mystery of The Adrenal X-ZoneDocument13 pagesThe Transient Cortical Zone in The Adrenal Gland: The Mystery of The Adrenal X-ZoneCandle Jo'inNo ratings yet
- Developmental Evolution: This Side of Paradise: Anthony Graham and Imelda McgonnellDocument3 pagesDevelopmental Evolution: This Side of Paradise: Anthony Graham and Imelda McgonnellAroa ChansNo ratings yet
- Neuro 2Document5 pagesNeuro 2Ivan RisnaNo ratings yet
- TMP AABEDocument2 pagesTMP AABEFrontiersNo ratings yet
- Garces 2007Document6 pagesGarces 2007Peter CordovageNo ratings yet
- Pi Is 1878540914000462Document6 pagesPi Is 1878540914000462aandakuNo ratings yet
- Articulo de EmbrioDocument10 pagesArticulo de EmbrioGabriel Pineda CastroNo ratings yet
- Neuroanatomy of The Zebrafish Brain - A Topological Atlas 1996Document141 pagesNeuroanatomy of The Zebrafish Brain - A Topological Atlas 1996Daniel RuizNo ratings yet
- Splina Functii PDFDocument11 pagesSplina Functii PDFTotolici StefanNo ratings yet
- Stenotic Semilunar Valve in Persistent Truncus ArteriosusDocument6 pagesStenotic Semilunar Valve in Persistent Truncus ArteriosusAlma Grace Pineda TeránNo ratings yet
- Akash Aipmt Final 2011 SolutionDocument18 pagesAkash Aipmt Final 2011 Solutionsaurav guptaNo ratings yet
- Genes Regulating Lymphangiogenesis Control Venous Valve Formation and Maintenance in MiceDocument10 pagesGenes Regulating Lymphangiogenesis Control Venous Valve Formation and Maintenance in MiceGerardo OrtizNo ratings yet
- Ajpendo 00705 2006Document8 pagesAjpendo 00705 2006Fujiko SaavedraNo ratings yet
- 93 Welcome To The Revolution AgendaDocument1 page93 Welcome To The Revolution AgendaJamesComeyJustaBitch0% (2)
- 87-Triune Brain TheoryDocument7 pages87-Triune Brain TheoryJamesComeyJustaBitchNo ratings yet
- 86-Tp Scales TalesDocument19 pages86-Tp Scales TalesJamesComeyJustaBitchNo ratings yet
- The Evolution of The Amygdala: Basic FunctionDocument8 pagesThe Evolution of The Amygdala: Basic FunctionJamesComeyJustaBitchNo ratings yet
- 83-The Triune BrainDocument4 pages83-The Triune BrainJamesComeyJustaBitchNo ratings yet
- Suffixes: Introduction: Suffixes Are Syllables Added To The Ends of Words To Change Their Functions. For ExampleDocument5 pagesSuffixes: Introduction: Suffixes Are Syllables Added To The Ends of Words To Change Their Functions. For ExampleJamesComeyJustaBitchNo ratings yet
- Reptilian All The Way?: LetterDocument1 pageReptilian All The Way?: LetterJamesComeyJustaBitchNo ratings yet
- Avian and Reptilian Evaporative CompartmentalizationDocument6 pagesAvian and Reptilian Evaporative CompartmentalizationJamesComeyJustaBitchNo ratings yet
- 73-Reptilian Abductions in The..Document3 pages73-Reptilian Abductions in The..JamesComeyJustaBitchNo ratings yet
- 67 ParasiteIdentificationDocument1 page67 ParasiteIdentificationJamesComeyJustaBitchNo ratings yet
- 78 Reptillian AggDocument1 page78 Reptillian AggJamesComeyJustaBitchNo ratings yet
- 74-Reptilian Agenda Milky Way Galaxy Catastrophe Year 2370Document5 pages74-Reptilian Agenda Milky Way Galaxy Catastrophe Year 2370JamesComeyJustaBitch100% (1)
- 60 Marshall ArticleDocument7 pages60 Marshall ArticleJamesComeyJustaBitchNo ratings yet
- Environmental Persistence of Amphibian and Reptilian RanavirusesDocument8 pagesEnvironmental Persistence of Amphibian and Reptilian RanavirusesJamesComeyJustaBitchNo ratings yet
- 59 MARS Andrew D Basiago Reptoid Predation Found On Mars 4-12-09Document5 pages59 MARS Andrew D Basiago Reptoid Predation Found On Mars 4-12-09JamesComeyJustaBitchNo ratings yet
- Human Language and Our Reptilian Brain: The Subcortical Bases of Speech, Syntax, and ThoughtDocument20 pagesHuman Language and Our Reptilian Brain: The Subcortical Bases of Speech, Syntax, and ThoughtJamesComeyJustaBitchNo ratings yet
- 031 Bahasa - Innggris - 1Document28 pages031 Bahasa - Innggris - 1Tavip ZulaifahNo ratings yet
- Evolution of Respiratory SystemDocument10 pagesEvolution of Respiratory SystemWwwanand111No ratings yet
- The Animal Kingdom: Living On Planet EarthDocument19 pagesThe Animal Kingdom: Living On Planet EarthSri Latha Ganesh RaoNo ratings yet
- Evolution, Epidemiology and Etiology of Temporomandibular Joint DisordersDocument6 pagesEvolution, Epidemiology and Etiology of Temporomandibular Joint DisordersakNo ratings yet
- E.V.O Search For Eden DetonadoDocument51 pagesE.V.O Search For Eden DetonadoAlan KardecNo ratings yet
- Lola The Animal Helper ScienceDocument27 pagesLola The Animal Helper ScienceCryptoS hitNo ratings yet
- Fossils in Utah Booklet 1Document21 pagesFossils in Utah Booklet 1api-218354277No ratings yet
- Comparative Placentation: GlossaryDocument8 pagesComparative Placentation: GlossaryJosh Soriano IiNo ratings yet
- One Million Things Animal LifeDocument127 pagesOne Million Things Animal Lifesebibv100% (5)
- 2nd Quarter Summative Test No.2Document2 pages2nd Quarter Summative Test No.2Marilou KimayongNo ratings yet
- Declarative Programming ExercisesDocument19 pagesDeclarative Programming ExercisesadaNo ratings yet
- Unit 2 Lesson 8 Super Sea Animals CLIL ScienceDocument29 pagesUnit 2 Lesson 8 Super Sea Animals CLIL Sciencemylyn ortizNo ratings yet
- BSC Zoology Part II ReptiliaDocument5 pagesBSC Zoology Part II Reptiliayasahswi91No ratings yet
- 8-Bit Adventures - Space Bounty Hunters PDFDocument48 pages8-Bit Adventures - Space Bounty Hunters PDFJohn Churchill100% (1)
- Life-History Evolution in ReptilesDocument27 pagesLife-History Evolution in ReptilesPahal AgarwalNo ratings yet
- Classification of AnimalsDocument33 pagesClassification of Animalsrajeshmmahajan100% (1)
- PT - SCIENCE 6 - Q2 - FinalDocument5 pagesPT - SCIENCE 6 - Q2 - FinalIrine BrionesNo ratings yet
- An Updated Checklist of The Amphibians and Reptiles of NicaraguaDocument18 pagesAn Updated Checklist of The Amphibians and Reptiles of NicaraguaRicardo SozaNo ratings yet
- O P Jindal Modern School, Hisar: Worksheet No. 3 Subject-Evs NAME: ................... ROLL NO. . Class 5Document2 pagesO P Jindal Modern School, Hisar: Worksheet No. 3 Subject-Evs NAME: ................... ROLL NO. . Class 5Raghav BansalNo ratings yet
- DETAILED LESSON PLAN Hierachical System ClassificationsDocument22 pagesDETAILED LESSON PLAN Hierachical System ClassificationsMary Rose RamosNo ratings yet
- Natural HistoryDocument664 pagesNatural Historykiradiologija75% (4)
- PTS 2019 2020 40 Nomer SoalDocument6 pagesPTS 2019 2020 40 Nomer SoalRizki HariyantiNo ratings yet
- Unit 2 Exploring The Living World: Multiple-Choice QuestionsDocument18 pagesUnit 2 Exploring The Living World: Multiple-Choice QuestionsVincent NGNo ratings yet
- REPTILESDocument2 pagesREPTILESCharll Dezon Abcede AbaNo ratings yet
- A Detailed Lesson Plan in Mathematic VI g4 and 6Document12 pagesA Detailed Lesson Plan in Mathematic VI g4 and 6Ivy Novero SibuloNo ratings yet
- Tuatara-Climate-Change StudentbDocument5 pagesTuatara-Climate-Change Studentbapi-406290658No ratings yet
- Tugas Bahasa InggrisDocument5 pagesTugas Bahasa InggrisVvyynxNo ratings yet