Professional Documents
Culture Documents
Tutorial 3 - Question 6
Tutorial 3 - Question 6
Uploaded by
DiablofireZAOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tutorial 3 - Question 6
Tutorial 3 - Question 6
Uploaded by
DiablofireZACopyright:
Available Formats
x
2
0.922 = x
2
h
1
h
f133.55degC
h
g133.55degC
h
f133.55degC
:=
Solution:
h
g133.55degC
2725.3
kJ
kg
:= h
f133.55degC
561.47
kJ
kg
:= Point 2:
h
1
2.557 10
3
kJ
kg
= h
1
.9 h
g164.97degC
h
f164.97degC
( )
h
f164.97degC
+ :=
h
g164.97degC
2763.5
kJ
kg
:= h
f164.97degC
697.22
kJ
kg
:= Point 1:
Used Steam Tables in Cengel & Boles:
Thermophysical Properties:
T
2
133.55 degC := p
2
3 bar = p
2
2 1.0 + ( ) bar :=
T
1
164.97 degC := p
1
8 bar = p
1
7 1.0 + ( ) bar :=
To simplify the problem, assume that atmospheric pressure is 1 bar
Given:
kJ 1000 J degC K 273.16 K bar 100 kPa kPa 1000 Pa MPa 1000000 Pa MW 1000000 W
Definitions:
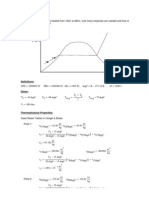
T [oC]
s [kJ/kg.K]
1
2
Steam with a dryness fraction of 0.9 at 7bar(g) passes through a reducing valve to a pressure of 2
bar(g). What is the dryness fraction of the steam downstream of the valve?
Tutorial 3 - Question 6
You might also like
- Steam Power Board ProblemsDocument6 pagesSteam Power Board ProblemsCaguioa Mark Anthony G.100% (5)
- Practice Examples Chapter 7 Thermochemistry Petrucci 10th EdDocument7 pagesPractice Examples Chapter 7 Thermochemistry Petrucci 10th EdHaggai NidarNo ratings yet
- Fokus Deutsch - Episode 01Document11 pagesFokus Deutsch - Episode 01DiablofireZA33% (3)
- ChemLec - Module 4.1 - 4.3Document23 pagesChemLec - Module 4.1 - 4.3Jerick JasperNo ratings yet
- Refrigeration Moran Shapiro Solution ManualDocument10 pagesRefrigeration Moran Shapiro Solution ManualNovaCastilloNo ratings yet
- Meneses, Keziah F.: Cop T T T T T T Where: T T T Cop COP 1.4696 1.47Document19 pagesMeneses, Keziah F.: Cop T T T T T T Where: T T T Cop COP 1.4696 1.47Eriane GarciaNo ratings yet
- Tutorial 3 - Question 7Document1 pageTutorial 3 - Question 7DiablofireZANo ratings yet
- Tutorial 3 - Question 8Document2 pagesTutorial 3 - Question 8DiablofireZANo ratings yet
- Tutorial 3 - Question 5Document1 pageTutorial 3 - Question 5DiablofireZANo ratings yet
- Tutorial 3 - Question 3Document1 pageTutorial 3 - Question 3DiablofireZANo ratings yet
- CH 09Document22 pagesCH 09hirenpatel_universalNo ratings yet
- Thermodynamics, Heat Transfer-Solutions PDFDocument5 pagesThermodynamics, Heat Transfer-Solutions PDFKemalMalovcicNo ratings yet
- LESSON 3.3 Calculating Free EnergyDocument25 pagesLESSON 3.3 Calculating Free EnergyQueenie TalabocNo ratings yet
- Chapter - 08 (RAC by Hipolito)Document6 pagesChapter - 08 (RAC by Hipolito)John Jonel CasupananNo ratings yet
- 15.1 250 KG/H of Air Saturated at 2°C Is Mixed With 50 KG/H of Air at 35°C and 80% RHDocument17 pages15.1 250 KG/H of Air Saturated at 2°C Is Mixed With 50 KG/H of Air at 35°C and 80% RHNathan EscobalNo ratings yet
- CH 10Document34 pagesCH 10hirenpatel_universalNo ratings yet
- Result Assignment ThermoDocument6 pagesResult Assignment ThermoSuraya JohariNo ratings yet
- Chapter 3 - Single-Stage Vapour Compression Refrigeration Cycle PDFDocument46 pagesChapter 3 - Single-Stage Vapour Compression Refrigeration Cycle PDFLộc ĐạiNo ratings yet
- 3 (A) Thermodynamics RelationsDocument6 pages3 (A) Thermodynamics Relationsananda narayananNo ratings yet
- Ian BAB 4Document6 pagesIan BAB 4NURUL SYUHADA BT ISMAIL HAJARNo ratings yet
- Tut 3 - Question 2Document2 pagesTut 3 - Question 2DiablofireZANo ratings yet
- PAC ES, MishaelDocument7 pagesPAC ES, Mishaelyeng botzNo ratings yet
- Thermo HWDocument6 pagesThermo HWMuhammad Fawwad ObaidaNo ratings yet
- Psychometric Properties and ProcessesDocument40 pagesPsychometric Properties and ProcessesUser140035No ratings yet
- Solution Week 9Document6 pagesSolution Week 9Ariadne ChuaNo ratings yet
- CH302 General Chemistry II Homework 3Document7 pagesCH302 General Chemistry II Homework 3Edward SpellingNo ratings yet
- q m C ΔT: SolutionDocument7 pagesq m C ΔT: SolutionMjhay Tanchiatco DavidNo ratings yet
- 15.docx 1 1Document21 pages15.docx 1 1Nathan EscobalNo ratings yet
- Higher Temperature Reservor, T Heat Engine Low Temperature Reservor, TDocument16 pagesHigher Temperature Reservor, T Heat Engine Low Temperature Reservor, THào Văn TríNo ratings yet
- Heat of Formation of SucroseDocument9 pagesHeat of Formation of SucroseLuluaNo ratings yet
- Refriger Ac I OnDocument15 pagesRefriger Ac I OnMiguel Angel MartinezNo ratings yet
- Thermochemistry Problem Set #1: Angelica Avrielle C. Arevalo Bsce 1FDocument6 pagesThermochemistry Problem Set #1: Angelica Avrielle C. Arevalo Bsce 1FAngelica Avrielle C. ArevaloNo ratings yet
- Enthalpy ExercisesDocument5 pagesEnthalpy ExercisesmpumelaqqNo ratings yet
- Tutorium Refrigeration SolutionDocument20 pagesTutorium Refrigeration SolutionwanpudinNo ratings yet
- Enriquez Rovie C. ThermochemistryDocument9 pagesEnriquez Rovie C. ThermochemistryENRIQUEZ, ROVIE C.No ratings yet
- ch14 PDFDocument8 pagesch14 PDFAkash ThummarNo ratings yet
- Chemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Solutions Manual 1Document36 pagesChemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Solutions Manual 1josephandersonxqwbynfjzk100% (29)
- Chemistry The Molecular Nature of Matter and Change 7Th Edition Silberberg Solutions Manual Full Chapter PDFDocument46 pagesChemistry The Molecular Nature of Matter and Change 7Th Edition Silberberg Solutions Manual Full Chapter PDFsusan.robleto221100% (17)
- Basic State Values of Matter: Example 1.1Document27 pagesBasic State Values of Matter: Example 1.1Warren CabunyagNo ratings yet
- Power Plant Design Plan ADocument21 pagesPower Plant Design Plan Akim deygabiNo ratings yet
- Applied Thermodynamics D201 Self Assessment Solutions Tutorial 5 Self Assessment Exercise No. 1Document4 pagesApplied Thermodynamics D201 Self Assessment Solutions Tutorial 5 Self Assessment Exercise No. 1Alexander MugabeNo ratings yet
- CH301 ThermoUnit Activity QuantifyingHeatGroupProblems KEYDocument2 pagesCH301 ThermoUnit Activity QuantifyingHeatGroupProblems KEYYen PradoNo ratings yet
- HVAC Assignment 1 Solutions (Updated)Document11 pagesHVAC Assignment 1 Solutions (Updated)Aleena Amin KhuwajaNo ratings yet
- Evaporative Cooling: David Edward Jason Ksatrio ReinhartDocument28 pagesEvaporative Cooling: David Edward Jason Ksatrio ReinhartEdward EdbergNo ratings yet
- MEC 4105 Test Marking GuideDocument4 pagesMEC 4105 Test Marking GuideBirimumaso DavidNo ratings yet
- Chapter 02Document14 pagesChapter 02stephen jamesNo ratings yet
- ch13 PDFDocument6 pagesch13 PDFAkash ThummarNo ratings yet
- Lunes, Herzon S.Document7 pagesLunes, Herzon S.yeng botzNo ratings yet
- Thermodynamics Chapter 3 Solution Sta Maria PDFDocument7 pagesThermodynamics Chapter 3 Solution Sta Maria PDFZandie Garcia75% (4)
- Section e To Be Solved Set 1Document14 pagesSection e To Be Solved Set 1Neil SequioNo ratings yet
- Chapter 5 Review SolutionDocument7 pagesChapter 5 Review SolutionSFDLSFHIOANo ratings yet
- Solutions To Homework Assignment #1 CHM 152 Spring 2002: F D 2 F D 2 F D 2Document4 pagesSolutions To Homework Assignment #1 CHM 152 Spring 2002: F D 2 F D 2 F D 2josegpaNo ratings yet
- Methane:: Δ ˙H +Δ ˙E Δ ˙E Q− ˙WDocument3 pagesMethane:: Δ ˙H +Δ ˙E Δ ˙E Q− ˙WchemicalgeeksNo ratings yet
- Thermodynamics 1 by Sta. Maria Chapter 3 Solution ManualDocument7 pagesThermodynamics 1 by Sta. Maria Chapter 3 Solution ManualAllen MalabarbasNo ratings yet
- Hess LawDocument24 pagesHess Lawactive learning educationNo ratings yet
- Chapter 17 - Rev PDFDocument13 pagesChapter 17 - Rev PDFalaa al sahmaraniNo ratings yet
- Thermodynamics ProbDocument7 pagesThermodynamics ProbJan Mae Beja AdolfoNo ratings yet
- Chapter 3Document16 pagesChapter 3Laurence Lee AdventoNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Fokus Deutsch - Series 104Document4 pagesFokus Deutsch - Series 104DiablofireZA100% (2)
- Tutorial 3 - Question 5Document1 pageTutorial 3 - Question 5DiablofireZANo ratings yet
- Tutorial 3 - Question 7Document1 pageTutorial 3 - Question 7DiablofireZANo ratings yet
- Renewable Energies 2Document29 pagesRenewable Energies 2DiablofireZANo ratings yet
- Tutorial 3 - Question 8Document2 pagesTutorial 3 - Question 8DiablofireZANo ratings yet
- Jacketed Pan: Tutorial 3 - Question 4Document1 pageJacketed Pan: Tutorial 3 - Question 4DiablofireZANo ratings yet
- Tut 3 - Question 2Document2 pagesTut 3 - Question 2DiablofireZANo ratings yet
- Tutorial 3 - Question 3Document1 pageTutorial 3 - Question 3DiablofireZANo ratings yet
- Energiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!Document1 pageEnergiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!DiablofireZANo ratings yet
- Energiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!Document1 pageEnergiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!DiablofireZANo ratings yet
- Tut 3Document5 pagesTut 3DiablofireZANo ratings yet