Professional Documents
Culture Documents
Graphs From Lab
Graphs From Lab
Uploaded by
Mohammed Elsaadi0 ratings0% found this document useful (0 votes)
7 views4 pagesgraphs from labwork
Original Title
graphs from lab
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentgraphs from labwork
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
7 views4 pagesGraphs From Lab
Graphs From Lab
Uploaded by
Mohammed Elsaadigraphs from labwork
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 4
Results and Calculations:
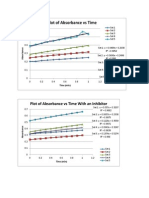
Figure 1: Absorbance vs Time(min) for no inhibitor data sets:
Table 1: Equations of lines of best fit:
Data Set: Equation of line of best fit
1 Set 1: y = 0.0489x + 0.2038 R = 0.9852
2 Set 2: y = 0.0698x + 0.2496 R = 0.998
3 Set 3: y = 0.1062x + 0.291 R = 0.9961
4 Set 4: y = 0.1548x + 0.3903 R = 0.9874
5 Set 5: y = 0.1509x + 0.391 R = 0.9408
Table 2: Rates of Reactions:
0
0.1
0.2
0.3
0.4
0.5
0.6
0 0.2 0.4 0.6 0.8 1 1.2
A
b
s
o
r
b
a
n
c
e
Time (min)
Absorbance vs Time (No Inhibitor)
Set 1
Set 2
Set 3
Set 4
Set 5
Linear (Set 1)
Linear (Set 2)
Linear (Set 3)
Linear (Set 4)
Linear (Set 5)
Data Set Rate of Reaction
(A/min)
1 0.0489
2 0.0698
3 0.1062
4 0.1548
5 0.1509
Figure 2: Plot of Absorbance vs Time(min) for data sets with inhibitor:
Table 3: Equations of lines of best fit:
Data sets: Equations of lines:
1 y = 0.057x + 0.3207 R = 0.9882
2 y = 0.0657x + 0.2338 R = 0.9975
3 y = 0.1128x + 0.3559 R = 0.9971
4 y = 0.0943x + 0.3247 R = 0.9839
5 y = 0.1374x + 0.5274 R = 0.998
Table 4: Rates of Reactions:
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0 0.2 0.4 0.6 0.8 1 1.2
A
b
s
o
r
b
a
n
c
e
Time (min)
Absorbance vs Time (with inhibitor)
Set 1
Set 2
Set 3
Set 4
Set 5
Linear (Set 1)
Linear (Set 2 )
Linear (Set 3)
Linear (Set 4)
Linear (Set 5)
Data Sets Rate of Reaction
(A/min)
1 0.057
2 0.0657
3 0.1128
4 0.0943
5 0.1374
Table 5: Velocities of the data sets and the reciprocal velocities:
Data Sets: V
o
(mM/min) 1/V
o
(min/mM)
No
inhibitor
1 0.01358 73.64
2 0.01940 51.55
3 0.0295 33.90
4 0.043 23.26
5 0.0419 23.87
With
Inhibitor
1 0.0158 63.29
2 0.0183 54.64
3 0.031 32.26
4 0.0262 38.17
5 0.0382 26.18
Sample calculation: Converting A/min to V
o
:
Using Beer's law: A =cl where A = A/min, =3600 L/mol cm, l = 1cm, x 1000mM/1M to convert units
Using Set 1 of no inhibitor:
V
o
= c = A/l = [(0.0489 min
-1
)/(3600 L/mol cm)(1 cm)] x (1000mM/1M)
V
o
= 0.01358 mM/min
Table 6: Substrate Calculations and the reciprocal Concentrations:
Data Sets: Substrate Concentration [S] (mM) Reciprocal Substrate Concentration 1/[S]
(mM
-1
)
No
inhibitor
1 0.8 1.25
2 1.6 0.625
3 2.4 0.417
4 3.2 0.3125
5 4.0 0.25
With
Inhibitor
1 0.8 1.25
2 1.6 0.625
3 2.4 0.417
4 3.2 0.3125
5 4.0 0.25
Sample Calculations: Calculating Substrate Concentrations [S]:
Using C
1
V
1
=C
2
V
2
where C
1
= 8 mM, V
1
= 100L, V
2
= 1000L
Solving for C
2
: C
2
= C
1
V
1
/V
2
= (8)(100)/1000 = 0.8 mM
Figure 3: Michaelis-Menten Plot of Tyrosinase-Catalyzed Reactions:
Figure 4: Lineweaver-Burk plot of Tyrinosinase-Catalyzed Reactions:
Without Inhibitor:
y = 0.01x + 0.0054
R = 0.9311
With Inhibitor:
y = 0.0066x + 0.0101
R = 0.8237
0
0.01
0.02
0.03
0.04
0.05
0 1 2 3 4 5
V
o
(
m
M
/
m
i
n
)
Substrate Concentration [S] (mM)
Michaelis-Menten Plot of
Tyrosinase-Catalyzed Reactions
No Inhibitor
with Inhibitor
Without Inhibitor:
y = 51.572x + 11.802
R = 0.9531
With Inhibitor:
y = 34.534x + 23.193
R = 0.8095
0
10
20
30
40
50
60
70
80
90
0 0.5 1 1.5
1
/
V
o
(
m
i
n
/
m
M
)
Recipricol of Substrate Concentration 1/[S] (mM
-1
)
Lineweaver-Burk Plot of
Tyrosinase-Catalyzed Reactions
No Inhibitor
with Inhibitor
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Tle - Food Processing 8: Division of City of San FernandoDocument43 pagesTle - Food Processing 8: Division of City of San FernandoJoanne Manlises100% (7)
- Glycogen QuizDocument2 pagesGlycogen QuizMohammed Elsaadi100% (1)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Ewm Production IntegrationDocument78 pagesEwm Production IntegrationDipak BanerjeeNo ratings yet
- 500 1000 1500 2000 2500 3000 3500 Wavenumber cm-1: Page 1/1Document1 page500 1000 1500 2000 2500 3000 3500 Wavenumber cm-1: Page 1/1Mohammed ElsaadiNo ratings yet
- Plot of Absorbance Vs Time With An InhibitorDocument2 pagesPlot of Absorbance Vs Time With An InhibitorMohammed ElsaadiNo ratings yet
- Standard Plot of Absorbance Vs TimeDocument1 pageStandard Plot of Absorbance Vs TimeMohammed ElsaadiNo ratings yet
- Docusign: Customer Success Case Study SamplerDocument7 pagesDocusign: Customer Success Case Study Samplerozge100% (1)
- 865-RUGC Manuscript-2653-1-10-20200306Document4 pages865-RUGC Manuscript-2653-1-10-20200306Bal Deep SharmaNo ratings yet
- Fuel EnergizerDocument29 pagesFuel EnergizeratulsemiloNo ratings yet
- Density Estimation From The Sonic Log: A Case Study Sl2.3: James P. Disiena and Fred J. HiltermanDocument4 pagesDensity Estimation From The Sonic Log: A Case Study Sl2.3: James P. Disiena and Fred J. Hiltermanlulalala8888No ratings yet
- 300 Compact/1233: ManualDocument22 pages300 Compact/1233: ManualbogdanNo ratings yet
- DX DiagDocument35 pagesDX Diagdiddyaw150No ratings yet
- Superline & Implantium: Surgical / Prosthesis ManualDocument51 pagesSuperline & Implantium: Surgical / Prosthesis Manualk4ssdcNo ratings yet
- SG250HX User+Manual V15 20201106Document78 pagesSG250HX User+Manual V15 20201106specopbookieNo ratings yet
- First Conditional ChainDocument3 pagesFirst Conditional ChainSonia DivileNo ratings yet
- Metaphors and Similes Lesson PlanDocument4 pagesMetaphors and Similes Lesson Planapi-242439128No ratings yet
- Chapter 1 Transaction Management and Concurrency Control Lec 1 andDocument68 pagesChapter 1 Transaction Management and Concurrency Control Lec 1 andFiromsa DineNo ratings yet
- Element Main - JsDocument51 pagesElement Main - JsjaaritNo ratings yet
- Model 200-30A200P-31-21A Solenoid ValveDocument4 pagesModel 200-30A200P-31-21A Solenoid Valveemuno008No ratings yet
- Design of A Dumping GrateDocument13 pagesDesign of A Dumping GrateABDULLAH MAQBOOL100% (1)
- The Roots of Modern Feminism: Mary Wollstonecraft and The French RevolutionDocument16 pagesThe Roots of Modern Feminism: Mary Wollstonecraft and The French RevolutionShirlya LimaNo ratings yet
- Assignment 1Document4 pagesAssignment 1Nada AbbadyNo ratings yet
- What Is StrategyDocument21 pagesWhat Is StrategyPuttyErwinaNo ratings yet
- Pca. Inglés 6toDocument15 pagesPca. Inglés 6toJuan Carlos Amores GuevaraNo ratings yet
- Bockman, Johanna (2013) NeoliberalismDocument3 pagesBockman, Johanna (2013) NeoliberalismJosh VeigaNo ratings yet
- LVL 0 - Practical - 2 Contouring Cut and Fill For EarthworksDocument4 pagesLVL 0 - Practical - 2 Contouring Cut and Fill For Earthworksnuraina aqilahNo ratings yet
- A Robust Optimal Zero-Watermarking Technique For Secret Watermark SharingDocument12 pagesA Robust Optimal Zero-Watermarking Technique For Secret Watermark SharingzaidsaebNo ratings yet
- Ship Maintenance Planning With NavCadDocument4 pagesShip Maintenance Planning With NavCadtheleepiper8830No ratings yet
- MKT 005 Module 6 SASDocument5 pagesMKT 005 Module 6 SASconandetic123No ratings yet
- Komatsu Views 2014 No.31 (Ing)Document3 pagesKomatsu Views 2014 No.31 (Ing)Carlos Alfredo LauraNo ratings yet
- Korean WaveDocument12 pagesKorean WaveJm PogiNo ratings yet
- Bar Bending Schedule (Service BLDG)Document20 pagesBar Bending Schedule (Service BLDG)Rania SaiedNo ratings yet
- CJR FILSAFAT Sem 1Document17 pagesCJR FILSAFAT Sem 1Jesica RaivitaNo ratings yet
- Gyroscopic PrinciplesDocument38 pagesGyroscopic PrinciplesAli Abu Shhiwa100% (1)