Professional Documents
Culture Documents
Chemistry Unit 4 Equations
Chemistry Unit 4 Equations
Uploaded by
AnjumJahan0 ratings0% found this document useful (0 votes)
171 views2 pagesThe document summarizes several chemical equations relating to carboxylic acids and derivatives:
1) It describes equations for the synthesis of hydroxynitriles from hydrogen cyanide and carbonyls, and carboxylic acids from aldehydes and dichromate ions with heat.
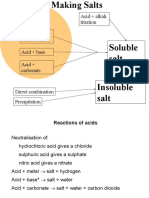
2) Equations are provided for forming salts and water from reactions of carboxylic acids with alkalis or carbonates.
3) Additional equations cover forming acyl chlorides from carboxylic acids and phosphorus chloride, esters from carboxylic acids and alcohols with acid catalysts and heat, and derivatives from reactions of esters, acyl chlorides, and amines.
Original Description:
Notes on edexcel chem 4 A2
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document summarizes several chemical equations relating to carboxylic acids and derivatives:
1) It describes equations for the synthesis of hydroxynitriles from hydrogen cyanide and carbonyls, and carboxylic acids from aldehydes and dichromate ions with heat.
2) Equations are provided for forming salts and water from reactions of carboxylic acids with alkalis or carbonates.
3) Additional equations cover forming acyl chlorides from carboxylic acids and phosphorus chloride, esters from carboxylic acids and alcohols with acid catalysts and heat, and derivatives from reactions of esters, acyl chlorides, and amines.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
171 views2 pagesChemistry Unit 4 Equations
Chemistry Unit 4 Equations
Uploaded by
AnjumJahanThe document summarizes several chemical equations relating to carboxylic acids and derivatives:
1) It describes equations for the synthesis of hydroxynitriles from hydrogen cyanide and carbonyls, and carboxylic acids from aldehydes and dichromate ions with heat.
2) Equations are provided for forming salts and water from reactions of carboxylic acids with alkalis or carbonates.
3) Additional equations cover forming acyl chlorides from carboxylic acids and phosphorus chloride, esters from carboxylic acids and alcohols with acid catalysts and heat, and derivatives from reactions of esters, acyl chlorides, and amines.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
Chemistry Unit 4 Equations
Hydrogen Cyanide + Carbonyls ---- Hydroxynitriles
Aldehyde + Acidified dichromate (VI) ions ---- Carboxylic acid (Heat &
reflux)
Carboxylic acids + Aq. Alkali ---- Salt + H20
CH3COOH + NaOH ---- CH3COONa + H20
Carboxylic acids + Carbonates ---- Salt + Water + CO2
2CH3COOH (aq) + NA2C03 (s) ---- 2CH3COONa(aq) + H20 (l) + CO2 (g)
CH3COOH (aq) + NaHCO3 (s) ---- CH3COONa (aq) + H20 (l) + C02 (g)
Phosphorus (V) Chloride + Carboxylic acid ---- Acyl chloride + POCl3 + HCl
Carboxylic acid + Alcohol <<----- Ester + Water (Acid catalyst & heat)
(Esterification)
Ethanoic acid + Ethanol <<------ Ethyl ethanoate + H2O
Ester + Dilute alkali <<----- Carboxylate ion + Alcohol (Reflux)
Ethyl ethanoate + OH
-
<<---- Ethanoate + Ethanol
Ester + Water <<---- Acid + Alcohol (Dilute acid & reflux)
Ethyl ethanoate + H2O <<---- Ethanoic acid + Ethanol
Acyl chlorides + Water ---- Carboxylic acid + Hydrogen chloride (Cold
water)
Ethanoyl chloride + H2O ---- Ethanoic acid + Hydrogen chloride
Acyl chlorides + Alcohols ---- Ester + Hydrogen Chloride (Reflux)
Ethanoyl chloride + Methanol ---- Methyl ethanoate + HCl
Acyl chlorides + Ammonia ---- Amide + Hydrogen chloride (r.t.p)
Ethanoyl chloride + Ammonia ---- Ethanamide + HCl
Acyl chlorides + Amines ---- N-substituted amide + Hydrogen chloride
Ethanoyl chloride + Amine ---- N-methylethanamide + HCl
You might also like
- Common Chemical Formula ListDocument3 pagesCommon Chemical Formula Listaran9286% (7)
- Chemistry HSC FormulasDocument6 pagesChemistry HSC Formulashpgc101100% (1)
- Chem RevisionDocument8 pagesChem RevisionclinquantNo ratings yet
- Heat of Solution Data PDFDocument2 pagesHeat of Solution Data PDFdodofan2000No ratings yet
- Eat of Solution Data For Aqueous SolutionsDocument2 pagesEat of Solution Data For Aqueous SolutionsJúlio Gabriel Queiroz dos SantosNo ratings yet
- Acid and Bases Notes 1Document5 pagesAcid and Bases Notes 1ignacy.jaroszewicz1No ratings yet
- CARBON COMPOUND (Asid Carboxylic)Document24 pagesCARBON COMPOUND (Asid Carboxylic)Shirley SimonNo ratings yet
- AlcaniDocument3 pagesAlcanibroscutzatNo ratings yet
- CALCIUMDocument2 pagesCALCIUMAshleyn Mary SandersNo ratings yet
- notesG11Week1 1Document24 pagesnotesG11Week1 1Damonte HenryNo ratings yet
- Reaksi Pemisahan KationDocument2 pagesReaksi Pemisahan KationAnisa Nursella TrunodikromoNo ratings yet
- 7 Unit - 2 - Mod - 1 - Carboxylic - Acids - and - Derivatives PDFDocument8 pages7 Unit - 2 - Mod - 1 - Carboxylic - Acids - and - Derivatives PDFKrisNo ratings yet
- Week 7 (B) ChemistryDocument3 pagesWeek 7 (B) ChemistryBeyonce SkekelNo ratings yet
- Reaction List v002Document5 pagesReaction List v002cecil3414No ratings yet
- Summary Buffer SolutionDocument3 pagesSummary Buffer Solutionelcha_putraNo ratings yet
- Acid Base Conjugate SDocument1 pageAcid Base Conjugate SNick DonatelliNo ratings yet
- Properties of Carboxylic AcidsDocument1 pageProperties of Carboxylic AcidsOrevelNo ratings yet
- Alcohols and Carboxylic AcidsDocument22 pagesAlcohols and Carboxylic AcidsJordan BelfortNo ratings yet
- Carboxylic AcidDocument33 pagesCarboxylic AcidRika Yulliyani RNo ratings yet
- 17 Strength of Acids and Alkalis Teacher PDFDocument17 pages17 Strength of Acids and Alkalis Teacher PDFVanessa LeungNo ratings yet
- 6 AcidDocument22 pages6 AcidhaslimiNo ratings yet
- Salts and AcidsDocument3 pagesSalts and AcidskmbgtssnbmNo ratings yet
- Alcohol, Phenol & EtherDocument23 pagesAlcohol, Phenol & EtherRahul PrajapatiNo ratings yet
- All As Chem ReactionsDocument11 pagesAll As Chem Reactionsklo9d ninjaNo ratings yet
- Elements and Compounds g8Document5 pagesElements and Compounds g8Fahad RashidNo ratings yet
- Ald&KetoneDocument41 pagesAld&KetoneFeng SpencerNo ratings yet
- Trong 36 Ke Chay La Thuong SachDocument1 pageTrong 36 Ke Chay La Thuong Sachnippasuppa7No ratings yet
- Ald&Ketone IDocument41 pagesAld&Ketone IreinitavanyNo ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Year 9Document6 pagesYear 9f78mdnrds9No ratings yet
- Carbonyl Compounds: Carboxylic Acids & EsterDocument28 pagesCarbonyl Compounds: Carboxylic Acids & Esterrustam effendyNo ratings yet
- 21 Organic ReactionsDocument3 pages21 Organic ReactionsAlliah Jasmine AlcalaNo ratings yet
- ChemistryDocument3 pagesChemistrypearlynpuayNo ratings yet
- Revision of C6 Chemical SynthesisDocument55 pagesRevision of C6 Chemical Synthesisagustina simorangkirNo ratings yet
- Aliphatic XIIDocument45 pagesAliphatic XIISUYOG K.C.No ratings yet
- AS Chemistry Organic An Inorganic ReactionsDocument14 pagesAS Chemistry Organic An Inorganic Reactionsdenithmayon1No ratings yet
- Carboxylic Acids and Their Derivatives NewDocument18 pagesCarboxylic Acids and Their Derivatives Newxinying94No ratings yet
- Chemical Properties of Carbon CompoundsDocument7 pagesChemical Properties of Carbon CompoundsAnonymous HLkNDToNo ratings yet
- Ald&ketone IDocument41 pagesAld&ketone Iasney2512No ratings yet
- Acids, Bases and Salts AKHS 2020 - Complete NotesDocument27 pagesAcids, Bases and Salts AKHS 2020 - Complete NotesKim SewoonNo ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidAbir DuttaNo ratings yet
- Acids and BasesDocument97 pagesAcids and Basesapi-683027695No ratings yet
- Chapter 3Document50 pagesChapter 3Fatin Aisyah SulaimanNo ratings yet
- Carbonyl Compounds 230Document60 pagesCarbonyl Compounds 230mohtasim hasanNo ratings yet
- NeutralizationDocument6 pagesNeutralizationbrahmhoNo ratings yet
- Acids: Common Acids in Daily LifeDocument47 pagesAcids: Common Acids in Daily LifeElanIe InspiritNo ratings yet
- Chemical Equation WorksheetDocument3 pagesChemical Equation WorksheetRichardoBrandonNo ratings yet
- Tugas Kimia Hidrolisis GaramDocument4 pagesTugas Kimia Hidrolisis GaramWelsya CahyaniNo ratings yet
- Chemical EquationsDocument10 pagesChemical Equationsme-elormNo ratings yet
- Writing and Balancing Chemical EquationsDocument2 pagesWriting and Balancing Chemical EquationsZainNo ratings yet
- 8A Salts - AnswerDocument14 pages8A Salts - AnswerFrankieNgNo ratings yet
- Bibliography Books: Telescope. Retrieved 8 March 2011Document6 pagesBibliography Books: Telescope. Retrieved 8 March 2011Ariel MermyNo ratings yet
- R-Cooh, R-Co H,: À Ant À VinegarDocument43 pagesR-Cooh, R-Co H,: À Ant À VinegarArvin MarasiganNo ratings yet
- Chemical EquationDocument2 pagesChemical EquationChris McLeanNo ratings yet
- Reducing Reagents Oxidizing AgentsDocument3 pagesReducing Reagents Oxidizing AgentsCamha NguyenNo ratings yet
- 6 Carboxilic Acid and EsterDocument27 pages6 Carboxilic Acid and EsterIna FadhlinaNo ratings yet
- Carboxylic AcidDocument21 pagesCarboxylic AcidShalsabila NHNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)