Professional Documents
Culture Documents
Liquid
Liquid
Uploaded by
AditholeCopyright:
Available Formats
You might also like
- Introduction To Robotics Mechanics and Control 4th Edition Craig Solutions ManualDocument3 pagesIntroduction To Robotics Mechanics and Control 4th Edition Craig Solutions Manuala3905733700% (5)
- Fluid Friction in PipesDocument15 pagesFluid Friction in PipesMradul Yadav0% (1)
- CE 5014 Design of Selected StrsDocument65 pagesCE 5014 Design of Selected StrsTAMIL100% (1)
- A7 B7 Locomotive Brake Equipment TestDocument14 pagesA7 B7 Locomotive Brake Equipment TestFrancesca Parrino100% (1)
- Trident 2 PowerscreenDocument9 pagesTrident 2 PowerscreenRomuald PogorzelczykNo ratings yet
- 1.fluid Mechanics IntroductionDocument6 pages1.fluid Mechanics IntroductionJohn Lester PaltepNo ratings yet
- Matter in The Liquid Phase1Document6 pagesMatter in The Liquid Phase1Angel Rose NarcenaNo ratings yet
- ICE - Unit 3 - RECDocument17 pagesICE - Unit 3 - RECspidey dudeNo ratings yet
- Fluids For Hydraulic SystemsDocument16 pagesFluids For Hydraulic SystemsAimee SpearsNo ratings yet
- Hydraulics System PDFDocument177 pagesHydraulics System PDFsushantreshmaNo ratings yet
- Module 1 - Flow and Fluid PropertiesDocument5 pagesModule 1 - Flow and Fluid Propertieskdnji123No ratings yet
- Fluid Mechanics and Machinery NotesDocument137 pagesFluid Mechanics and Machinery NotesapsrtsNo ratings yet
- 7Document42 pages7bilalNo ratings yet
- Fluid MechanichDocument5 pagesFluid Mechanichasishraj2003No ratings yet
- Fluid 2 Project 1Document11 pagesFluid 2 Project 1Sanjay RagupathyNo ratings yet
- Visco CityDocument10 pagesVisco CityTanjila HasanNo ratings yet
- Properties of FluidDocument7 pagesProperties of FluidAnonymous QM0NLqZONo ratings yet
- Hydraulic SystemDocument20 pagesHydraulic SystemCECIL JOSEF C. LIGANNo ratings yet
- Abe 106 - 02Document6 pagesAbe 106 - 02emmanuelNo ratings yet
- Qtheory VuitDocument8 pagesQtheory VuitCHANDRASEKAR RNo ratings yet
- FM BesDocument91 pagesFM Bessce civilNo ratings yet
- Liquid: Jump To Navigationjump To SearchDocument12 pagesLiquid: Jump To Navigationjump To SearchLaura Maria GalanNo ratings yet
- Fluid FlowDocument8 pagesFluid Flowking khanNo ratings yet
- Fluid MechanicsDocument25 pagesFluid MechanicsDominic GograNo ratings yet
- Lec 1 Properties of Fluids MechDocument35 pagesLec 1 Properties of Fluids MechJJG ABVNo ratings yet
- Workfile 2Document9 pagesWorkfile 2MISNo ratings yet
- 6 Fluid MechanicsDocument14 pages6 Fluid MechanicsbalajigandhirajanNo ratings yet
- Introduction To Fluid MechanicsDocument85 pagesIntroduction To Fluid MechanicsKaul PatrickNo ratings yet
- Remove Materials ThesisDocument12 pagesRemove Materials ThesisSameera RajpoutNo ratings yet
- Fluid Mechanics IntroductionDocument4 pagesFluid Mechanics IntroductionAvinash GarikapatiNo ratings yet
- Properties of Liquids and Intermolecular Forces PDFDocument15 pagesProperties of Liquids and Intermolecular Forces PDFpie100% (1)
- Lab 9 - Fluid Properties (Viscosity)Document22 pagesLab 9 - Fluid Properties (Viscosity)Amanda AmaniNo ratings yet
- ME080 Section 1 - Hydraulic System BasicsDocument31 pagesME080 Section 1 - Hydraulic System BasicsAhmed FaragNo ratings yet
- Fluid Dynamics: (Relative Density)Document8 pagesFluid Dynamics: (Relative Density)Aidilmann AshrafeiderNo ratings yet
- Fluid Mechanics Basics 1Document12 pagesFluid Mechanics Basics 1Hashmi AshmalNo ratings yet
- Chapter1-Fluid MechanicsDocument84 pagesChapter1-Fluid MechanicsSrujan Chowdary100% (1)
- Properties of Water and Intermolecular ForcesDocument34 pagesProperties of Water and Intermolecular Forcesjoshua gollosoNo ratings yet
- 001 l#1 Intro To FluidmechanicsDocument76 pages001 l#1 Intro To FluidmechanicsEng Faysal AhmedNo ratings yet
- FM Unit1 3semDocument19 pagesFM Unit1 3semIan PrayarNo ratings yet
- Fluid Mechanics: School of Mechanical and Industrial EngineeringDocument57 pagesFluid Mechanics: School of Mechanical and Industrial EngineeringAbel YohannesNo ratings yet
- Fluid Mechanics: Mechanical Engineering DepartmentDocument85 pagesFluid Mechanics: Mechanical Engineering DepartmentVictoria ObulorNo ratings yet
- Note Mce 112 - 1Document24 pagesNote Mce 112 - 1Abraham OmomhenleNo ratings yet
- Compressible VS Incompressible FluidsDocument2 pagesCompressible VS Incompressible FluidsAbdulhamid DaudaNo ratings yet
- Hydraulic Fluids 1Document2 pagesHydraulic Fluids 1Andres Felipe Ospino CatañoNo ratings yet
- Fluid Mechanics Unit1Document100 pagesFluid Mechanics Unit1anjuNo ratings yet
- Ice 5Document9 pagesIce 5Gabriel CortesNo ratings yet
- Roy Preparation of Ink ChemistryDocument13 pagesRoy Preparation of Ink ChemistryMeyy SNo ratings yet
- Rheology: Newtonian FluidsDocument4 pagesRheology: Newtonian FluidsarabbbbbbbkNo ratings yet
- Fluid Mechanics I CE-407: Basic Concepts, Properties of Fluid and Fluid StaticsDocument26 pagesFluid Mechanics I CE-407: Basic Concepts, Properties of Fluid and Fluid Staticstaha zafarNo ratings yet
- Fluid Properties LabDocument18 pagesFluid Properties LabsilasNo ratings yet
- Mechanics of Fluids: Introduction To Fluid MechanicsDocument46 pagesMechanics of Fluids: Introduction To Fluid Mechanicsearl pannilaNo ratings yet
- Topic 1 - Fluid PropertiesDocument40 pagesTopic 1 - Fluid PropertiesNazhan HaziqNo ratings yet
- Water Resources: Dr. Hiba A. AbbasDocument9 pagesWater Resources: Dr. Hiba A. AbbasMuhammad SadiqNo ratings yet
- Fluid Mechanics. Chapter 1. Introduction To Fluid MechanicsDocument8 pagesFluid Mechanics. Chapter 1. Introduction To Fluid MechanicsCHRISTIAN JAY TESNADONo ratings yet
- Covenant GEC 223 Lecture Note 1 Week 2-MoodleDocument53 pagesCovenant GEC 223 Lecture Note 1 Week 2-MoodleAdeolu-Idowu AbiolaNo ratings yet
- Chapter 1 - Physical Properties of LiquidDocument21 pagesChapter 1 - Physical Properties of LiquidGSaurav DahalNo ratings yet
- Science Exam Study NotesDocument9 pagesScience Exam Study Notescainicole22No ratings yet
- Rhe OlogyDocument3 pagesRhe OlogyAldiDamoraNo ratings yet
- Power-Law FluidDocument4 pagesPower-Law FluidaryanNo ratings yet
- Fluid Mechanics ApplicationsDocument29 pagesFluid Mechanics ApplicationsHASSAN ARSHADNo ratings yet
- Week 4 (Lecture 1)Document28 pagesWeek 4 (Lecture 1)muhammadtalhabilal6666No ratings yet
- Fluid Mechanics GEC 223 Lecture NoteDocument53 pagesFluid Mechanics GEC 223 Lecture NoteCHIBUIKE UDENTANo ratings yet
- Liquid Drops and Globules, Their Formation and Movements: Three lectures delivered to popular audiencesFrom EverandLiquid Drops and Globules, Their Formation and Movements: Three lectures delivered to popular audiencesNo ratings yet
- Datasheet Flygt 2201 012 HT-MT 60hz En-UsDocument2 pagesDatasheet Flygt 2201 012 HT-MT 60hz En-UsCristhian Vilca SilvestreNo ratings yet
- PneumaticsPneumatics, Air Conditioning, & PressurizationDocument9 pagesPneumaticsPneumatics, Air Conditioning, & PressurizationAnastasios Pavlou100% (3)
- VRCA Relief ValveDocument8 pagesVRCA Relief Valvenenad135No ratings yet
- Analysis and Optimal Design of Wind Boosters For Vertical Axis Wind PDFDocument10 pagesAnalysis and Optimal Design of Wind Boosters For Vertical Axis Wind PDFulil mohamad albabNo ratings yet
- Rigid Pavement DesignDocument11 pagesRigid Pavement DesignAjit P. SinghNo ratings yet
- Sistema Inyección CR BoschDocument8 pagesSistema Inyección CR BoschRodolfo Leiva100% (1)
- Wrinklebends andDocument179 pagesWrinklebends androdholfhoNo ratings yet
- Induction Motor AnalysisDocument123 pagesInduction Motor AnalysisatmegamicroNo ratings yet
- SicemoursestudyguideDocument12 pagesSicemoursestudyguideZakariya AbdiNo ratings yet
- Pages From Switched Reluctance Motor PDFDocument10 pagesPages From Switched Reluctance Motor PDFHAMID SULIAMANNo ratings yet
- Dynamic Analysis of An Overhead Transmission Line Subject To Gusty Wind Loading Predicted by Wind-Conductor InteractionDocument10 pagesDynamic Analysis of An Overhead Transmission Line Subject To Gusty Wind Loading Predicted by Wind-Conductor Interactionsara fernandesNo ratings yet
- Re Lease: D TC: ".) - Istributio0N 0F This 0UNDocument62 pagesRe Lease: D TC: ".) - Istributio0N 0F This 0UNJennifer Small100% (1)
- Elearn - Ecu Pins PDFDocument6 pagesElearn - Ecu Pins PDFJavierPariNo ratings yet
- Water Meter Guidelines & SpecificationsDocument19 pagesWater Meter Guidelines & SpecificationsSrujan GuptaNo ratings yet
- Sci 4N2 S PDFDocument3 pagesSci 4N2 S PDFArchie PérezNo ratings yet
- Demech Antistick TDSDocument4 pagesDemech Antistick TDSAtharva UlangwarNo ratings yet
- Appendix A Definitions of TermsDocument7 pagesAppendix A Definitions of TermsSantiago SeveinNo ratings yet
- Lec 4 - Properties of Natural Gas-IDocument26 pagesLec 4 - Properties of Natural Gas-IVishnu CnairNo ratings yet
- JGERE 2020-7-2 PG 96-102Document7 pagesJGERE 2020-7-2 PG 96-102SeasonNo ratings yet
- BSI-British Standard ValvesDocument3 pagesBSI-British Standard ValvesVincent RajNo ratings yet
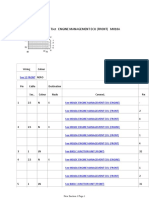
- MTU20 V 4000 G43, 20 V 4000 G83L, 20 V 4000 G63, 20 V 4000 G63L, 20 V 4000 G83, 20 4000 G23 Operating Instructions ManualDocument181 pagesMTU20 V 4000 G43, 20 V 4000 G83L, 20 V 4000 G63, 20 V 4000 G63L, 20 V 4000 G83, 20 4000 G23 Operating Instructions ManualAlexNo ratings yet
- Manual tm-10098 Rev 05-27-08Document203 pagesManual tm-10098 Rev 05-27-08Alhgasjsghjagjsdajlsd Asdalsdlkaksd100% (1)
- LEAP Component Maintenance ManualsDocument12 pagesLEAP Component Maintenance ManualsNurdyas RamadhonNo ratings yet
- NTQ Electric ActuatorDocument19 pagesNTQ Electric ActuatorJennerousNo ratings yet
- Tilt Up DesignDocument27 pagesTilt Up DesigntwinniesNo ratings yet
- Lec 2 Best PPT On Jet EnginesDocument25 pagesLec 2 Best PPT On Jet Enginesahmet yıldızNo ratings yet
Liquid
Liquid
Uploaded by
AditholeOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Liquid
Liquid
Uploaded by
AditholeCopyright:
Available Formats
Liquid
Liquid is one of the four primary states of matter, with the others being solid, gas and plasma. A
liquid is a fluid. Unlike a solid, the molecules in a liquid have a much greater freedom to move.
The forces that bind the molecules together in a solid are only temporary in a liquid, allowing a
liquid to flow while a solid remains rigid.
A liquid, like a gas, displays the properties of a fluid. A liquid can flow, assume the shape of a
container, and, if placed in a sealed container, will distribute applied pressure evenly to every
surface in the container. Unlike a gas, a liquid may not always mix readily with another liquid,
will not always fill every space in the container, forming its own surface, and will not compress
significantly, except under extremely high pressures. These properties make a liquid suitable for
applications such as hydraulics.
Liquid particles are bound firmly but not rigidly. They are able to move around one another
freely, resulting in a limited degree of particle mobility. As the temperature increases, the
increased vibrations of the molecules causes distances between the molecules to increase. hen
a liquid reaches its boiling point, the cohesive forces that bind the molecules closely together
break, and the liquid changes to its gaseous state !unless superheating occurs". #f the temperature
is decreased, the distances between the molecules become smaller. hen the liquid reaches its
free$ing point the molecules will usually lock into a very specific order, called crystalli$ing, and
the bonds between them become more rigid, changing the liquid into its solid state !unless
supercooling occurs".
Examples
%nly two elements are liquid at standard conditions for temperature and pressure& mercury and
bromine. 'our more elements have melting points slightly above room temperature& francium,
caesium, gallium and rubidium.
()*
+etal alloys that are liquid at room temperature include ,a-,
a sodium.potassium metal alloy, galinstan, a fusible alloy liquid, and some amalgams !alloys
involving mercury".
/ure substances that are liquid under normal conditions include water, ethanol and many other
organic solvents. Liquid water is of vital importance in chemistry and biology0 it is believed to be
a necessity for the existence of life.
#norganic liquids include water, inorganic nonaqueous solvents and many acids.
#mportant everyday liquids include aqueous solutions like household bleach, other mixtures of
different substances such as mineral oil and gasoline, emulsions like vinaigrette or mayonnaise,
suspensions like blood, and colloids like paint and milk.
+any gases can be liquefied by cooling, producing liquids such as liquid oxygen, liquid
nitrogen, liquid hydrogen and liquid helium. ,ot all gases can be liquified at atmospheric
pressure, for example carbon dioxide can only be liquified at pressures above 1.) atm.
2ome materials cannot be classified within the classical three states of matter0 they possess solid.
like and liquid.like properties. 3xamples include liquid crystals, used in L45 displays, and
biological membranes.
Applications
Liquids have a variety of uses, as lubricants, solvents, and coolants. #n hydraulic systems, liquid
is used to transmit power.
#n tribology, liquids are studied for their properties as lubricants. Lubricants such as oil are
chosen for viscosity and flow characteristics that are suitable throughout the operating
temperature range of the component. %ils are often used in engines, gear boxes, metalworking,
and hydraulic systems for their good lubrication properties.
(6*
+any liquids are used as solvents, to dissolve other liquids or solids. 2olutions are found in a
wide variety of applications, including paints, sealants, and adhesives. ,aptha and acetone are
used frequently in industry to clean oil, grease, and tar from parts and machinery. 7ody fluids are
water based solutions.
2urfactants are commonly found in soaps and detergents. 2olvents like alcohol are often used as
antimicrobials. They are found in cosmetics, inks, and liquid dye lasers. They are used in the
food industry, in processes such as the extraction of vegetable oil.
(8*
Liquids tend to have better thermal conductivity than gases, and the ability to flow makes a
liquid suitable for removing excess heat from mechanical components. The heat can be removed
by channeling the liquid through a heat exchanger, such as a radiator, or the heat can be removed
with the liquid during evaporation.
(9*
ater or glycol coolants are used to keep engines from
overheating.
(1*
The coolants used in nuclear reactors include water or liquid metals, such as
sodium or bismuth.
(:*
Liquid propellant films are used to cool the thrust chambers of rockets.
(;*
#n
machining, water and oils are used to remove the excess heat generated, which can quickly ruin
both the work piece and the tooling. 5uring perspiration, sweat removes heat from the human
body by evaporating. #n the heating, ventilation, and air.conditioning industry !<=A4", liquids
such as water are used to transfer heat from one area to another.
(>*
Liquid is the primary component of hydraulic systems, which take advantage of /ascal?s law to
provide fluid power. 5evices such as pumps and waterwheels have been used to change liquid
motion into mechanical work since ancient times. %ils are forced through hydraulic pumps,
which transmit this force to hydraulic cylinders. <ydraulics can be found in many applications,
such as automotive brakes and transmissions, heavy equipment, and airplane control systems.
=arious hydraulic presses are used extensively in repair and manufacturing, for lifting, pressing,
clamping and forming.
(@*
Liquids are sometimes used in measuring devices. A thermometer often uses the thermal
expansion of liquids, such as mercury, combined with their ability to flow to indicate
temperature. A manometer uses the weight of the liquid to indicate air pressure.
()A*
Mechanical properties
Volume
Buantities of liquids are commonly measured in units of volume. These include the 2# unit cubic
metre !m
8
" and its divisions, in particular the cubic decimetre, more commonly called the litre !)
dm
8
C ) L C A.AA) m
8
", and the cubic centimetre, also called millilitre !) cm
8
C ) mL C A.AA) L C
)A
D:
m
8
".
The volume of a quantity of liquid is fixed by its temperature and pressure. Liquids generally
expand when heated, and contract when cooled. ater between A E4 and 9 E4 is a notable
exception. Liquids have little compressibility& water, for example, requires a pressure of the
order of 6AA bar to increase its density by )F)AAA. #n the study of fluid dynamics, liquids are
often treated as incompressible, especially when studying incompressible flow.
Pressure and buoyancy
+ain article& fluid statics
#n a gravitational field, liquids exert pressure on the sides of a container as well as on anything
within the liquid itself. This pressure is transmitted in all directions and increases with depth. #f a
liquid is at rest in a uniform gravitational field, the pressure, p, at any depth, z, is given by
where&
is the density of the liquid !assumed constant"
is the gravitational acceleration.
,ote that this formula assumes that the pressure at the free surface is $ero, and that surface
tension effects may be neglected.
%bGects immersed in liquids are subGect to the phenomenon of buoyancy. !7uoyancy is also
observed in other fluids, but is especially strong in liquids due to their high density."
Surfaces
+ain article& surface science
2urface waves in water
Unless the volume of a liquid exactly matches the volume of its container, one or more surfaces
are observed. The surface of a liquid behaves like an elastic membrane in which surface tension
appears, allowing the formation of drops and bubbles. 2urface waves, capillary action, wetting,
and ripples are other consequences of surface tension.
Flow
+ain article& fluid mechanics
+ain article& fluid dynamics
=iscosity measures the resistance of a liquid which is being deformed by either shear stress or
extensional stress.
hen a liquid is supercooled towards the glass transition, the viscosity increases dramatically.
The liquid then becomes a viscoelastic medium that shows both the elasticity of a solid and the
fluidity of a liquid, depending on the time scale of observation or on the frequency of
perturbation.
Sound propagation
+ain article& speed of sound H 2peed of sound in liquids
#n a fluid the only non.$ero stiffness is to volumetric deformation !a fluid does not sustain shear
forces". <ence the speed of sound in a fluid is given by where K is the bulk
modulus of the fluid, and the density. To give a typical value, in fresh water cC)9@; mFs at
61 E4.
hermodynamics
Phase transitions
+ain article& boiling
+ain article& boiling point
+ain article& melting
+ain article& melting point
A typical phase diagram. The dotted line gives the anomalous behaviour of water. The green
lines show how the free$ing point can vary with pressure, and the blue line shows how the
boiling point can vary with pressure. The red line shows the boundary where sublimation or
deposition can occur.
At a temperature below the boiling point, any matter in liquid form will evaporate until the
condensation of gas above reach an equilibrium. At this point the gas will condense at the same
rate as the liquid evaporates. Thus, a liquid cannot exist permanently if the evaporated liquid is
continually removed. A liquid at its boiling point will evaporate more quickly than the gas can
condense at the current pressure. A liquid at or above its boiling point will normally boil, though
superheating can prevent this in certain circumstances.
At a temperature below the free$ing point, a liquid will tend to crystalli$e, changing to its solid
form. Unlike the transition to gas, there is no equilibrium at this transition under constant
pressure, so unless supercooling occurs, the liquid will eventually completely crystalli$e. ,ote
that this is only true under constant pressure, so e.g. water and ice in a closed, strong container
might reach an equilibrium where both phases coexist. 'or the opposite transition from solid to
liquid, see melting.
Solutions
+ain article& solution
Liquids can display immiscibility. The most familiar mixture of two immiscible liquids in
everyday life is the vegetable oil and water in #talian salad dressing. A familiar set of miscible
liquids is water and alcohol. Liquid components in a mixture can often be separated from one
another via fractional distillation.
Microscopic properties
Static structure factor
+ain article& structure of liquids and glasses
2tructure of a classical monatomic liquid. Atoms have many nearest neighbors in contact, yet no
long.range order is present.
#n a liquid, atoms do not form a crystalline lattice, nor do they show any other form of long.
range order. This is evidenced by the absence of 7ragg peaks in I.ray and neutron diffraction.
Under normal conditions, the diffraction pattern has circular symmetry, expressing the isotropy
of the liquid. #n radial direction, the diffraction intensity smoothly oscillates. This is usually
described by the static structure factor S(q), with wavenumber qC!9JFK"sinL given by the
wavelength K of the probe !photon or neutron" and the 7ragg angle L. The oscillations of S(q)
express the near order of the liquid, i.e. the correlations between an atom and a few shells of
nearest, second nearest, ... neighbors.
A more intuitive description of these correlations is given by the radial distribution function g(r),
which is basically the 'ourier transform of S(q). #t represents a spatial average of a temporal
snapshot of pair correlations in the liquid.
Madial distribution function of the Lennard.Nones model fluid.
Sound dispersion and structural relaxation
The above expression for the sound velocity contains the bulk modulus K. #f K is
frequency independent then the liquid behaves as a linear medium, so that sound propagates
without dissipation and without mode coupling. #n reality, any liquid shows some dispersion&
with increasing frequency, K crosses over from the low.frequency, liquid.like limit to the
high.frequency, solid.like limit . #n normal liquids, most of this cross over takes place at
frequencies between O<$ and T<$, sometimes called hypersound.
At sub.O<$ frequencies, a normal liquid cannot sustain shear waves& the $ero.frequency limit of
the shear modulus is . This is sometimes seen as the defining property of a liquid.
())*()6*
<owever, Gust as the bulk modulus K, the shear modulus G is frequency dependent, and at
hypersound frequencies it shows a similar cross over from the liquid.like limit to a solid.like,
non.$ero limit .
According to the -ramers.-ronig relation, the dispersion in the sound velocity !given by the real
part of K or G" goes along with a maximum in the sound attenuation !dissipation, given by the
imaginary part of K or G". According to linear response theory, the 'ourier transform of K or G
describes how the system returns to equilibrium after an external perturbation0 for this reason,
the dispersion step in the O<$..T<$ region is also called structural relaxation. According the
fluctuation.dissipation theorem, relaxation towards equilibrium is intimately connected to
fluctuations in equilibrium. The density fluctuations associated with sound waves can be
experimentally observed by 7rillouin scattering.
%n supercooling a liquid towards the glass transition, the crossover from liquid.like to solid.like
response moves from O<$ to +<$, k<$, <$, ...0 equivalently, the characteristic time of structural
relaxation increases from ns to Ps, ms, s, ... This is the microscopic explanation for the above
mentioned viscoelastic behaviour of glass.forming liquids.
Effects of association
The mechanisms of atomicFmolecular diffusion !or particle displacement" in solids are closely
related to the mechanisms of viscous flow and solidification in liquid materials. 5escriptions of
viscosity in terms of molecular Qfree spaceQ within the liquid
()8*
were modified as needed in order
to account for liquids whose molecules are known to be QassociatedQ in the liquid state at
ordinary temperatures. hen various molecules combine together to form an associated
molecule, they enclose within a semi.rigid system a certain amount of space which before was
available as free space for mobile molecules. Thus, increase in viscosity upon cooling due to the
tendency of most substances to become associated on cooling.
()9*
2imilar arguments could be used to describe the effects of pressure on viscosity, where it may be
assumed that the viscosity is chiefly a function of the volume for liquids with a finite
compressibility. An increasing viscosity with rise of pressure is therefore expected. #n addition, if
the volume is expanded by heat but reduced again by pressure, the viscosity remains the same.
The local tendency to orientation of molecules in small groups lends the liquid !as referred to
previously" a certain degree of association. This association results in a considerable Qinternal
pressureQ within a liquid, which is due almost entirely to those molecules which, on account of
their temporary low velocities !following the +axwell distribution" have coalesced with other
molecules. The internal pressure between several such molecules might correspond to that
between a group of molecules in the solid form.
!eferences
). Theodore Oray, The 3lements& A =isual 3xploration of 3very -nown Atom in the
Universe ,ew Rork& orkman /ublishing, 6AA@ p. )6; #27, ).1;@)6.>)9.@
6. Theo +ang, ilfried 5ressel SSLubricants and lubricationSS, iley.=4< 6AA;
#27, 8.16;.8)9@;.A
8. Oeorge ypych SS<andbook of solventsSS illiam Andrew /ublishing 6AA) pp.
>9;T>>) #27, ).>@1)@>.69.A
9. ,. 7. =argaftik SS<andbook of thermal conductivity of liquids and gasesSS 4M4
/ress )@@9 #27, A.>9@8.@891.A
1. Nack 3rGavec SSAutomotive technology& a systems approachSS 5elmar Learning
6AAA p. 8A@ #27, ).9A)>.9>8).)
:. Oerald endt SSThe prospects of nuclear power and technologySS 5. =an ,ostrand
4ompany )@1; p. 6::
;. SS+odern engineering for design of liquid.propellant rocket enginesSS by 5ieter -.
<u$el, 5avid <. <uang T American #nstitute of Aeronautics and Astronautics )@@6 p. @@
#27, ).1:89;.A)8.:
>. Thomas 3 +ull SS<=A4 principles and applications manualSS +cOraw.<ill )@@;
#27, A.A;.A9991).I
@. M. -eith +obley Fluid power dynamics 7utterworth.<einemann 6AAA p. vii
#27, A.;1A:.;);9.6
)A. 7ela O. Liptak SS#nstrument engineersS handbook& process controlSS 4M4 /ress
)@@@ p. >A; #27, A.>9@8.)A>).9
)). 7orn, +ax !)@9A". Q%n the stability of crystal latticesQ. Mathematical
Proceedings !4ambridge /hilosophical 2ociety" "# !6"& ):AT);6.
doi&)A.)A);F2A8A1AA9)AAA);)8>.
)6. 7orn, +ax !)@8@". QThermodynamics of 4rystals and +eltingQ. Journal o
!hemical Physics $ !>"& 1@)T:A9. doi&)A.)A:8F).);1A9@;.
)8. 5.7. +acleod !)@68". Q%n a relation between the viscosity of a liquid and its
coefficient of expansionQ. "rans# Farad# Soc# %&& :. doi&)A.)A8@Ftf@68)@AAAA:.
)9. O. 2tewart !)@8A". QThe 4ybotactic !+olecular Oroup" 4ondition in Liquids0
the Association of +oleculesQ. Phys# $e%# "' !;"& ;6:. 7ibcode&)@8A/hMv...81..;6:2.
doi&)A.))A8F/hysMev.81.;6:.
You might also like
- Introduction To Robotics Mechanics and Control 4th Edition Craig Solutions ManualDocument3 pagesIntroduction To Robotics Mechanics and Control 4th Edition Craig Solutions Manuala3905733700% (5)
- Fluid Friction in PipesDocument15 pagesFluid Friction in PipesMradul Yadav0% (1)
- CE 5014 Design of Selected StrsDocument65 pagesCE 5014 Design of Selected StrsTAMIL100% (1)
- A7 B7 Locomotive Brake Equipment TestDocument14 pagesA7 B7 Locomotive Brake Equipment TestFrancesca Parrino100% (1)
- Trident 2 PowerscreenDocument9 pagesTrident 2 PowerscreenRomuald PogorzelczykNo ratings yet
- 1.fluid Mechanics IntroductionDocument6 pages1.fluid Mechanics IntroductionJohn Lester PaltepNo ratings yet
- Matter in The Liquid Phase1Document6 pagesMatter in The Liquid Phase1Angel Rose NarcenaNo ratings yet
- ICE - Unit 3 - RECDocument17 pagesICE - Unit 3 - RECspidey dudeNo ratings yet
- Fluids For Hydraulic SystemsDocument16 pagesFluids For Hydraulic SystemsAimee SpearsNo ratings yet
- Hydraulics System PDFDocument177 pagesHydraulics System PDFsushantreshmaNo ratings yet
- Module 1 - Flow and Fluid PropertiesDocument5 pagesModule 1 - Flow and Fluid Propertieskdnji123No ratings yet
- Fluid Mechanics and Machinery NotesDocument137 pagesFluid Mechanics and Machinery NotesapsrtsNo ratings yet
- 7Document42 pages7bilalNo ratings yet
- Fluid MechanichDocument5 pagesFluid Mechanichasishraj2003No ratings yet
- Fluid 2 Project 1Document11 pagesFluid 2 Project 1Sanjay RagupathyNo ratings yet
- Visco CityDocument10 pagesVisco CityTanjila HasanNo ratings yet
- Properties of FluidDocument7 pagesProperties of FluidAnonymous QM0NLqZONo ratings yet
- Hydraulic SystemDocument20 pagesHydraulic SystemCECIL JOSEF C. LIGANNo ratings yet
- Abe 106 - 02Document6 pagesAbe 106 - 02emmanuelNo ratings yet
- Qtheory VuitDocument8 pagesQtheory VuitCHANDRASEKAR RNo ratings yet
- FM BesDocument91 pagesFM Bessce civilNo ratings yet
- Liquid: Jump To Navigationjump To SearchDocument12 pagesLiquid: Jump To Navigationjump To SearchLaura Maria GalanNo ratings yet
- Fluid FlowDocument8 pagesFluid Flowking khanNo ratings yet
- Fluid MechanicsDocument25 pagesFluid MechanicsDominic GograNo ratings yet
- Lec 1 Properties of Fluids MechDocument35 pagesLec 1 Properties of Fluids MechJJG ABVNo ratings yet
- Workfile 2Document9 pagesWorkfile 2MISNo ratings yet
- 6 Fluid MechanicsDocument14 pages6 Fluid MechanicsbalajigandhirajanNo ratings yet
- Introduction To Fluid MechanicsDocument85 pagesIntroduction To Fluid MechanicsKaul PatrickNo ratings yet
- Remove Materials ThesisDocument12 pagesRemove Materials ThesisSameera RajpoutNo ratings yet
- Fluid Mechanics IntroductionDocument4 pagesFluid Mechanics IntroductionAvinash GarikapatiNo ratings yet
- Properties of Liquids and Intermolecular Forces PDFDocument15 pagesProperties of Liquids and Intermolecular Forces PDFpie100% (1)
- Lab 9 - Fluid Properties (Viscosity)Document22 pagesLab 9 - Fluid Properties (Viscosity)Amanda AmaniNo ratings yet
- ME080 Section 1 - Hydraulic System BasicsDocument31 pagesME080 Section 1 - Hydraulic System BasicsAhmed FaragNo ratings yet
- Fluid Dynamics: (Relative Density)Document8 pagesFluid Dynamics: (Relative Density)Aidilmann AshrafeiderNo ratings yet
- Fluid Mechanics Basics 1Document12 pagesFluid Mechanics Basics 1Hashmi AshmalNo ratings yet
- Chapter1-Fluid MechanicsDocument84 pagesChapter1-Fluid MechanicsSrujan Chowdary100% (1)
- Properties of Water and Intermolecular ForcesDocument34 pagesProperties of Water and Intermolecular Forcesjoshua gollosoNo ratings yet
- 001 l#1 Intro To FluidmechanicsDocument76 pages001 l#1 Intro To FluidmechanicsEng Faysal AhmedNo ratings yet
- FM Unit1 3semDocument19 pagesFM Unit1 3semIan PrayarNo ratings yet
- Fluid Mechanics: School of Mechanical and Industrial EngineeringDocument57 pagesFluid Mechanics: School of Mechanical and Industrial EngineeringAbel YohannesNo ratings yet
- Fluid Mechanics: Mechanical Engineering DepartmentDocument85 pagesFluid Mechanics: Mechanical Engineering DepartmentVictoria ObulorNo ratings yet
- Note Mce 112 - 1Document24 pagesNote Mce 112 - 1Abraham OmomhenleNo ratings yet
- Compressible VS Incompressible FluidsDocument2 pagesCompressible VS Incompressible FluidsAbdulhamid DaudaNo ratings yet
- Hydraulic Fluids 1Document2 pagesHydraulic Fluids 1Andres Felipe Ospino CatañoNo ratings yet
- Fluid Mechanics Unit1Document100 pagesFluid Mechanics Unit1anjuNo ratings yet
- Ice 5Document9 pagesIce 5Gabriel CortesNo ratings yet
- Roy Preparation of Ink ChemistryDocument13 pagesRoy Preparation of Ink ChemistryMeyy SNo ratings yet
- Rheology: Newtonian FluidsDocument4 pagesRheology: Newtonian FluidsarabbbbbbbkNo ratings yet
- Fluid Mechanics I CE-407: Basic Concepts, Properties of Fluid and Fluid StaticsDocument26 pagesFluid Mechanics I CE-407: Basic Concepts, Properties of Fluid and Fluid Staticstaha zafarNo ratings yet
- Fluid Properties LabDocument18 pagesFluid Properties LabsilasNo ratings yet
- Mechanics of Fluids: Introduction To Fluid MechanicsDocument46 pagesMechanics of Fluids: Introduction To Fluid Mechanicsearl pannilaNo ratings yet
- Topic 1 - Fluid PropertiesDocument40 pagesTopic 1 - Fluid PropertiesNazhan HaziqNo ratings yet
- Water Resources: Dr. Hiba A. AbbasDocument9 pagesWater Resources: Dr. Hiba A. AbbasMuhammad SadiqNo ratings yet
- Fluid Mechanics. Chapter 1. Introduction To Fluid MechanicsDocument8 pagesFluid Mechanics. Chapter 1. Introduction To Fluid MechanicsCHRISTIAN JAY TESNADONo ratings yet
- Covenant GEC 223 Lecture Note 1 Week 2-MoodleDocument53 pagesCovenant GEC 223 Lecture Note 1 Week 2-MoodleAdeolu-Idowu AbiolaNo ratings yet
- Chapter 1 - Physical Properties of LiquidDocument21 pagesChapter 1 - Physical Properties of LiquidGSaurav DahalNo ratings yet
- Science Exam Study NotesDocument9 pagesScience Exam Study Notescainicole22No ratings yet
- Rhe OlogyDocument3 pagesRhe OlogyAldiDamoraNo ratings yet
- Power-Law FluidDocument4 pagesPower-Law FluidaryanNo ratings yet
- Fluid Mechanics ApplicationsDocument29 pagesFluid Mechanics ApplicationsHASSAN ARSHADNo ratings yet
- Week 4 (Lecture 1)Document28 pagesWeek 4 (Lecture 1)muhammadtalhabilal6666No ratings yet
- Fluid Mechanics GEC 223 Lecture NoteDocument53 pagesFluid Mechanics GEC 223 Lecture NoteCHIBUIKE UDENTANo ratings yet
- Liquid Drops and Globules, Their Formation and Movements: Three lectures delivered to popular audiencesFrom EverandLiquid Drops and Globules, Their Formation and Movements: Three lectures delivered to popular audiencesNo ratings yet
- Datasheet Flygt 2201 012 HT-MT 60hz En-UsDocument2 pagesDatasheet Flygt 2201 012 HT-MT 60hz En-UsCristhian Vilca SilvestreNo ratings yet
- PneumaticsPneumatics, Air Conditioning, & PressurizationDocument9 pagesPneumaticsPneumatics, Air Conditioning, & PressurizationAnastasios Pavlou100% (3)
- VRCA Relief ValveDocument8 pagesVRCA Relief Valvenenad135No ratings yet
- Analysis and Optimal Design of Wind Boosters For Vertical Axis Wind PDFDocument10 pagesAnalysis and Optimal Design of Wind Boosters For Vertical Axis Wind PDFulil mohamad albabNo ratings yet
- Rigid Pavement DesignDocument11 pagesRigid Pavement DesignAjit P. SinghNo ratings yet
- Sistema Inyección CR BoschDocument8 pagesSistema Inyección CR BoschRodolfo Leiva100% (1)
- Wrinklebends andDocument179 pagesWrinklebends androdholfhoNo ratings yet
- Induction Motor AnalysisDocument123 pagesInduction Motor AnalysisatmegamicroNo ratings yet
- SicemoursestudyguideDocument12 pagesSicemoursestudyguideZakariya AbdiNo ratings yet
- Pages From Switched Reluctance Motor PDFDocument10 pagesPages From Switched Reluctance Motor PDFHAMID SULIAMANNo ratings yet
- Dynamic Analysis of An Overhead Transmission Line Subject To Gusty Wind Loading Predicted by Wind-Conductor InteractionDocument10 pagesDynamic Analysis of An Overhead Transmission Line Subject To Gusty Wind Loading Predicted by Wind-Conductor Interactionsara fernandesNo ratings yet
- Re Lease: D TC: ".) - Istributio0N 0F This 0UNDocument62 pagesRe Lease: D TC: ".) - Istributio0N 0F This 0UNJennifer Small100% (1)
- Elearn - Ecu Pins PDFDocument6 pagesElearn - Ecu Pins PDFJavierPariNo ratings yet
- Water Meter Guidelines & SpecificationsDocument19 pagesWater Meter Guidelines & SpecificationsSrujan GuptaNo ratings yet
- Sci 4N2 S PDFDocument3 pagesSci 4N2 S PDFArchie PérezNo ratings yet
- Demech Antistick TDSDocument4 pagesDemech Antistick TDSAtharva UlangwarNo ratings yet
- Appendix A Definitions of TermsDocument7 pagesAppendix A Definitions of TermsSantiago SeveinNo ratings yet
- Lec 4 - Properties of Natural Gas-IDocument26 pagesLec 4 - Properties of Natural Gas-IVishnu CnairNo ratings yet
- JGERE 2020-7-2 PG 96-102Document7 pagesJGERE 2020-7-2 PG 96-102SeasonNo ratings yet
- BSI-British Standard ValvesDocument3 pagesBSI-British Standard ValvesVincent RajNo ratings yet
- MTU20 V 4000 G43, 20 V 4000 G83L, 20 V 4000 G63, 20 V 4000 G63L, 20 V 4000 G83, 20 4000 G23 Operating Instructions ManualDocument181 pagesMTU20 V 4000 G43, 20 V 4000 G83L, 20 V 4000 G63, 20 V 4000 G63L, 20 V 4000 G83, 20 4000 G23 Operating Instructions ManualAlexNo ratings yet
- Manual tm-10098 Rev 05-27-08Document203 pagesManual tm-10098 Rev 05-27-08Alhgasjsghjagjsdajlsd Asdalsdlkaksd100% (1)
- LEAP Component Maintenance ManualsDocument12 pagesLEAP Component Maintenance ManualsNurdyas RamadhonNo ratings yet
- NTQ Electric ActuatorDocument19 pagesNTQ Electric ActuatorJennerousNo ratings yet
- Tilt Up DesignDocument27 pagesTilt Up DesigntwinniesNo ratings yet
- Lec 2 Best PPT On Jet EnginesDocument25 pagesLec 2 Best PPT On Jet Enginesahmet yıldızNo ratings yet