Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
52 viewsEggosmosislab
Eggosmosislab
Uploaded by
Cynthia Lingosmosis
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5825)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Experiment 2: Calorimetry Calorimetry Is A Method of Measuring The Heat Transfer Within A Chemical Reaction or OtherDocument7 pagesExperiment 2: Calorimetry Calorimetry Is A Method of Measuring The Heat Transfer Within A Chemical Reaction or OtherRianna Ramos67% (3)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- HW 04Document2 pagesHW 04Icy45No ratings yet
- Biology EssaysDocument26 pagesBiology EssaysCynthia LingNo ratings yet
- Chapter 4 Protein Mind Map PDFDocument1 pageChapter 4 Protein Mind Map PDFCynthia LingNo ratings yet
- Science Fair Projects: Capillary ActionDocument1 pageScience Fair Projects: Capillary ActionCynthia LingNo ratings yet
- Sains - Biology Form 4Document77 pagesSains - Biology Form 4Sekolah Portal97% (30)
- CHE 218 Resonance - StructuresDocument4 pagesCHE 218 Resonance - StructuresEmmy OlabosipoNo ratings yet
- Recent Trends in Non-Traditional Machining Processes: Unit - 5Document12 pagesRecent Trends in Non-Traditional Machining Processes: Unit - 5DISHA VNo ratings yet
- 64j0105-C-Piping ClassDocument85 pages64j0105-C-Piping ClassDubois100% (2)
- Woodward's RuleDocument48 pagesWoodward's RuleDeshan Wolfey100% (1)
- Jacs 2c07482Document14 pagesJacs 2c07482Rob ZunneciNo ratings yet
- At TN 03 Critical Micelle ConcentrationDocument2 pagesAt TN 03 Critical Micelle ConcentrationedwinNo ratings yet
- Morales - Jaime, 2019. Activity Coeff KClO4 + Poly (Ethylene Glycol) + H2ODocument10 pagesMorales - Jaime, 2019. Activity Coeff KClO4 + Poly (Ethylene Glycol) + H2OYahaira Barrueto JhonsonNo ratings yet
- Energies 16 02918Document21 pagesEnergies 16 02918Jesús MateoNo ratings yet
- EmulsionsDocument44 pagesEmulsionsMax SinghNo ratings yet
- Mole-1 JEE Advanced Level MCQsDocument8 pagesMole-1 JEE Advanced Level MCQswhoeverNo ratings yet
- Electronegativity A Force or EnergyDocument21 pagesElectronegativity A Force or EnergyEditor IJTSRDNo ratings yet
- FT&HXDocument55 pagesFT&HXKelvin TanNo ratings yet
- 7.0 Reaction Kinetics 2019Document62 pages7.0 Reaction Kinetics 2019salman khanNo ratings yet
- An Improved Method For Calculating Bottomhole Pressures in Flowing Gas Wells With Liquid PresentDocument13 pagesAn Improved Method For Calculating Bottomhole Pressures in Flowing Gas Wells With Liquid PresentAdan Martinez RiveraNo ratings yet
- Chepter Wise QuestionsDocument240 pagesChepter Wise QuestionsVinay Tyagi100% (1)
- BS Physics SyllabusDocument45 pagesBS Physics SyllabusManzoor Ahmad PashteenNo ratings yet
- Electrochemistry - 2 - 1Document6 pagesElectrochemistry - 2 - 1Mandeep PediredlaNo ratings yet
- Desiccant Wheel Dehumidification 2Document19 pagesDesiccant Wheel Dehumidification 2ovidiu73No ratings yet
- Chemical Basis of LifeDocument14 pagesChemical Basis of LifebigbangNo ratings yet
- 4ConversionPotenciales BSQ11-1Document4 pages4ConversionPotenciales BSQ11-1ElenaNo ratings yet
- Shape Control of Ag Shell Growth On Au NanodisksDocument4 pagesShape Control of Ag Shell Growth On Au NanodisksAdrianoDSNo ratings yet
- Characterization TechniquesDocument8 pagesCharacterization TechniquesMingqing ZuoNo ratings yet
- Direct DyesDocument27 pagesDirect DyesrehanabbaciNo ratings yet
- Pipe Heat Loss CalculationDocument10 pagesPipe Heat Loss CalculationhoangpalestineNo ratings yet
- Chemical and Optical Properties of Mno2 Thin Films Prepared by Reactive Evaporation of ManganeseDocument9 pagesChemical and Optical Properties of Mno2 Thin Films Prepared by Reactive Evaporation of ManganeseesatjournalsNo ratings yet
- PHD Thesis On Photonic CrystalDocument6 pagesPHD Thesis On Photonic Crystalaragunikd100% (2)
- GS2011 QP ChemistryDocument17 pagesGS2011 QP ChemistryAkash AgarwalNo ratings yet
- Students - Sheet 2Document6 pagesStudents - Sheet 2basemhazemNo ratings yet
Eggosmosislab
Eggosmosislab
Uploaded by
Cynthia Ling0 ratings0% found this document useful (0 votes)
52 views5 pagesosmosis
Original Title
eggosmosislab.doc
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentosmosis
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
0 ratings0% found this document useful (0 votes)
52 views5 pagesEggosmosislab
Eggosmosislab
Uploaded by
Cynthia Lingosmosis
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
You are on page 1of 5
MEASURING THE RATE OF OSMOSIS USING DESHELLED CHICKEN EGGS
(Effect of Solute Concentraton U!on Rate"De#ree of O$%o$$ n C&c'en E##$()
Intro*ucton
If a cell is to perform its functions, it must maintain a steady state in the midst of an ever-
changing environment. This constancy is maintained by the regulation of movement of materials
into and out of the shell. To achieve this control, cells are bounded by a delicate membrane that
differentiates between different substances, slowing down the movement of some while allowing
others to pass through. Since not all substances penetrate the membrane equally well, the
membrane is said to be differentially permeable.
The external and internal environment of cells is an aqueous solution of dissolved inorganic and
organic molecules. Movement of these molecules, both in the solution and through the cell
membrane, involves a physical process called diffusion a spontaneous process by which
molecules move from a region in which they are highly concentrated to a region in which their
concentration is lower.
! special "ind of diffusion is the phenomenon of osmosis. Simply defined in biological systems,
osmosis is the diffusion of water through a differentially permeable membrane from a region in
which it is highly concentrated to a region in which its concentration is lower. More often,
however, osmosis is defined in terms of the effects that solutes have on the thermodynamic
activity of water #i.e., the activity of the water molecule due to the "inetic energy of motion$.
%or example, the addition of a solute to water tends to decrease the activity of the water. In other
words, as more water molecules are displaced by solute molecules, the activity of the water goes
down. Thus, in thermodynamic terms, water diffuses across membranes from a region in which
the thermodynamic activity of water is high #low solute concentration$ to one in which the
thermodynamic activity is low #high solute concentration$.
&e use the terms hypotonic, hypertonic and isotonic in referring to the relative concentrations of
solute particles of different solutions. 'elow are the definitions of the three terms according to
(urtis, )*+,.
-ypotonic #.". hypo, under / tonos, tension$0 1f two solutions of different
concentration, the solution that contains the lower concentration of solute
particles2 water moves across a semi-permeable membrane into a
hypertonic solution.
-ypertonic #.". hyper, above / tonos, tension$0 1f two solutions of different
concentration, the solution that contains the higher concentration of solute
particles2 water moves across a semi-permeable membrane into a
hypertonic solution.
Isotonic #.". isos, equal / tonos, tension$0 -aving the same concentration of
solutes as another solution. If two isotonic solutions are separated by a
semi-permeable membrane, there will be no net flow of water across the
membrane.
It should be noted that the number of solute particles is the thing that affects the relative activity
of the water, not the "ind of particles.
-eat increases the motion of molecules. Therefore, we expect an increase in temperature to
speed up the rate of osmosis, regardless of which direction the solvent is moving. This is
diagrammatically shown below.
!. &ater enters cell '. &ater leaves cell
3.)M solute 3.*M solute
particles particles
#i.e., sucrose$ #i.e., sucrose$
3.4M solute 3.4M solute
particles particles
#i.e., proteins$ #i.e., proteins$
-51 -51
#-51 activity #-51 activity
lower than higher than
outside$ outside$
#-51 activity #-51 activity
higher than lower than
inside cell$ inside cell$
-671T18I( -679:T18I(
THE E+,ERIMENT
7art I0 9ffect of Solute (oncentration
9ach group of students will be given ; chic"en eggs from which the shell has been dissolved
away. The remaining membrane #the shell membrane$ is differentially permeable. &e will
assume that each egg has approximately the same concentration of solute in this membrane, and
based on the rate of osmosis, will attempt to determine what this concentration must be.
&eight each egg separately to the nearest 3.)g and record the weights in Table ) at time <3=.
7lace eggs ), 5, ,, >, 4, and ; into separate bea"ers containing solutions of distilled water, )3?
sucrose, 53? sucrose, ,3? sucrose, >3? sucrose and un"nown sucrose solution respectively. !t
)4 minute intervals, that is after )4, ,3, >4, ;3, and @4 minutes, remove the eggs from the
bea"ers2 carefully wipe off all excess water2 and again weigh each egg separately. :ecord the
weight in Table ). 7lot the changes in weight of each of the eggs against time on a piece of
graph paper.
&hich solutions would you say were hypotonic to that of the eggsA &hich of them were
hypertonicA IsotonicA &hat would you expect to happen if an egg was put into a sixth bea"er
containing a 43? sucrose solutionA
Ta-le I. /e#&t C&an#e of E##$ (#( 0$ T%e (%nute$(
T%e (Mn)( 12 $uc 312 $uc 412 $uc 512 $uc 612 $uc Un'no7n
3
)4
,3
>4
;3
@4
Ta-le II. /e#&t C&an#e of E##$ (#( 0$ T%e (%nute$(
T%e (Mn)( 12 $uc 312 $uc 412 $uc 512 $uc 612 $uc Un'no7n
3 3 3 3 3 3 3
)4
,3
>4
;3
@4
Ta-le III. Total /e#&t C&an#e of E##$ (#( 0$ Sucro$e Concentraton (2(
Sucrose (onc. 3?Suc )3?Suc 53?Suc ,3?Suc >3?Suc
Tot. &t.
(hange
In order to fill out table ,, extract data on the @4
th
minute row of table 5 and place them on table
,.
Bab :eport for Movement of Materials !cross (ell Membranes
To assist you in your understanding of this laboratory, you are as"ed to prepare the following
graphs using Microsoft excel and answer the questions at the end of each section.
). 7repare a graph in which you plot the weight change of the six #;$ eggs #use data from
table 5$, which have been placed in solutions of varying solute #sucrose$ concentrations,
as a function of time. 6ou will have six #;$ lines on this graph #one for each egg$. This
graph indicates the weight change of eggs #g$ vs time #minutes$
#/$
&eight (hange
of 9ggs #grams$ 3
#-$
3 )4 ,3 >4 ;3 @4
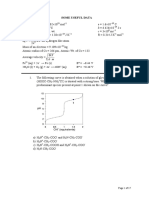
a. &hat conclusion can you draw from the data in this graphA
b. &hich solutions were hypotonicA -ypertonicA IsotonicA
5. 7repare a graph in which you plot total 7e#&t c&an#e of the eggs #g$ placed in varying
solute #sucrose$ solutions against $ucro$e concentraton #?$. 6ou, in effect, will be
constructing a type of $tan*ar* cur0e) Cse the data in table , to construct this graph.
#/$
Total &eight
(hange #grams$ 3
#-$
3 )3 53 ,3 >3
Sucrose (oncentration #?$
Csing this graph, you should be able to0
). Determine the $otonc !ont of the contents of a chic"en egg. #-int0 where your
curve crosses the 3 line, read down to the concentration axis and record the value you
obtain.$
5. Determine the concentration of the un"nown solution. #-int0 locate on the total
weight change axis, the value representing the total weight change of the egg placed
in the un"nown solution. :ead across to your standard curve and then down to the
concentration axis and record the value you obtain.$
Expected tables and graphs
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5825)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Experiment 2: Calorimetry Calorimetry Is A Method of Measuring The Heat Transfer Within A Chemical Reaction or OtherDocument7 pagesExperiment 2: Calorimetry Calorimetry Is A Method of Measuring The Heat Transfer Within A Chemical Reaction or OtherRianna Ramos67% (3)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- HW 04Document2 pagesHW 04Icy45No ratings yet
- Biology EssaysDocument26 pagesBiology EssaysCynthia LingNo ratings yet
- Chapter 4 Protein Mind Map PDFDocument1 pageChapter 4 Protein Mind Map PDFCynthia LingNo ratings yet
- Science Fair Projects: Capillary ActionDocument1 pageScience Fair Projects: Capillary ActionCynthia LingNo ratings yet
- Sains - Biology Form 4Document77 pagesSains - Biology Form 4Sekolah Portal97% (30)
- CHE 218 Resonance - StructuresDocument4 pagesCHE 218 Resonance - StructuresEmmy OlabosipoNo ratings yet
- Recent Trends in Non-Traditional Machining Processes: Unit - 5Document12 pagesRecent Trends in Non-Traditional Machining Processes: Unit - 5DISHA VNo ratings yet
- 64j0105-C-Piping ClassDocument85 pages64j0105-C-Piping ClassDubois100% (2)
- Woodward's RuleDocument48 pagesWoodward's RuleDeshan Wolfey100% (1)
- Jacs 2c07482Document14 pagesJacs 2c07482Rob ZunneciNo ratings yet
- At TN 03 Critical Micelle ConcentrationDocument2 pagesAt TN 03 Critical Micelle ConcentrationedwinNo ratings yet
- Morales - Jaime, 2019. Activity Coeff KClO4 + Poly (Ethylene Glycol) + H2ODocument10 pagesMorales - Jaime, 2019. Activity Coeff KClO4 + Poly (Ethylene Glycol) + H2OYahaira Barrueto JhonsonNo ratings yet
- Energies 16 02918Document21 pagesEnergies 16 02918Jesús MateoNo ratings yet
- EmulsionsDocument44 pagesEmulsionsMax SinghNo ratings yet
- Mole-1 JEE Advanced Level MCQsDocument8 pagesMole-1 JEE Advanced Level MCQswhoeverNo ratings yet
- Electronegativity A Force or EnergyDocument21 pagesElectronegativity A Force or EnergyEditor IJTSRDNo ratings yet
- FT&HXDocument55 pagesFT&HXKelvin TanNo ratings yet
- 7.0 Reaction Kinetics 2019Document62 pages7.0 Reaction Kinetics 2019salman khanNo ratings yet
- An Improved Method For Calculating Bottomhole Pressures in Flowing Gas Wells With Liquid PresentDocument13 pagesAn Improved Method For Calculating Bottomhole Pressures in Flowing Gas Wells With Liquid PresentAdan Martinez RiveraNo ratings yet
- Chepter Wise QuestionsDocument240 pagesChepter Wise QuestionsVinay Tyagi100% (1)
- BS Physics SyllabusDocument45 pagesBS Physics SyllabusManzoor Ahmad PashteenNo ratings yet
- Electrochemistry - 2 - 1Document6 pagesElectrochemistry - 2 - 1Mandeep PediredlaNo ratings yet
- Desiccant Wheel Dehumidification 2Document19 pagesDesiccant Wheel Dehumidification 2ovidiu73No ratings yet
- Chemical Basis of LifeDocument14 pagesChemical Basis of LifebigbangNo ratings yet
- 4ConversionPotenciales BSQ11-1Document4 pages4ConversionPotenciales BSQ11-1ElenaNo ratings yet
- Shape Control of Ag Shell Growth On Au NanodisksDocument4 pagesShape Control of Ag Shell Growth On Au NanodisksAdrianoDSNo ratings yet
- Characterization TechniquesDocument8 pagesCharacterization TechniquesMingqing ZuoNo ratings yet
- Direct DyesDocument27 pagesDirect DyesrehanabbaciNo ratings yet
- Pipe Heat Loss CalculationDocument10 pagesPipe Heat Loss CalculationhoangpalestineNo ratings yet
- Chemical and Optical Properties of Mno2 Thin Films Prepared by Reactive Evaporation of ManganeseDocument9 pagesChemical and Optical Properties of Mno2 Thin Films Prepared by Reactive Evaporation of ManganeseesatjournalsNo ratings yet
- PHD Thesis On Photonic CrystalDocument6 pagesPHD Thesis On Photonic Crystalaragunikd100% (2)
- GS2011 QP ChemistryDocument17 pagesGS2011 QP ChemistryAkash AgarwalNo ratings yet
- Students - Sheet 2Document6 pagesStudents - Sheet 2basemhazemNo ratings yet