Professional Documents
Culture Documents
E-Cigs SF Posterboard 2
E-Cigs SF Posterboard 2
Uploaded by
api-2409661730 ratings0% found this document useful (0 votes)
2K views1 pageOriginal Title
e-cigs sf posterboard 2
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
2K views1 pageE-Cigs SF Posterboard 2
E-Cigs SF Posterboard 2
Uploaded by
api-240966173Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
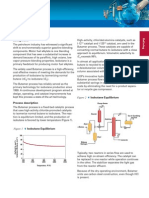
DETECTION AND QUANTIFICATION OF NICOTINE IN ELECTRONIC CIGARETTES BY HPLC
Electronic cigarettes are a new and widely popular, but fairly
untested and unregulated technology. This project aims to
put electronic cigarette liquid manufacturers to the test by
determining the amount of nicotine in their products. Using
reverse-phased high performance liquid chromatography,
standard solutions of nicotine at concentrations of 50 ppm,
100 ppm, 200 ppm, 400 ppm, and 600 ppm were run in order
to determine a standard calibration curve. By comparing
these standards to five different electronic cigarette liquids,
nicotine was detected and quantified.
ABSTRACT
INTRODUCTION
Materials
Preparation of Nicotine Standards
Standards were prepared using a serial dilution method, with 50:50 MeOH H2O acting as
the solvent. Astock solution of 600 ppm nicotine was made from a Sigma-Aldrich 1mg/
ml nicotine solution. From this stock, samples of 400 ppm, 200 ppm, 100 ppm, and 50
ppm were created. Solutions were made in eppendorf tubes and filtered through 0.45m
filters using syringes upon transfer to HPLC vials.
Preparation of Electronic Cigarette Liquids:
Electronic cigarette liquids of two different flavors and brands were purchased. From
Savor Vapor, menthol refill liquid bottles of 0mg, 6mg, and 12mg were purchased, and
from iFill Vape, Bahama Mama liquids of 0mg and 6mg were purchased. Approximately
1ml of each concentration and flavor was filtered through a 0.45m syringe filter and
into HPLC vials.
Preparation of Mobile Phase:
An isocratic method was used, initially with a 25:75 MeOH H2O mobile phase (with
0.05% formic acid to adjust pH). Later runs, however, were done using a 20:80 MeOH
H2O mobile phase which was filtered prior to running.
MATERIALS AND METHODS
Given these results, we can conclude that there is, in fact, more nicotine per bottle of refill
liquid than these companies are claiming. Every liquid tested showed more nicotine than
advertised, including those labeled as nicotine free. We can therefore further conclude that
our hypothesis was correct.
DATA
CONCLUSION
REFERENCES
Setting for HPLC Reverse-phase
Column Zorbax Eclipse XDB-C8 (4.6 x 150mm) 5-micron
Mobile Phase 20:80 MeOH H2O (with 0.05% formic acid to adjust pH)
Injection Volume 5 l
Retention time 2 minutes
Wavelength 254 nm
Flow Rate 0.7 ml/min
OBJECTIVE/HYPOTHESIS
Objective:
To determine amount of nicotine in electronic cigarette refill
liquids.
Hypothesis:
We hypothesize that the electronic cigarette refill liquids are
improperly labeled and contain more nicotine than advertised.
ACKNOWLEDGEMENTS
RESULTS/DISCUSSION
Wed like to thank Dr. Malhotra for her constant counsel and support, Dr. Tannaci for
providing us with the chemicals we needed, Karen Kearsley of Agilent Technologies for
generously donating a column, and Dr. Cauchon for his HPLC expertise. Without the
guidance of these great mentors and professionals, our project would not have been the
great learning experience it was.
y = 9.5448x - 9.7338
R! = 0.9981
-1000
0
1000
2000
3000
4000
5000
6000
7000
0 100 200 300 400 500 600 700
P
e
a
k
A
r
e
a
Concentration (ppm)
Nicotine Standard Calibration Curve
Concentration
(ppm)
Nicotine (g) per
5 l Injection
Nicotine (mg) per
15 ml Bottle
Menthol 0mg 44.54 0.2227 0.681
Menthol 6mg 1330.172 6.651 19.953
Menthol 12mg 3987.691 19.938 59.814
BM 0mg 90.96 .453 1.359
BM 6mg 1469.906 7.35 22.05
0
10
20
30
40
50
60
70
Bahama
Mama
0mg
Bahama
Mama
6mg
Menthol
12mg
Menthol
6mg
Menthol
0mg
N
ic
o
tin
e
(m
g
) p
e
r
B
o
ttle
Electronic Cigarette
Comparison of Nicotine Levels
Labeled Amount
Amount We Detected
y = 0.005x
0
0.5
1
1.5
2
2.5
3
3.5
0 100 200 300 400 500 600 700
M
a
s
s
(u
g
) p
e
r
I
n
je
c
tio
n
Concentration (ppm)
Nicotine Standard Injection Curve
FURTHER WORK
You might also like
- Exp6 chm260Document11 pagesExp6 chm260Syfkh Nsr100% (1)
- Lab Report SQC7008 ParabensDocument8 pagesLab Report SQC7008 ParabenstamilarasiganasanNo ratings yet
- HPLC - Determination of Caffeine in SodaDocument4 pagesHPLC - Determination of Caffeine in SodaAnonymous bA866TNo ratings yet
- Gravimetric Analysis WorksheetDocument2 pagesGravimetric Analysis WorksheetShurlandJamesJr.50% (2)
- Diesel Oxidation Catalyst - TheoryDocument34 pagesDiesel Oxidation Catalyst - TheoryApoorva Bhatt100% (1)
- Estimation of Nebivolol Hydro Chloride by Using RPDocument5 pagesEstimation of Nebivolol Hydro Chloride by Using RPkarthik613No ratings yet
- Cofein + ParacetamolDocument8 pagesCofein + Paracetamollia_imyoonaNo ratings yet
- Met AnaDocument17 pagesMet AnaMadhuri YakkalaNo ratings yet
- Validation of Analytical Method For Determination of Synthetic Sweeteners and Caffeine in Juices and Carbonated Beverages by HPLC With Photodiode Array DetectionDocument7 pagesValidation of Analytical Method For Determination of Synthetic Sweeteners and Caffeine in Juices and Carbonated Beverages by HPLC With Photodiode Array DetectionAnggiNo ratings yet
- Determination of Caffeine in Soda Using HPLCDocument8 pagesDetermination of Caffeine in Soda Using HPLCYolanda De GuzmanNo ratings yet
- High Performance Liquid Chromatography: Chemistry 321L ManualDocument3 pagesHigh Performance Liquid Chromatography: Chemistry 321L Manuala d e eNo ratings yet
- S Determination of Caffeine in BeveragesDocument5 pagesS Determination of Caffeine in BeveragesVioleta Grigoras100% (1)
- Development and Validation by RP-HPLC For The In-Vitro Release of Lercanidipine Hydrochloride in Tablet Dosages Form.Document8 pagesDevelopment and Validation by RP-HPLC For The In-Vitro Release of Lercanidipine Hydrochloride in Tablet Dosages Form.Baru Chandrasekhar RaoNo ratings yet
- Lab 4 HPLCDocument5 pagesLab 4 HPLCSyahirah YahyaNo ratings yet
- Analysis Ascorbic Acid Citric Acid Benzoic Acid in Orange JuiceDocument12 pagesAnalysis Ascorbic Acid Citric Acid Benzoic Acid in Orange JuiceHuong Nguyen100% (1)
- HPLC Method For The Analysis of Paracetamol Caffeine and Dipyron-1Document9 pagesHPLC Method For The Analysis of Paracetamol Caffeine and Dipyron-1Đoàn Lê Thuý HiềnNo ratings yet
- Determination Paraben in ShampooDocument5 pagesDetermination Paraben in ShampooromaincharlesNo ratings yet
- APP_Analysis-of-Capsaicin-and-Dihydrocapsaicin-in-Chili-Peppers-Using-Altus-HPLC-012226_01Document5 pagesAPP_Analysis-of-Capsaicin-and-Dihydrocapsaicin-in-Chili-Peppers-Using-Altus-HPLC-012226_01RocketManNo ratings yet
- HPLC Analysis of Caffeine Content in Energy DrinksDocument8 pagesHPLC Analysis of Caffeine Content in Energy DrinksDennis WrinNo ratings yet
- AnalysisDocument30 pagesAnalysisSai SaiNo ratings yet
- 09 KhabbazDocument6 pages09 KhabbazpreetysimpleNo ratings yet
- Lab Report Basic Instrumental Exp 6Document7 pagesLab Report Basic Instrumental Exp 6Azli AzmanNo ratings yet
- Technical Package OF XXXXXX: Submitted byDocument18 pagesTechnical Package OF XXXXXX: Submitted bySabbir Hossain ImranNo ratings yet
- Determination of Food Preservative1Document23 pagesDetermination of Food Preservative1Online NinaNo ratings yet
- STABILITY INDICATING ASSAY METHOD DEVELOPMENT AND VALIDATION OF PREGABALIN IN PHARMACEUTICAL DOSAGE FORMS BY RP-HPLC P.Sneha, Prathima SrinivasDocument10 pagesSTABILITY INDICATING ASSAY METHOD DEVELOPMENT AND VALIDATION OF PREGABALIN IN PHARMACEUTICAL DOSAGE FORMS BY RP-HPLC P.Sneha, Prathima SrinivasiajpsNo ratings yet
- Cefoperazone & Sulbactam InjectionDocument3 pagesCefoperazone & Sulbactam Injectionpatel_346879839No ratings yet
- Chromatography HPLCDocument16 pagesChromatography HPLCFilia YunizaNo ratings yet
- CHM260 Experiment 6Document12 pagesCHM260 Experiment 6Muhammad Azri HaziqNo ratings yet
- Application Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerDocument4 pagesApplication Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerPrianurraufikachmadNo ratings yet
- BioQuant2 (Schreibgeschützt)Document10 pagesBioQuant2 (Schreibgeschützt)Laorencia PraptiwiNo ratings yet
- Pharmaceutics-I - Practical Record - 1st Sem-M.pharmDocument44 pagesPharmaceutics-I - Practical Record - 1st Sem-M.pharmVenkatesh VenkateshNo ratings yet
- UA2 enDocument4 pagesUA2 enhilmayuniarNo ratings yet
- Chemical Testing of Hedysarum Alpinum Seeds For The Neurotoxin beta-ODAPDocument8 pagesChemical Testing of Hedysarum Alpinum Seeds For The Neurotoxin beta-ODAPalatnabirdNo ratings yet
- 22 PDFDocument7 pages22 PDFWidya Dwi Arini100% (1)
- Determination of Bovine Lactoferrin in Food by HPLC With A Heparin Affinity Column For Sample PreparationDocument6 pagesDetermination of Bovine Lactoferrin in Food by HPLC With A Heparin Affinity Column For Sample PreparationRusbel Andres RodriguezNo ratings yet
- Application Hand Sanitizer Analysis 8860 Fid 5994 2089en AgilentDocument8 pagesApplication Hand Sanitizer Analysis 8860 Fid 5994 2089en AgilentsedammullaogluNo ratings yet
- HPLC Determination of Caffeine in Coffee BeverageDocument7 pagesHPLC Determination of Caffeine in Coffee Beveragemuhammad ihklasulNo ratings yet
- Determination of Synthetic Food Colors, Caffeine, Sodium Benzoate and Potassium Sorbate in Sports DrinksDocument6 pagesDetermination of Synthetic Food Colors, Caffeine, Sodium Benzoate and Potassium Sorbate in Sports DrinksSurendra RamkissoonNo ratings yet
- Determination of Collagen in Cosmetics by HPLCDocument3 pagesDetermination of Collagen in Cosmetics by HPLCDaniel Camilo CarreñoNo ratings yet
- Determination of Benzalkonium Chloride in Nasal Drops by High-Performance Liquid ChromatographyDocument7 pagesDetermination of Benzalkonium Chloride in Nasal Drops by High-Performance Liquid ChromatographyRirin Arsita Pramita SariNo ratings yet
- HPLC - Determination of Caffeine in SodaDocument4 pagesHPLC - Determination of Caffeine in SodasusanaNo ratings yet
- HPLC - Determination of Caffeine in SodaDocument4 pagesHPLC - Determination of Caffeine in SodasusanaNo ratings yet
- Sop of Interleukin-6 (IL-6) Testing: A.PurposeDocument7 pagesSop of Interleukin-6 (IL-6) Testing: A.PurposeUMMID WashimNo ratings yet
- Direct Isolation: Promega DNA Analysis NotebookDocument6 pagesDirect Isolation: Promega DNA Analysis NotebookAndreea DuduNo ratings yet
- Quantitative Analysis of Active Constituent of ParacetamolDocument14 pagesQuantitative Analysis of Active Constituent of ParacetamolHennah UsmanNo ratings yet
- 4 RJPT 14-2-2021 Akshata ResearchDocument5 pages4 RJPT 14-2-2021 Akshata ResearchNutan Desai RaoNo ratings yet
- Bai Tap Phan Tich Cong Cu 17 385 1432152272011502127882Document5 pagesBai Tap Phan Tich Cong Cu 17 385 1432152272011502127882Huong ThuNo ratings yet
- Natamycin 6Document6 pagesNatamycin 6Ευαγγελία ΘεοχάρηNo ratings yet
- Experiment 1: Determination of Total Acidity of Vinegar: Final Laboratory ReportDocument13 pagesExperiment 1: Determination of Total Acidity of Vinegar: Final Laboratory ReportEunice OpinioNo ratings yet
- Molecular Biology LabDocument18 pagesMolecular Biology LabKristin MoserNo ratings yet
- 147-150 (Buslig) PDFDocument4 pages147-150 (Buslig) PDFTeguh M IhsanNo ratings yet
- Determination of Caffeine in Coke Soda.Document7 pagesDetermination of Caffeine in Coke Soda.WaitheraNo ratings yet
- 216 2017 256 Moesm1 EsmDocument12 pages216 2017 256 Moesm1 EsmmariusNo ratings yet
- 14 CreatinineDocument8 pages14 CreatinineAzhar Clinical Laboratory TubeNo ratings yet
- AlcoholDocument1 pageAlcoholtuan vănNo ratings yet
- MMC Da Kwa DDocument0 pagesMMC Da Kwa DNunu NouraaNo ratings yet
- 19 0426 - wwPTFE GHP - AN LockedDocument6 pages19 0426 - wwPTFE GHP - AN LockedChristian SFNo ratings yet
- Quetiapine FumarateDocument9 pagesQuetiapine FumarateVinaya SnehalathaNo ratings yet
- LONG CAFFEINE UVLong Caffeine UvDocument8 pagesLONG CAFFEINE UVLong Caffeine UvzaNo ratings yet
- Methods of Analysis For FluconazoleDocument6 pagesMethods of Analysis For FluconazoleJuan PerezNo ratings yet
- Caffeine Determination by HPLCDocument3 pagesCaffeine Determination by HPLCnga_yan0% (1)
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)
- The Center - Tiers - Final - May 2016Document1 pageThe Center - Tiers - Final - May 2016api-240966173No ratings yet
- Fall RetreatDocument1 pageFall Retreatapi-240966173No ratings yet
- Sample Course of Study: 9 Grade 10 Grade 11 Grade 12 GradeDocument1 pageSample Course of Study: 9 Grade 10 Grade 11 Grade 12 Gradeapi-240966173No ratings yet
- Photo Release FormDocument1 pagePhoto Release Formapi-240966173No ratings yet
- Financial Aid Night 2015Document28 pagesFinancial Aid Night 2015api-240966173No ratings yet
- Ncaa PowerpointDocument22 pagesNcaa Powerpointapi-240966173No ratings yet
- Future Lancer Night - New UpdateDocument17 pagesFuture Lancer Night - New Updateapi-240966173No ratings yet
- 8th To 9th Registration - Spring 2015Document17 pages8th To 9th Registration - Spring 2015api-240966173No ratings yet
- Handbook of Pollution Control and Waste Minimization Ed. Abbas GhassemiDocument516 pagesHandbook of Pollution Control and Waste Minimization Ed. Abbas Ghassemianhtuan66100% (1)
- Tubacero Catalogo OMBDocument52 pagesTubacero Catalogo OMB3189XNo ratings yet
- Chemistry 101A General College Chemistry: Torrey GlennDocument604 pagesChemistry 101A General College Chemistry: Torrey GlennKishore SurampalliNo ratings yet
- Supermix PC550 PDFDocument2 pagesSupermix PC550 PDFmanavNo ratings yet
- TM 9-850 (1951)Document153 pagesTM 9-850 (1951)degardin lucNo ratings yet
- Cleaning Validation in Pharmaceutical IndustriesDocument5 pagesCleaning Validation in Pharmaceutical IndustriesAbhishek RajNo ratings yet
- Multi Purpose Floor Coating - Nippon PaintDocument2 pagesMulti Purpose Floor Coating - Nippon PaintNippon Paint Total Coating and Construction SolutionsNo ratings yet
- Rock SaltDocument10 pagesRock SaltnananthNo ratings yet
- GOVPUB-C13 - PreparationDocument100 pagesGOVPUB-C13 - PreparationManoranjan MohapatraNo ratings yet
- Mud ViscosityDocument5 pagesMud Viscosityhindn162No ratings yet
- Design and Analysis Procedures For Shafts and Splines: Paul E. BurkeDocument21 pagesDesign and Analysis Procedures For Shafts and Splines: Paul E. BurkeBalasrinivasan Murugan100% (3)
- Nichrome60 Wire Data SheetDocument2 pagesNichrome60 Wire Data SheetvvingtsabtaNo ratings yet
- Inorganic Guide For PlasticsDocument8 pagesInorganic Guide For PlasticscarlonewmannNo ratings yet
- 52 BMA Question Solution by DESIGN INTEGRITYDocument5 pages52 BMA Question Solution by DESIGN INTEGRITYMd.Towhidul IslamNo ratings yet
- Weld Neck Body Flange Design Calculation: HE-CGI, HE-CG, Spiral Wound Gaskets For Heat ExchangersDocument25 pagesWeld Neck Body Flange Design Calculation: HE-CGI, HE-CG, Spiral Wound Gaskets For Heat ExchangersLipika GayenNo ratings yet
- Technical Data Sheet: Polyethylene Terephthalate FlakesDocument2 pagesTechnical Data Sheet: Polyethylene Terephthalate Flakesdorra snoussiNo ratings yet
- Evaporation Principles & Black Liquor PropertiesDocument15 pagesEvaporation Principles & Black Liquor PropertiesNaveenGoyalNo ratings yet
- TPD ABCIL CAUSTIC PLANT MANUAL RevDocument42 pagesTPD ABCIL CAUSTIC PLANT MANUAL Revjavier pividoriNo ratings yet
- Koc-P-002 Part 1 Rev 3Document29 pagesKoc-P-002 Part 1 Rev 3Hari KrishnanNo ratings yet
- 01 - En1999 - IntroductionDocument51 pages01 - En1999 - Introductionnebojsadj6411No ratings yet
- UOP ButamerDocument2 pagesUOP Butamerphaniraj_c100% (1)
- 6 Different Types of Electrical ConduitsDocument2 pages6 Different Types of Electrical Conduitskash30No ratings yet
- Paper ChromatographyDocument7 pagesPaper ChromatographySEHAR KHAN100% (1)
- Interconnection Challenge in Wire Bonding - Ag Alloy WireDocument25 pagesInterconnection Challenge in Wire Bonding - Ag Alloy WireChih Chen LeeNo ratings yet
- Conferinte Sectiunea I - cnc2018Document15 pagesConferinte Sectiunea I - cnc2018Cristina IoanaNo ratings yet
- Kiln Area Learning ReportDocument24 pagesKiln Area Learning ReportAbasiemekaNo ratings yet
- Dna Extraction MethodsDocument23 pagesDna Extraction MethodsWARDAH SHOAIBNo ratings yet