Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
59 viewsDiabetic Retinopathy Is Retinopathy (Damage To The Retina) Caused by Complications
Diabetic Retinopathy Is Retinopathy (Damage To The Retina) Caused by Complications
Uploaded by
Sasadara PramuditaDiabetic retinopathy is a complication of diabetes that damages the retina and can lead to blindness. It affects up to 80% of patients who have had diabetes for 10 years or more. The longer a person has diabetes, the higher their risk of developing diabetic retinopathy. Left untreated, it is responsible for 12% of blindness in the United States each year and is the leading cause of blindness for people aged 20-64. Proper monitoring and treatment can reduce the risk of vision loss from diabetic retinopathy by at least 90%.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Notes - Notes - Ophthalmology PDFDocument30 pagesNotes - Notes - Ophthalmology PDFRumana Ali100% (2)
- Diabetic RetinopathyDocument5 pagesDiabetic RetinopathyJayvee CornelioNo ratings yet
- Seminar On Opt 521 MainDocument13 pagesSeminar On Opt 521 MainCHIMA ONWUKA MONGNo ratings yet
- Diabetic ThyDocument3 pagesDiabetic ThynfacmaNo ratings yet
- Diabetic Retinopathy: Clinical Findings and Management: Review ArticleDocument4 pagesDiabetic Retinopathy: Clinical Findings and Management: Review ArticlewadejackNo ratings yet
- Symptoms and Stages of Diabetic RetinopathyDocument2 pagesSymptoms and Stages of Diabetic RetinopathyIshaani GargNo ratings yet
- Diabetes AND The Eye: Juliana Bentil Deborah AddoDocument50 pagesDiabetes AND The Eye: Juliana Bentil Deborah Addogideon A. owusuNo ratings yet
- Natural History of Diabetic RetinopathyDocument1 pageNatural History of Diabetic Retinopathyputri nirmalaNo ratings yet
- Chapter 4 Beginner's Guide To The Retina - TimRoDocument3 pagesChapter 4 Beginner's Guide To The Retina - TimRoFarah FarahNo ratings yet
- Diabetes and The EyeDocument7 pagesDiabetes and The EyeAndhita Satya Pratama GiovanniNo ratings yet
- RAV Nonproliferative Diabetic Retinopathy NPDRDocument1 pageRAV Nonproliferative Diabetic Retinopathy NPDRRidha Surya NugrahaNo ratings yet
- Diabetic RetinopathyDocument3 pagesDiabetic RetinopathyfynneroNo ratings yet
- Diabetic Retinopathy - Practice Essentials, Pathophysiology, EtiologyDocument11 pagesDiabetic Retinopathy - Practice Essentials, Pathophysiology, EtiologyIrma Kurniawati100% (1)
- Diabetic Retinopathy Presentations (Can Still Use The Older Classification)Document6 pagesDiabetic Retinopathy Presentations (Can Still Use The Older Classification)gdudex118811No ratings yet
- Abp EstadisticDocument6 pagesAbp EstadisticLaura López CastroNo ratings yet
- Deep Learing Technique For Diabetic Retinopath ClassificationDocument10 pagesDeep Learing Technique For Diabetic Retinopath ClassificationIJRASETPublicationsNo ratings yet
- MEDS 1. Diabetic Retinopathy: Practice Essentials, Pathophysiology, EtiologyDocument14 pagesMEDS 1. Diabetic Retinopathy: Practice Essentials, Pathophysiology, EtiologyVivi DeviyanaNo ratings yet
- Diabetic RetinopathyDocument55 pagesDiabetic RetinopathyMuhammad Bilal SaifulhaqNo ratings yet
- Diabetic RetinopathyDocument18 pagesDiabetic Retinopathymohelshiekh100% (3)
- Diabetic Retinopathy Symptoms Causes RisDocument7 pagesDiabetic Retinopathy Symptoms Causes Riskenneth MoragaNo ratings yet
- Diabetic Retinopathy by-DuaneBryantMDDocument7 pagesDiabetic Retinopathy by-DuaneBryantMDduanebryantmdNo ratings yet
- RetinopathyDocument64 pagesRetinopathySiti NcitNo ratings yet
- Diabetic With The EyeDocument50 pagesDiabetic With The EyeJianhua ShiNo ratings yet
- Diabetic Retinopathy DR KatariaDocument65 pagesDiabetic Retinopathy DR KatariaDr Sandeep Kataria100% (1)
- Diabetic Retinopathy - StudentDocument24 pagesDiabetic Retinopathy - StudentaulioraraNo ratings yet
- Diabetic RetinopathyDocument8 pagesDiabetic RetinopathyGul SapiNo ratings yet
- Diabetic RetinopathyDocument13 pagesDiabetic RetinopathyjaysonNo ratings yet
- UntitledDocument2 pagesUntitledBen YangNo ratings yet
- FinalijserScenario of Diabetic RetinopathyDocument10 pagesFinalijserScenario of Diabetic RetinopathysavithriNo ratings yet
- Lecture 11 Retina 1Document44 pagesLecture 11 Retina 1ؤيؤييسيNo ratings yet
- Retinal Vascular DisordersDocument52 pagesRetinal Vascular Disorderssushma shresthaNo ratings yet
- Diabetic RetinopathyDocument4 pagesDiabetic RetinopathybencleeseNo ratings yet
- To Study The Prevalence of Eye Complications Among Diabetic PatientDocument10 pagesTo Study The Prevalence of Eye Complications Among Diabetic PatientMuna Hassan MustafaNo ratings yet
- 02 DR-Classification PDFDocument5 pages02 DR-Classification PDFArghirescuNo ratings yet
- Nonproliferative Diabetic Retinopathy and Diabetic Macular EdemaDocument37 pagesNonproliferative Diabetic Retinopathy and Diabetic Macular EdemaBhumika RathNo ratings yet
- Diabetes and EyesDocument13 pagesDiabetes and EyesRishabh TickooNo ratings yet
- Retinopati Diabetikum: Arso Pranindyo UtomoDocument14 pagesRetinopati Diabetikum: Arso Pranindyo Utomosusilo hadi wNo ratings yet
- Ignacio - 6TH Paper SGD OphthaDocument4 pagesIgnacio - 6TH Paper SGD OphthaRichelle IgnacioNo ratings yet
- DM SeminarDocument79 pagesDM Seminarendalew mulugetaNo ratings yet
- Diabetic RetinopathyDocument5 pagesDiabetic RetinopathyAndreiNo ratings yet
- Diabetic RetinopathyDocument25 pagesDiabetic RetinopathyThe Eye Digest100% (2)
- Diabetic Retinopathy: From Diagnosis to TreatmentFrom EverandDiabetic Retinopathy: From Diagnosis to TreatmentRating: 3 out of 5 stars3/5 (3)
- Biochemical and Molecular Mechanisms of Diabetic RetinopathyDocument9 pagesBiochemical and Molecular Mechanisms of Diabetic RetinopathyNor Ubudiah SetiNo ratings yet
- Westjmed00159 0115Document3 pagesWestjmed00159 0115bangd1f4nNo ratings yet
- Practice: Diabetic RetinopathyDocument1 pagePractice: Diabetic RetinopathyRina ChairunnisaNo ratings yet
- Diabetic Retinopathy: by Mohamad Faizul Abu Hanifa Tillai SharimalaDocument13 pagesDiabetic Retinopathy: by Mohamad Faizul Abu Hanifa Tillai SharimalaMohamad Faizul Abu HanifaNo ratings yet
- Jurnal Retinopati DiabetikDocument11 pagesJurnal Retinopati DiabetikAzwaydaRayhanaSariNo ratings yet
- Diabetic Retinopathy - Classification and Clinical Features - UpToDateDocument38 pagesDiabetic Retinopathy - Classification and Clinical Features - UpToDateRachmatBimanjayaNo ratings yet
- Retinopathy, Diabetic, BackgroundDocument12 pagesRetinopathy, Diabetic, BackgroundmyusuffrNo ratings yet
- Diabetes ComplicationsDocument7 pagesDiabetes ComplicationstalaNo ratings yet
- Diabetic RetinopathyDocument2 pagesDiabetic RetinopathySanal KumarNo ratings yet
- Diabetic Retinopathy - Aetiopathogenesis, Clinical Presentation andDocument83 pagesDiabetic Retinopathy - Aetiopathogenesis, Clinical Presentation andOlayemi Olorundare0% (1)
- Ocular Issues and Endocrine Diseases in Small Animal Practice (Canine Eye Disease)Document11 pagesOcular Issues and Endocrine Diseases in Small Animal Practice (Canine Eye Disease)maddierogersNo ratings yet
- Diabetic Retinopathy (Wills Eye Manual)Document9 pagesDiabetic Retinopathy (Wills Eye Manual)Yuri YogyaNo ratings yet
- Refrat Retinopati-DiabetikDocument20 pagesRefrat Retinopati-DiabetikDea NabilaNo ratings yet
- Retinopati DiabetikDocument5 pagesRetinopati DiabetikMuhammad Afriadi HamdanNo ratings yet
- Diabetic RetinopathyDocument55 pagesDiabetic RetinopathyDrSaid Hussein GediNo ratings yet
- How Does Hipertension Affects Your Eye - Anure 2011Document13 pagesHow Does Hipertension Affects Your Eye - Anure 2011Germano Freire Bezerra FilhoNo ratings yet
- Diabetic Retinopathy: Introduction to Novel Treatment StrategiesFrom EverandDiabetic Retinopathy: Introduction to Novel Treatment StrategiesNo ratings yet
- The Masculoskeletal SystemDocument6 pagesThe Masculoskeletal Systemapi-296199660No ratings yet
- UAV - NPTEL - IIT RoorkeeDocument14 pagesUAV - NPTEL - IIT Roorkeesankalp chopkarNo ratings yet
- Nasscom RecommendationsDocument11 pagesNasscom RecommendationsSANTANU DASNo ratings yet
- Modeling and Analysis of DC-DC Converters Under Pulse Skipping ModulationDocument6 pagesModeling and Analysis of DC-DC Converters Under Pulse Skipping Modulationad duybgNo ratings yet
- A Detailed Lesson Plan in English 9: (Using Adverbs in Narration)Document8 pagesA Detailed Lesson Plan in English 9: (Using Adverbs in Narration)Faith Malinao100% (1)
- En LNG Air Products Floating LNG Plant CapabilitiesDocument2 pagesEn LNG Air Products Floating LNG Plant CapabilitiesMshelia M.No ratings yet
- Master Degree InformationDocument3 pagesMaster Degree InformationBivash NiroulaNo ratings yet
- Process Design and Economics For The Conversion of The Lignocellulosic To HydrocarbonsDocument133 pagesProcess Design and Economics For The Conversion of The Lignocellulosic To HydrocarbonsCristhian Camilo Vargas QuinteroNo ratings yet
- Naiad GettingstartedDocument37 pagesNaiad GettingstartedYurivanovNo ratings yet
- AND8331/D Quasi-Resonant Current-Mode Controller For High - Power Ac-Dc AdaptersDocument16 pagesAND8331/D Quasi-Resonant Current-Mode Controller For High - Power Ac-Dc AdaptersLucía MitchellNo ratings yet
- No Smoking in The TriangleDocument60 pagesNo Smoking in The TriangleThomas L HaydenNo ratings yet
- Indian ClimateDocument7 pagesIndian ClimatePrakash Kumar Kumar100% (1)
- Administrative Management in Education: Mark Gennesis B. Dela CernaDocument36 pagesAdministrative Management in Education: Mark Gennesis B. Dela CernaMark Gennesis Dela CernaNo ratings yet
- 5.2 Extreme Value Theorem, Global Versus Local Extrema, and CriticalDocument26 pages5.2 Extreme Value Theorem, Global Versus Local Extrema, and Criticalazzamomar2018No ratings yet
- AFATL-TR-72-401 - Developement of 20MM and 30MM Plastic-Aluminium Cartridge Cases (1972)Document91 pagesAFATL-TR-72-401 - Developement of 20MM and 30MM Plastic-Aluminium Cartridge Cases (1972)defendercc130No ratings yet
- How To Uncompress and List An Initramfs Content On LinuxDocument10 pagesHow To Uncompress and List An Initramfs Content On Linuxvpalmar8871No ratings yet
- Module 4 ResearchDocument9 pagesModule 4 ResearchJegg AsisNo ratings yet
- 13.n-p-n TransistorDocument4 pages13.n-p-n TransistorkirtiNo ratings yet
- Schallert 2019Document7 pagesSchallert 2019Dian Putri NingsihNo ratings yet
- BC23000122Document1 pageBC23000122azharNo ratings yet
- Int Org 2Document60 pagesInt Org 2Alara MelnichenkoNo ratings yet
- Ce Booklet Fall 14Document28 pagesCe Booklet Fall 14api-279863771No ratings yet
- Eaton Guide Specification Notes and Instructions To SpecwriterDocument17 pagesEaton Guide Specification Notes and Instructions To SpecwriterIsrael Luna PenasNo ratings yet
- ISO 22301 Lead Implementer - Two Page BrochureDocument2 pagesISO 22301 Lead Implementer - Two Page BrochurePECBCERTIFICATIONNo ratings yet
- Ap18 English Literature q1 PDFDocument12 pagesAp18 English Literature q1 PDFMartin CoscolluelaNo ratings yet
- BillDocument3 pagesBillTha OoNo ratings yet
- Development: CHAPTEK Green Governance: Sustainable HumanDocument24 pagesDevelopment: CHAPTEK Green Governance: Sustainable HumanAlisha VarandaniNo ratings yet
- Shirish - Kumar - Software - Engineer NewDocument1 pageShirish - Kumar - Software - Engineer NewAbhishek KumarNo ratings yet
- Diasporic Writing QuestionsDocument31 pagesDiasporic Writing QuestionsMEIMOONA HUSNAIN100% (1)
- Trompenaars' Cultural Dimensions in France: February 2021Document15 pagesTrompenaars' Cultural Dimensions in France: February 2021Như Hảo Nguyễn NgọcNo ratings yet
Diabetic Retinopathy Is Retinopathy (Damage To The Retina) Caused by Complications
Diabetic Retinopathy Is Retinopathy (Damage To The Retina) Caused by Complications
Uploaded by
Sasadara Pramudita0 ratings0% found this document useful (0 votes)
59 views15 pagesDiabetic retinopathy is a complication of diabetes that damages the retina and can lead to blindness. It affects up to 80% of patients who have had diabetes for 10 years or more. The longer a person has diabetes, the higher their risk of developing diabetic retinopathy. Left untreated, it is responsible for 12% of blindness in the United States each year and is the leading cause of blindness for people aged 20-64. Proper monitoring and treatment can reduce the risk of vision loss from diabetic retinopathy by at least 90%.
Original Description:
dggha
Original Title
Diabetic Retinopathy
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentDiabetic retinopathy is a complication of diabetes that damages the retina and can lead to blindness. It affects up to 80% of patients who have had diabetes for 10 years or more. The longer a person has diabetes, the higher their risk of developing diabetic retinopathy. Left untreated, it is responsible for 12% of blindness in the United States each year and is the leading cause of blindness for people aged 20-64. Proper monitoring and treatment can reduce the risk of vision loss from diabetic retinopathy by at least 90%.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
59 views15 pagesDiabetic Retinopathy Is Retinopathy (Damage To The Retina) Caused by Complications
Diabetic Retinopathy Is Retinopathy (Damage To The Retina) Caused by Complications
Uploaded by
Sasadara PramuditaDiabetic retinopathy is a complication of diabetes that damages the retina and can lead to blindness. It affects up to 80% of patients who have had diabetes for 10 years or more. The longer a person has diabetes, the higher their risk of developing diabetic retinopathy. Left untreated, it is responsible for 12% of blindness in the United States each year and is the leading cause of blindness for people aged 20-64. Proper monitoring and treatment can reduce the risk of vision loss from diabetic retinopathy by at least 90%.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 15
Diabetic Retinopathy
Diabetic retinopathy is retinopathy (damage to the retina) caused by complications
of diabetes, which can eventually lead to blindness.
It is an ocular manifestation of diabetes, a systemic disease, which affects up to 80
percent of all patients who have had diabetes for 10 years or more. Despite these
intimidating statistics, research indicates that at least 90% of these new cases could
be reduced if there was proper and vigilant treatment and monitoring of the eyes.
The longer a person has diabetes, the higher his or her chances of developing
diabetic retinopathy. Each year in the United States, diabetic retinopathy accounts
for 12% of all new cases of blindness. It is also the leading cause of blindness for
people aged 20 to 64 years.
Etiology
Duration of diabetes
In patients with type I diabetes, no clinically significant retinopathy can be seen in
the first 5 years after the initial diagnosis of diabetes is made. After 10-15 years, 25-
50% of patients show some signs of retinopathy. This prevalence increases to 75-
95% after 15 years and approaches 100% after 30 years of diabetes. Proliferative
diabetic retinopathy (PDR) is rare within the first decade of type I diabetes diagnosis
but increases to 14-17% by 15 years, rising steadily thereafter.
In patients with type II diabetes, the incidence of diabetic retinopathy increases with
the disease duration. Of patients with type II diabetes, 23% have nonproliferative
diabetic retinopathy (NPDR) after 11-13 years, 41% have NPDR after 14-16 years,
and 60% have NPDR after 16 years.
Hypertension and hyperlipidemia
Systemic hypertension, in the setting of diabetic nephropathy, correlates well with
the presence of retinopathy. Independently, hypertension also may complicate
diabetes in that it may result in hypertensive retinal vascular changes superimposed
on the preexisting diabetic retinopathy, further compromising retinal blood flow.
Proper management of hyperlipidemia (elevated serum lipids) may result in less
retinal vessel leakage and hard exudate formation, but the reason behind this is
unclear.
Pregnancy
Pregnant women with proliferative diabetic retinopathy do poorly without
treatment, but those who have had prior panretinal photocoagulation remain stable
throughout pregnancy. Pregnant women without diabetic retinopathy run a 10% risk
of developing NPDR during their pregnancy; of those with preexisting NPDR, 4%
progress to the proliferative type.
Epidemiology
Of the approximately 16 million Americans with diabetes, 50% are unaware that
they have it. Of those who know they have diabetes, only half receive appropriate
eye care. Thus, it is not surprising that diabetic retinopathy is the leading cause of
new blindness in persons aged 25-74 years in the United States.
Approximately 700,000 Americans have proliferative diabetic retinopathy, with an
annual incidence of 65,000. Approximately 500,000 persons have clinically significant
macular edema, with an annual incidence of 75,000.
Diabetes is responsible for approximately 8000 eyes becoming blinded each year,
meaning that diabetes is responsible for 12% of blindness.[17] The rate is even
higher among certain ethnic groups. An increased risk of diabetic retinopathy
appears to exist in patients of Native American, Hispanic, and African American
heritage.
With increasing duration of diabetes or with increasing age since its onset, there is a
higher risk of developing diabetic retinopathy and its complications, including
diabetic macular edema or proliferative diabetic retinopathy.
Sign and symptoms
Diabetic retinopathy often has no early warning signs. Even macular edema, which
may cause vision loss more rapidly, may not have any warning signs for some time.
In general, however, a person with macular edema is likely to have blurred vision,
making it hard to do things like read or drive. In some cases, the vision will get better
or worse during the day.
In the first stage which is called non-proliferative diabetic retinopathy (NPDR) there
are no symptoms, it is not visible to the naked eye and patients will have 20/20
vision. The only way to detect NPDR is by fundus photography, in which
microaneurysms (microscopic blood-filled bulges in the artery walls) can be seen. If
there is reduced vision, fluorescein angiography can be done to see the back of the
eye. Narrowing or blocked retinal blood vessels can be seen clearly and this is called
retinal ischemia (lack of blood flow).
Macular edema may occur in which blood vessels leak contents into the macular
region can happen at all stages of NPDR. The macular edema symptoms are blurring,
darkening or distorted images with not the same between two eyes. 10 percent of
diabetic patients will get vision loss related with macular edema. Optical Coherence
Tomography can show areas of retinal thickening (fluid accumulation) of macular
edema.[7]
On the second stage, as abnormal new blood vessels (neovascularisation) form at
the back of the eye as a part of proliferative diabetic retinopathy (PDR), they can
burst and bleed (vitreous hemorrhage) and blur vision, because the new blood
vessels are weak. The first time this happens, it may not be very severe. In most
cases, it will leave just a few specks of blood, or spots, floating in a person's visual
field, though the spots often go away after a few hours.
These spots are often followed within a few days or weeks by a much greater
leakage of blood, which blurs vision. In extreme cases, a person will only be able to
tell light from dark in that eye. It may take the blood anywhere from a few days to
months or even years to clear from the inside of the eye, and in some cases the
blood will not clear. These types of large hemorrhages tend to happen more than
once, often during sleep.
On funduscopic exam, a doctor will see cotton wool spots, flame hemorrhages
(similar lesions are also caused by the alpha-toxin of Clostridium novyi), and dot-blot
hemorrhages.
Pathogenesis
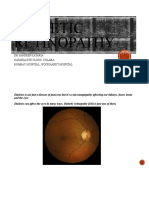
Illustration depicting diabetic retinopathy
Diabetic retinopathy is the result of microvascular retinal changes. Hyperglycemia-
induced intramural pericyte death and thickening of the basement membrane lead
to incompetence of the vascular walls. These damages change the formation of the
blood-retinal barrier and also make the retinal blood vessels become more
permeable.
The pericyte death is caused when "hyperglycemia persistently activates protein
kinase C- (PKC-, encoded by Prkcd) and p38 mitogen-activated protein kinase
(MAPK) to increase the expression of a previously unknown target of PKC- signaling,
Src homology-2 domaincontaining phosphatase-1 (SHP-1), a protein tyrosine
phosphatase. This signaling cascade leads to PDGF receptor- dephosphorylation and
a reduction in downstream signaling from this receptor, resulting in pericyte
apoptosis"
Small blood vessels such as those in the eye are especially vulnerable to poor
blood sugar (blood glucose) control. An overaccumulation of glucose and/or fructose
damages the tiny blood vessels in the retina. During the initial stage, called
nonproliferative diabetic retinopathy (NPDR), most people do not notice any change
in their vision. Early changes that are reversible and do not threaten central vision
are sometimes termed simplex retinopathy or background retinopathy.
Some people develop a condition called macular edema. It occurs when the
damaged blood vessels leak fluid and lipids onto the macula, the part of the retina
that lets us see detail. The fluid makes the macula swell, which blurs vision.
Proliferative diabetic retinopathy
As the disease progresses, severe nonproliferative diabetic retinopathy enters an
advanced, or proliferative (PDR), stage when blood vessels proliferate (i.e. grow).
The lack of oxygen in the retina causes fragile, new, blood vessels to grow along the
retina and in the clear, gel-like vitreous humour that fills the inside of the eye.
Without timely treatment, these new blood vessels can bleed, cloud vision, and
destroy the retina. Fibrovascular proliferation can also cause tractional retinal
detachment. The new blood vessels can also grow into the angle of the anterior
chamber of the eye and cause neovascular glaucoma.
Nonproliferative diabetic retinopathy shows up as cotton wool spots, or
microvascular abnormalities or as superficial retinal hemorrhages. Even so, the
advanced proliferative diabetic retinopathy (PDR) can remain asymptomatic for a
very long time, and so should be monitored closely with regular checkups.
Pathophysiology
Fundus photograph of early background diabetic retinopathy showing multiple
microaneurysms.
The exact mechanism by which diabetes causes retinopathy remains unclear, but
several theories have been postulated to explain the typical course and history of
the disease.[1, 2]
Growth hormone
Growth hormone appears to play a causative role in the development and
progression of diabetic retinopathy. Diabetic retinopathy has been shown to be
reversible in women who had postpartum hemorrhagic necrosis of the pituitary
gland (Sheehan syndrome). This led to the controversial practice of pituitary ablation
to treat or prevent diabetic retinopathy in the 1950s. This technique has since been
abandoned because of numerous systemic complications and the discovery of the
effectiveness of laser treatment.
Platelets and blood viscosity
The variety of hematologic abnormalities seen in diabetes, such as increased
erythrocyte aggregation, decreased red blood cell deformability, increased platelet
aggregation, and adhesion, predispose the patient to sluggish circulation, endothelial
damage, and focal capillary occlusion. This leads to retinal ischemia, which, in turn,
contributes to the development of diabetic retinopathy.
Aldose reductase and vasoproliferative factors
Fundamentally, diabetes mellitus (DM) causes abnormal glucose metabolism as a
result of decreased levels or activity of insulin. Increased levels of blood glucose are
thought to have a structural and physiologic effect on retinal capillaries causing them
to be both functionally and anatomically incompetent.
A persistent increase in blood glucose levels shunts excess glucose into the aldose
reductase pathway in certain tissues, which converts sugars into alcohol (eg, glucose
into sorbitol, galactose to dulcitol). Intramural pericytes of retinal capillaries seem to
be affected by this increased level of sorbitol, eventually leading to the loss of their
primary function (ie, autoregulation of retinal capillaries). This results in weakness
and eventual saccular outpouching of capillary walls. These microaneurysms are the
earliest detectable signs of DM retinopathy. (See the image below.)
Fundus photograph of early background diabetic retinopathy showing multiple
microaneurysms.
Using nailfold video capillaroscopy, a high prevalence of capillary changes is detected
in patients with diabetes, particularly those with retinal damage. This reflects a
generalized microvessel involvement in both type 1 and type 2 diabetes.[15]
Ruptured microaneurysms result in retinal hemorrhages either superficially (flame-
shaped hemorrhages) or in deeper layers of the retina (blot and dot hemorrhages).
(See the image below.)
Retinal findings in background diabetic retinopathy, including blot hemorrhages
(long arrow), microaneurysms (short arrow), and hard exudates (arrowhead).
Increased permeability of these vessels results in leakage of fluid and proteinaceous
material, which clinically appears as retinal thickening and exudates. If the swelling
and exudation involve the macula, a diminution in central vision may be
experienced.
Macular edema
Macular edema is the most common cause of vision loss in patients with
nonproliferative diabetic retinopathy (NPDR). However, it is not exclusively seen in
patients with NPDR; it may also complicate cases of proliferative diabetic
retinopathy.
Fluorescein angiogram demonstrating foveal dye leakage caused by macular edema.
Fundus photograph of clinically significant macular edema demonstrating retinal
exudates within the fovea.
Another theory to explain the development of macular edema focuses on the
increased levels of diacylglycerol from the shunting of excess glucose. This is thought
to activate protein kinase C, which, in turn, affects retinal blood dynamics, especially
permeability and flow, leading to fluid leakage and retinal thickening.
Hypoxia
As the disease progresses, eventual closure of the retinal capillaries occurs, leading
to hypoxia. Infarction of the nerve fiber layer leads to the formation of cotton-wool
spots, with associated stasis in axoplasmic flow.
More extensive retinal hypoxia triggers compensatory mechanisms in the eye to
provide enough oxygen to tissues. Venous caliber abnormalities, such as venous
beading, loops, and dilation, signify increasing hypoxia and almost always are seen
bordering the areas of capillary nonperfusion. Intraretinal microvascular
abnormalities represent either new vessel growth or remodeling of preexisting
vessels through endothelial cell proliferation within the retinal tissues to act as
shunts through areas of nonperfusion.
Neovascularization
Further increases in retinal ischemia trigger the production of vasoproliferative
factors that stimulate new vessel formation. The extracellular matrix is broken down
first by proteases, and new vessels arising mainly from the retinal venules penetrate
the internal limiting membrane and form capillary networks between the inner
surface of the retina and the posterior hyaloid face. (See the images below.)
New vessel formation on the surface of the retina (neovascularization elsewhere)
An area of neovascularization that leaks fluorescein on angiography.
Boat-shaped preretinal hemorrhage associated with neovascularization elsewhere.
In patients with proliferative diabetic retinopathy (PDR), nocturnal intermittent
hypoxia/reoxygenation that results from sleep-disordered breathing may be a risk
factor for iris and/or angle neovascularization.[16]
Neovascularization is most commonly observed at the borders of perfused and
nonperfused retina and most commonly occurs along the vascular arcades and at
the optic nerve head. The new vessels break through and grow along the surface of
the retina and into the scaffold of the posterior hyaloid face. By themselves, these
vessels rarely cause visual compromise, but they are fragile and highly permeable.
These delicate vessels are disrupted easily by vitreous traction, which leads to
hemorrhage into the vitreous cavity or the preretinal space.
These new blood vessels initially are associated with a small amount of fibroglial
tissue formation. However, as the density of the neovascular frond increases, so
does the degree of fibrous tissue formation.
In later stages, the vessels may regress, leaving only networks of avascular fibrous
tissue adherent to both the retina and the posterior hyaloid face. As the vitreous
contracts, it may exert tractional forces on the retina via these fibroglial connections.
Traction may cause retinal edema, retinal heterotropia, and both tractional retinal
detachments and retinal tear formation with subsequent detachment.
Risk factors
All people with diabetes mellitus are at risk those with Type I diabetes and those
with Type II diabetes. The longer a person has diabetes, the higher the risk of
developing some ocular problem. Between 40 to 45 percent of Americans diagnosed
with diabetes have some stage of diabetic retinopathy. After 20 years of diabetes,
nearly all patients with Type I diabetes and >60% of patients with Type II diabetes
have some degree of retinopathy; however, these statistics were published in 2002
using data from four years earlier, limiting the usefulness of the research. The
subjects would have been diagnosed with diabetes in the late 1970s, before modern
fast acting insulin and home glucose testing.
Prior studies had also assumed a clear glycemic threshold between people at high
and low risk of diabetic retinopathy.
However, it has been shown that the widely accepted WHO and American Diabetes
Association diagnostic cutoff for diabetes of a fasting plasma glucose 7.0 mmol/l
(126 mg/dl) does not accurately identify diabetic retinopathy among patients.[14]
The cohort study included a multi-ethnic, cross-sectional adult population sample in
the US, as well as two cross-sectional adult populations in Australia. For the US-
based component of the study, the sensitivity was 34.7% and specificity was 86.6%.
For patients at similar risk to those in this study (15.8% had diabetic retinopathy),
this leads to a positive predictive value of 32.7% and negative predictive value of
87.6%.
Published rates vary between trials, the proposed explanation being differences in
study methods and reporting of prevalence rather than incidence values.
During pregnancy, diabetic retinopathy may also be a problem for women with
diabetes. It is recommended, that all pregnant women with diabetes have dilated
eye examinations each trimester to protect their vision.
People with Down's syndrome, who have three copies of chromosome 21, almost
never acquire diabetic retinopathy. This protection appears to be due to the
elevated levels of endostatin, an anti-angiogenic protein, derived from collagen XVIII.
The collagen XVIII gene is located on chromosome 21.
Physical Examinations
The mainstay of diagnosing diabetic retinopathy is a complete ophthalmic
examination and dilated retinal examination by an ophthalmologist or retina
specialist or retina surgeon.
Outreach screening has the potential to increase screening coverage of high-risk
patients with diabetic retinopathy in remote and resource-poor settings or in areas
in which no ophthalmologist or retina specialist is available, without the risk of
missing diabetic retinopathy and the opportunity to prevent vision loss.[20]
Microaneurysms
Microaneurysms are the earliest clinical sign of diabetic retinopathy and occur
secondary to capillary wall outpouching due to pericyte loss. They appear as small
red dots in the superficial retinal layers, and there is fibrin and red blood cell
accumulation in the microaneurysm lumen. A rupture produces blot/flame
hemorrhages. Affected areas may appear yellowish in time, as endothelial cells
proliferate and produce basement membrane.
Dot and blot hemorrhages
Dot and blot hemorrhages occur as microaneurysms rupture in the deeper layers of
the retina, such as the inner nuclear and outer plexiform layers. These appear similar
to microaneurysms if they are small; fluorescein angiography may be needed to
distinguish between the two.
Flame-shaped hemorrhages
Flame-shaped hemorrhages are splinter hemorrhages that occur in the more
superficial nerve fiber layer.
Retinal edema and hard exudates
Retinal edema and hard exudates are caused by the breakdown of the blood-retina
barrier, allowing leakage of serum proteins, lipids, and protein from the vessels.
Cotton-wool spots
Cotton-wool spots are nerve fiber layer infarctions from occlusion of precapillary
arterioles. With the use of fluorescein angiography, there is no capillary perfusion.
These are frequently bordered by microaneurysms and vascular hyperpermeability.
Venous loops and venous beading
Venous loops and venous beading frequently occur adjacent to areas of
nonperfusion and reflect increasing retinal ischemia. Their occurrence is the most
significant predictor of progression to proliferative diabetic retinopathy.
Intraretinal microvascular abnormalities
Intraretinal microvascular abnormalities are remodeled capillary beds without
proliferative changes. These collateral vessels do not leak on fluorescein angiography
and can usually be found on the borders of the nonperfused retina.
Macular edema
Macular edema is the leading cause of visual impairment in patients with diabetes. A
reported 75,000 new cases of macular edema are diagnosed annually. This may be
due to functional damage and necrosis of retinal capillaries.
Clinically significant macular edema is defined as any of the following:
Retinal thickening located 500 m or less from the center of the foveal
avascular zone (FAZ)
Hard exudates with retinal thickening 500 m or less from the center of the
FAZ
Retinal thickening 1 disc area or larger in size located within 1 disc diameter
of the FAZ
Nonproliferative diabetic retinopathy
Mild nonproliferative diabetic retinopathy (NPDR) is indicated by the presence of at
least 1 microaneurysm. Mild NPDR reflects structural changes in the retina caused by
the physiological and anatomical effects of diabetes.
More advanced stages of NPDR reflect the increasing retinal ischemia, setting the
stage for proliferative changes.
Moderate nonproliferative diabetic retinopathy includes the presence of
hemorrhages, microaneurysms, and hard exudates. With this condition, soft
exudates, venous beading, and intraretinal microvascular abnormalities (IRMA)
occur less frequently than with severe NPDR.
Severe NPDR (4-2-1) is characterized by hemorrhages and microaneurysms in 4
quadrants, with venous beading in at least 2 quadrants and IRMA in at least 1
quadrant.
Proliferative diabetic retinopathy
Neovascularization is the hallmark of PDR. It most often occurs near the optic disc
(neovascularization of the disc [NVD]) or within 3 disc diameters of the major retinal
vessels (neovascularization elsewhere [NVE]). (See the image below.)
New vessel formation on the surface of the retina (neovascularization elsewhere)
Preretinal hemorrhages appear as pockets of blood within the potential space
between the retina and the posterior hyaloid face. As blood pools within this space,
they may appear boat shaped. (See the image below.)
Boat-shaped preretinal hemorrhage associated with neovascularization elsewhere.
Hemorrhage into the vitreous may appear as a diffuse haze or as clumps of blood
clots within the gel.
Fibrovascular tissue proliferation is usually seen associated with the neovascular
complex and also may appear avascular when the vessels have already regressed.
(See the images below.)
Fibrovascular proliferations within the vitreous cavity
Extensive fibrovascular proliferations within and around the optic disc
Traction retinal detachments usually appear tented up, immobile, and concave, as
compared to rhegmatogenous retinal detachments, which are bullous, mobile, and
convex. A combination of both mechanisms is not an uncommon finding, however.
Macular edema is the leading cause of visual impairment in patients with diabetes. It
may result from functional damage and necrosis of retinal capillaries. In cases of
PDR, edema also may be caused by retinal traction if the retina is sufficiently
elevated away from the retinal pigment epithelium.
Proliferative diabetic retinopathy is classified as early or high risk.[21] In early PDR,
new vessels are present, but they do not meet the criteria for high-risk PDR. In high-
risk PDR, NVD is one-third to one-half, or greater, of the disc area (DA); there may be
any amount of NVD with vitreous or preretinal hemorrhage; and NVE is one-half or
greater of the DA, with preretinal or vitreous hemorrhage.
Diagnosis
Diabetic retinopathy is detected during an eye examination that includes:
Visual acuity test: This test uses an eye chart to measure how well a person
sees at various distances (i.e., visual acuity).
Pupil dilation: The eye care professional places drops into the eye to widen
the pupil. This allows him or her to see more of the retina and look for signs
of diabetic retinopathy. After the examination, close-up vision may remain
blurred for several hours.
Ophthalmoscopy or fundus photography: Ophthalmoscopy is an examination
of the retina in which the eye care professional: (1) looks through a slit lamp
biomicroscope with a special magnifying lens that provides a narrow view of
the retina, or (2) wearing a headset (indirect ophthalmoscope) with a bright
light, looks through a special magnifying glass and gains a wide view of the
retina. Hand-held ophthalmoscopy is insufficient to rule out significant and
treatable diabetic retinopathy. Fundus photography generally recreate
considerably larger areas of the fundus, and has the advantage of photo
documentation for future reference, as well as availing the image to be
examined by a specialist at another location and/or time.
Fundus Fluorescein angiography (FFA): This is an imaging technique which
relies on the circulation of Fluorescein dye to show staining, leakage, or non-
perfusion of the retinal and choroidal vasculature.
Optical coherence tomography (OCT): This is an optical imaging modality
based upon interference, and analogous to ultrasound. It produces cross-
sectional images of the retina (B-scans) which can be used to measure the
thickness of the retina and to resolve its major layers, allowing the
observation of swelling.
Digital Retinal Screening Programs: Systematic programs for the early
detection of eye disease including diabetic retinopathy are becoming more
common, such as in the UK, where all people with diabetes are offered
retinal screening at least annually. This involves digital image capture and
transmission of the images to a digital reading center for evaluation and
treatment referral. See Vanderbilt Ophthalmic Imaging Center[17] and the
NHS Diabetic Eye Screening Programme[18] The name Diabetic Retinopathy
Screening Service (DRSS) is also used.[19]
Computer Vision Approach: It is a System developed by Researchers at IIT
Kharagpur in collaboration with IBM India. It uses data analytics capabilities
to automatically compare and analyse retina images of the patient. It can tell
if the patient has DR and also provides risk categorisation ranging from low to
medium and high.[20]
Slit Lamp Biomicroscopy Retinal Screening Programs: Systematic programs
for the early detection of diabetic retinopathy using slit-lamp biomicroscopy.
These exist either as a standalone scheme or as part of the Digital program
(above) where the digital photograph was considered to lack enough clarity
for detection and/or diagnosis of any retinal abnormality.
The eye care professional will look at the retina for early signs of the disease, such
as:
1. leaking blood vessels,
2. retinal swelling, such as macular edema,
3. pale, fatty deposits on the retina (exudates) signs of leaking blood vessels,
4. damaged nerve tissue (neuropathy), and
5. any changes in the blood vessels.
If macular edema is suspected, FFA and sometimes OCT may be performed.
According to a DRSS user manual, poor quality images (which may apply to other
methods) may be caused by cataract, poor dilation, ptosis, external ocular condition,
or learning difficulties. There may be artefacts caused by dust, dirt, condensation, or
smudge.
Management
There are three major treatments for diabetic retinopathy, which are very
effective
[
citation needed
]
in reducing vision loss from this disease. In fact, even
people with advanced retinopathy have a 90 percent chance of keeping their vision
when they get treatment before the retina is severely damaged. These three
treatments are laser surgery, injection of corticosteroids or Anti-VEGF into the eye,
and vitrectomy.
Although these treatments are very successful (in slowing or stopping further vision
loss), they do not cure diabetic retinopathy. Caution should be exercised in
treatment with laser surgery since it causes a loss of retinal tissue. It is often more
prudent to inject triamcinolone or Anti-VEGF. In some patients it results in a marked
increase of vision, especially if there is an edema of the macula.
Avoiding tobacco use and correction of associated hypertension are important
therapeutic measures in the management of diabetic retinopathy.
The best way of addressing diabetic retinopathy is to monitor it vigilantly and
achieve euglycemia.
Since 2008 there have been other drugs (e.g. kinase inhibitors and anti-VEGF)
available.
Laser photocoagulation
Laser photocoagulation can be used in two scenarios for the treatment of diabetic
retinopathy. It can be used to treat macular edema by creating a Modified Grid at
the posterior pole and it can be used for panretinal coagulation for controlling
neovascularization. It is widely used for early stages of proliferative retinopathy.
Modified Grid Laser photocoagulation
A 'C' shaped area around the macula is treated with low intensity small burns. This
helps in clearing the macular edema.
Panretinal photocoagulation
Panretinal photocoagulation, or PRP (also called scatter laser treatment), is used to
treat proliferative diabetic retinopathy (PDR). The goal is to create 1,600 - 2,000
burns in the retina with the hope of reducing the retina's oxygen demand, and hence
the possibility of ischemia. It is done in multiple sittings.
In treating advanced diabetic retinopathy, the burns are used to destroy the
abnormal blood vessels that form in the retina. This has been shown to reduce the
risk of severe vision loss for eyes at risk by 50%.<[3]
Before using the laser, the ophthalmologist dilates the pupil and applies anesthetic
drops to numb the eye. In some cases, the doctor also may numb the area behind
the eye to reduce discomfort. The patient sits facing the laser machine while the
doctor holds a special lens on the eye. The physician can use a single spot laser or a
pattern scan laser for two dimensional patterns such as squares, rings and arcs.
During the procedure, the patient will see flashes of light. These flashes often create
an uncomfortable stinging sensation for the patient. After the laser treatment,
patients should be advised not to drive for a few hours while the pupils are still
dilated. Vision will most likely remain blurry for the rest of the day. Though there
should not be much pain in the eye itself, an ice-cream headache like pain may last
for hours afterwards.
Patients will lose some of their peripheral vision after this surgery although it may be
barely noticeable by the patient. The procedure does however save the center of the
patient's sight. Laser surgery may also slightly reduce colour and night vision.
A person with proliferative retinopathy will always be at risk for new bleeding, as
well as glaucoma, a complication from the new blood vessels. This means that
multiple treatments may be required to protect vision.
Intravitreal triamcinolone acetonide
Triamcinolone is a long acting steroid preparation. When injected in the vitreous
cavity, it decreases the macular edema (thickening of the retina at the macula)
caused due to diabetic maculopathy, and results in an increase in visual acuity. The
effect of triamcinolone is transient, lasting up to three months, which necessitates
repeated injections for maintaining the beneficial effect. Best results of intravitreal
Triamcinolone have been found in eyes that have already undergone cataract
surgery. Complications of intravitreal injection of triamcinolone include cataract,
steroid-induced glaucoma and endophthalmitis.
Intravitreal Anti-VEGF
There are good results from multiple doses of intravitreal injections of Anti-VEGF
drugs such as bevacizumab. Present recommended treatment for diabetic macular
edema is Modified Grid laser photocoagulation combined with multiple injections of
Anti-VEGF.
Vitrectomy
Instead of laser surgery, some people require a vitrectomy to restore vision. A
vitrectomy is performed when there is a lot of blood in the vitreous. It involves
removing the cloudy vitreous and replacing it with a saline solution.
Studies show that people who have a vitrectomy soon after a large hemorrhage are
more likely to protect their vision than someone who waits to have the operation.
Early vitrectomy is especially effective in people with insulin-dependent diabetes,
who may be at greater risk of blindness from a hemorrhage into the eye.
Vitrectomy is often done under local anesthesia. The doctor makes a tiny incision in
the sclera, or white of the eye. Next, a small instrument is placed into the eye to
remove the vitreous and insert the saline solution into the eye.
Patients may be able to return home soon after the vitrectomy, or may be asked to
stay in the hospital overnight. After the operation, the eye will be red and sensitive,
and patients usually need to wear an eyepatch for a few days or weeks to protect
the eye. Medicated eye drops are also prescribed to protect against infection.
Vitrectomy is frequently combined with other modalities of treatment.
You might also like
- Notes - Notes - Ophthalmology PDFDocument30 pagesNotes - Notes - Ophthalmology PDFRumana Ali100% (2)
- Diabetic RetinopathyDocument5 pagesDiabetic RetinopathyJayvee CornelioNo ratings yet
- Seminar On Opt 521 MainDocument13 pagesSeminar On Opt 521 MainCHIMA ONWUKA MONGNo ratings yet
- Diabetic ThyDocument3 pagesDiabetic ThynfacmaNo ratings yet
- Diabetic Retinopathy: Clinical Findings and Management: Review ArticleDocument4 pagesDiabetic Retinopathy: Clinical Findings and Management: Review ArticlewadejackNo ratings yet
- Symptoms and Stages of Diabetic RetinopathyDocument2 pagesSymptoms and Stages of Diabetic RetinopathyIshaani GargNo ratings yet
- Diabetes AND The Eye: Juliana Bentil Deborah AddoDocument50 pagesDiabetes AND The Eye: Juliana Bentil Deborah Addogideon A. owusuNo ratings yet
- Natural History of Diabetic RetinopathyDocument1 pageNatural History of Diabetic Retinopathyputri nirmalaNo ratings yet
- Chapter 4 Beginner's Guide To The Retina - TimRoDocument3 pagesChapter 4 Beginner's Guide To The Retina - TimRoFarah FarahNo ratings yet
- Diabetes and The EyeDocument7 pagesDiabetes and The EyeAndhita Satya Pratama GiovanniNo ratings yet
- RAV Nonproliferative Diabetic Retinopathy NPDRDocument1 pageRAV Nonproliferative Diabetic Retinopathy NPDRRidha Surya NugrahaNo ratings yet
- Diabetic RetinopathyDocument3 pagesDiabetic RetinopathyfynneroNo ratings yet
- Diabetic Retinopathy - Practice Essentials, Pathophysiology, EtiologyDocument11 pagesDiabetic Retinopathy - Practice Essentials, Pathophysiology, EtiologyIrma Kurniawati100% (1)
- Diabetic Retinopathy Presentations (Can Still Use The Older Classification)Document6 pagesDiabetic Retinopathy Presentations (Can Still Use The Older Classification)gdudex118811No ratings yet
- Abp EstadisticDocument6 pagesAbp EstadisticLaura López CastroNo ratings yet
- Deep Learing Technique For Diabetic Retinopath ClassificationDocument10 pagesDeep Learing Technique For Diabetic Retinopath ClassificationIJRASETPublicationsNo ratings yet
- MEDS 1. Diabetic Retinopathy: Practice Essentials, Pathophysiology, EtiologyDocument14 pagesMEDS 1. Diabetic Retinopathy: Practice Essentials, Pathophysiology, EtiologyVivi DeviyanaNo ratings yet
- Diabetic RetinopathyDocument55 pagesDiabetic RetinopathyMuhammad Bilal SaifulhaqNo ratings yet
- Diabetic RetinopathyDocument18 pagesDiabetic Retinopathymohelshiekh100% (3)
- Diabetic Retinopathy Symptoms Causes RisDocument7 pagesDiabetic Retinopathy Symptoms Causes Riskenneth MoragaNo ratings yet
- Diabetic Retinopathy by-DuaneBryantMDDocument7 pagesDiabetic Retinopathy by-DuaneBryantMDduanebryantmdNo ratings yet
- RetinopathyDocument64 pagesRetinopathySiti NcitNo ratings yet
- Diabetic With The EyeDocument50 pagesDiabetic With The EyeJianhua ShiNo ratings yet
- Diabetic Retinopathy DR KatariaDocument65 pagesDiabetic Retinopathy DR KatariaDr Sandeep Kataria100% (1)
- Diabetic Retinopathy - StudentDocument24 pagesDiabetic Retinopathy - StudentaulioraraNo ratings yet
- Diabetic RetinopathyDocument8 pagesDiabetic RetinopathyGul SapiNo ratings yet
- Diabetic RetinopathyDocument13 pagesDiabetic RetinopathyjaysonNo ratings yet
- UntitledDocument2 pagesUntitledBen YangNo ratings yet
- FinalijserScenario of Diabetic RetinopathyDocument10 pagesFinalijserScenario of Diabetic RetinopathysavithriNo ratings yet
- Lecture 11 Retina 1Document44 pagesLecture 11 Retina 1ؤيؤييسيNo ratings yet
- Retinal Vascular DisordersDocument52 pagesRetinal Vascular Disorderssushma shresthaNo ratings yet
- Diabetic RetinopathyDocument4 pagesDiabetic RetinopathybencleeseNo ratings yet
- To Study The Prevalence of Eye Complications Among Diabetic PatientDocument10 pagesTo Study The Prevalence of Eye Complications Among Diabetic PatientMuna Hassan MustafaNo ratings yet
- 02 DR-Classification PDFDocument5 pages02 DR-Classification PDFArghirescuNo ratings yet
- Nonproliferative Diabetic Retinopathy and Diabetic Macular EdemaDocument37 pagesNonproliferative Diabetic Retinopathy and Diabetic Macular EdemaBhumika RathNo ratings yet
- Diabetes and EyesDocument13 pagesDiabetes and EyesRishabh TickooNo ratings yet
- Retinopati Diabetikum: Arso Pranindyo UtomoDocument14 pagesRetinopati Diabetikum: Arso Pranindyo Utomosusilo hadi wNo ratings yet
- Ignacio - 6TH Paper SGD OphthaDocument4 pagesIgnacio - 6TH Paper SGD OphthaRichelle IgnacioNo ratings yet
- DM SeminarDocument79 pagesDM Seminarendalew mulugetaNo ratings yet
- Diabetic RetinopathyDocument5 pagesDiabetic RetinopathyAndreiNo ratings yet
- Diabetic RetinopathyDocument25 pagesDiabetic RetinopathyThe Eye Digest100% (2)
- Diabetic Retinopathy: From Diagnosis to TreatmentFrom EverandDiabetic Retinopathy: From Diagnosis to TreatmentRating: 3 out of 5 stars3/5 (3)
- Biochemical and Molecular Mechanisms of Diabetic RetinopathyDocument9 pagesBiochemical and Molecular Mechanisms of Diabetic RetinopathyNor Ubudiah SetiNo ratings yet
- Westjmed00159 0115Document3 pagesWestjmed00159 0115bangd1f4nNo ratings yet
- Practice: Diabetic RetinopathyDocument1 pagePractice: Diabetic RetinopathyRina ChairunnisaNo ratings yet
- Diabetic Retinopathy: by Mohamad Faizul Abu Hanifa Tillai SharimalaDocument13 pagesDiabetic Retinopathy: by Mohamad Faizul Abu Hanifa Tillai SharimalaMohamad Faizul Abu HanifaNo ratings yet
- Jurnal Retinopati DiabetikDocument11 pagesJurnal Retinopati DiabetikAzwaydaRayhanaSariNo ratings yet
- Diabetic Retinopathy - Classification and Clinical Features - UpToDateDocument38 pagesDiabetic Retinopathy - Classification and Clinical Features - UpToDateRachmatBimanjayaNo ratings yet
- Retinopathy, Diabetic, BackgroundDocument12 pagesRetinopathy, Diabetic, BackgroundmyusuffrNo ratings yet
- Diabetes ComplicationsDocument7 pagesDiabetes ComplicationstalaNo ratings yet
- Diabetic RetinopathyDocument2 pagesDiabetic RetinopathySanal KumarNo ratings yet
- Diabetic Retinopathy - Aetiopathogenesis, Clinical Presentation andDocument83 pagesDiabetic Retinopathy - Aetiopathogenesis, Clinical Presentation andOlayemi Olorundare0% (1)
- Ocular Issues and Endocrine Diseases in Small Animal Practice (Canine Eye Disease)Document11 pagesOcular Issues and Endocrine Diseases in Small Animal Practice (Canine Eye Disease)maddierogersNo ratings yet
- Diabetic Retinopathy (Wills Eye Manual)Document9 pagesDiabetic Retinopathy (Wills Eye Manual)Yuri YogyaNo ratings yet
- Refrat Retinopati-DiabetikDocument20 pagesRefrat Retinopati-DiabetikDea NabilaNo ratings yet
- Retinopati DiabetikDocument5 pagesRetinopati DiabetikMuhammad Afriadi HamdanNo ratings yet
- Diabetic RetinopathyDocument55 pagesDiabetic RetinopathyDrSaid Hussein GediNo ratings yet
- How Does Hipertension Affects Your Eye - Anure 2011Document13 pagesHow Does Hipertension Affects Your Eye - Anure 2011Germano Freire Bezerra FilhoNo ratings yet
- Diabetic Retinopathy: Introduction to Novel Treatment StrategiesFrom EverandDiabetic Retinopathy: Introduction to Novel Treatment StrategiesNo ratings yet
- The Masculoskeletal SystemDocument6 pagesThe Masculoskeletal Systemapi-296199660No ratings yet
- UAV - NPTEL - IIT RoorkeeDocument14 pagesUAV - NPTEL - IIT Roorkeesankalp chopkarNo ratings yet
- Nasscom RecommendationsDocument11 pagesNasscom RecommendationsSANTANU DASNo ratings yet
- Modeling and Analysis of DC-DC Converters Under Pulse Skipping ModulationDocument6 pagesModeling and Analysis of DC-DC Converters Under Pulse Skipping Modulationad duybgNo ratings yet
- A Detailed Lesson Plan in English 9: (Using Adverbs in Narration)Document8 pagesA Detailed Lesson Plan in English 9: (Using Adverbs in Narration)Faith Malinao100% (1)
- En LNG Air Products Floating LNG Plant CapabilitiesDocument2 pagesEn LNG Air Products Floating LNG Plant CapabilitiesMshelia M.No ratings yet
- Master Degree InformationDocument3 pagesMaster Degree InformationBivash NiroulaNo ratings yet
- Process Design and Economics For The Conversion of The Lignocellulosic To HydrocarbonsDocument133 pagesProcess Design and Economics For The Conversion of The Lignocellulosic To HydrocarbonsCristhian Camilo Vargas QuinteroNo ratings yet
- Naiad GettingstartedDocument37 pagesNaiad GettingstartedYurivanovNo ratings yet
- AND8331/D Quasi-Resonant Current-Mode Controller For High - Power Ac-Dc AdaptersDocument16 pagesAND8331/D Quasi-Resonant Current-Mode Controller For High - Power Ac-Dc AdaptersLucía MitchellNo ratings yet
- No Smoking in The TriangleDocument60 pagesNo Smoking in The TriangleThomas L HaydenNo ratings yet
- Indian ClimateDocument7 pagesIndian ClimatePrakash Kumar Kumar100% (1)
- Administrative Management in Education: Mark Gennesis B. Dela CernaDocument36 pagesAdministrative Management in Education: Mark Gennesis B. Dela CernaMark Gennesis Dela CernaNo ratings yet
- 5.2 Extreme Value Theorem, Global Versus Local Extrema, and CriticalDocument26 pages5.2 Extreme Value Theorem, Global Versus Local Extrema, and Criticalazzamomar2018No ratings yet
- AFATL-TR-72-401 - Developement of 20MM and 30MM Plastic-Aluminium Cartridge Cases (1972)Document91 pagesAFATL-TR-72-401 - Developement of 20MM and 30MM Plastic-Aluminium Cartridge Cases (1972)defendercc130No ratings yet
- How To Uncompress and List An Initramfs Content On LinuxDocument10 pagesHow To Uncompress and List An Initramfs Content On Linuxvpalmar8871No ratings yet
- Module 4 ResearchDocument9 pagesModule 4 ResearchJegg AsisNo ratings yet
- 13.n-p-n TransistorDocument4 pages13.n-p-n TransistorkirtiNo ratings yet
- Schallert 2019Document7 pagesSchallert 2019Dian Putri NingsihNo ratings yet
- BC23000122Document1 pageBC23000122azharNo ratings yet
- Int Org 2Document60 pagesInt Org 2Alara MelnichenkoNo ratings yet
- Ce Booklet Fall 14Document28 pagesCe Booklet Fall 14api-279863771No ratings yet
- Eaton Guide Specification Notes and Instructions To SpecwriterDocument17 pagesEaton Guide Specification Notes and Instructions To SpecwriterIsrael Luna PenasNo ratings yet
- ISO 22301 Lead Implementer - Two Page BrochureDocument2 pagesISO 22301 Lead Implementer - Two Page BrochurePECBCERTIFICATIONNo ratings yet
- Ap18 English Literature q1 PDFDocument12 pagesAp18 English Literature q1 PDFMartin CoscolluelaNo ratings yet
- BillDocument3 pagesBillTha OoNo ratings yet
- Development: CHAPTEK Green Governance: Sustainable HumanDocument24 pagesDevelopment: CHAPTEK Green Governance: Sustainable HumanAlisha VarandaniNo ratings yet
- Shirish - Kumar - Software - Engineer NewDocument1 pageShirish - Kumar - Software - Engineer NewAbhishek KumarNo ratings yet
- Diasporic Writing QuestionsDocument31 pagesDiasporic Writing QuestionsMEIMOONA HUSNAIN100% (1)
- Trompenaars' Cultural Dimensions in France: February 2021Document15 pagesTrompenaars' Cultural Dimensions in France: February 2021Như Hảo Nguyễn NgọcNo ratings yet