Professional Documents
Culture Documents
Mtbe Tame-Coproduction Refc4 C5feeds-Cdethers
Mtbe Tame-Coproduction Refc4 C5feeds-Cdethers
Uploaded by
anhchangleloiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mtbe Tame-Coproduction Refc4 C5feeds-Cdethers
Mtbe Tame-Coproduction Refc4 C5feeds-Cdethers
Uploaded by
anhchangleloiCopyright:
Available Formats
C
4
/C
5
Raffinate
Ca t a l y t i c
Di s t i l l a t i on
Te c hnol ogi e s
MTBE/TAME Coproduction From Refinery C
4
/C
5
Feeds
T e c h n o l o g y P r o f i l e
Overview The CDEthers catalytic
distillation technology processes C
4
/C
5
streams from refinery units to produce
MTBE/TAME. CDEthers is one of a
family of process technologies devel-
oped and commercialized by Catalytic
Distillation Technologies (CDTECH) for
license to the petroleum refining and
petrochemical industries. CDTECH is a
partnership between Lummus Techno-
logy, a CB&I company, and Chemical
Research & Licensing, a CRI company.
MTBE/TAME Synthesis-General MTBE and TAME are coproduced by the
catalytic etherification of isobutylene and isoamylene with methanol. The patented
CDEthers process is based on a two-step reactor design, consisting of a boiling point
fixed bed reactor followed by final conversion in a catalytic distillation column. The
process utilizes an acidic ion exchange resin catalyst in both its fixed bed reactor and
proprietary catalytic distillation structures.
The boiling point reactor is designed so that the liquid is allowed to reach its boiling
point by absorbing the heat of reaction, after which a limited amount of vaporization
takes place thereby maintaining precise temperature control. The maximum temperature
is adjusted by setting the total system pressure. Since the reacting liquid mixture temper-
ature cannot exceed the boiling temperature, control is far superior to those systems in
which heat must be transferred by convection or conduction. This design retains the heat
of reaction as latent heat, reducing heat input requirements for the ensuing fractionation.
The unique catalytic distillation column combines reaction and fractionation in a single
unit operation. It allows simultaneous, high conversion of isobutylene and isoamylene
(exceeding fixed bed equilibrium limitations) to be achieved simply and economically. By
using distillation to separate the product from the reactants, the equilibrium limitation is
exceeded and higher conversion to ethers is achieved. Catalytic distillation also takes
advantage of the improved kinetics through increased temperature without penalizing
equilibrium conversion.
CDTECH's unique, proprietary technology is optimized for coproduction of MTBE and
TAME, and is an improved option for small refineries, where operating separate units for
these products is not economical.
CDEthers Process Flow Diagram
Feed Boiling Point Catalytic Methanol Methanol
Wash Reactor Distillation Extraction Recovery
Fresh Methanol Recycle Methanol
Water
Water and
Contaminants
Water
Mixed C
4
s/C
5
s
Methanol
and C
4
s/C
5
s
MTBE and TAME
Isobutylene conversion percent: 97+
Typical Overall Material Balance Isoamylene conversion percent: 90+
Feeds LB/HR
C
4
s (Isobutylene 16 wt. %) 63,714
C
5
s (Reactive Isoamylene 20.3 wt. %) 60,954
Methanol 10,808
Products
C
4
/C
5
Raffinate 102,103
MTBE/TAME product 33,373
MTBE/ TAME Product Composition (excluding C
6
+)
Wt.%
C
5
<4.0
Methanol <0.1
Dimers 0.5
Tertiary Alcohols 0.4
MTBE/TAME >95.0
Total 100.0
Ca t a l y t i c
Di s t i l l a t i on
Te c hnol ogi e s
Process Chemistry
CH
3
OH CH
3
OCH
3
CH
3
C
=
CH
2
CH
3
Isobutylene Methanol MTBE
CH
3
CH
3
Advantages
CDTECHs Boiling Point
reactor offers:
Simple and effective control
Elimination of hot spots
Long catalyst life
High flexibility
Low capital cost
Elimination of catalyst attrition
Most effective heat removal technique
Elimination of cooling water
requirement
CDTECHs catalytic
distillation offers:
Improved kinetics
High conversion
(beyond fixed bed equilibrium limit)
Low capital cost
Low utilities
Long catalyst life with
sustained high conversion
Reduced plot area
Coproduction of MTBE/TAME at high
conversion and low cost
CDTECH
3010 Briarpark Drive
Houston, TX 77042 USA
Tel: 713-821-5181
Fax: 713-821-3587
Etherification
CH
3
CH
3
Etherification
CH
2
=
CCH
2
CH
3
CH
3
C
=
CHCH
3
CH
3
MTBE/TAME Coproduction
From Refinery C
4
/ C
5
Feeds
CH
3
OH CH
3
CH
2
OCH
3
CH
3
2MB1 & 2MB2 Methanol TAME
Isoamylenes
2/08
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Piping Interview QuestionsDocument22 pagesPiping Interview Questionsmsaad289% (37)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- BP Process Safety Series - Safe Ups and Downs For Process Units (7th Edition)Document71 pagesBP Process Safety Series - Safe Ups and Downs For Process Units (7th Edition)anhchangleloi88% (8)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- CH 02Document37 pagesCH 02AndrésFelipeMeloNo ratings yet
- Tips For IELTS PDFDocument64 pagesTips For IELTS PDFanhchangleloiNo ratings yet
- Lab 4 - Updating: 4.1 Data Dates and ReschedulingDocument9 pagesLab 4 - Updating: 4.1 Data Dates and ReschedulinganhchangleloiNo ratings yet
- Sss Using Grammar BookDocument2 pagesSss Using Grammar BookanhchangleloiNo ratings yet
- 2009-03 CleanDieselHydroPTQ MustangDocument7 pages2009-03 CleanDieselHydroPTQ Mustanganhchangleloi100% (2)
- IELTSreadingexam 5Document14 pagesIELTSreadingexam 5anhchangleloi100% (1)
- Offshore ConceptsDocument195 pagesOffshore ConceptsZack Lee75% (4)
- Semi Submersible PlatformDocument140 pagesSemi Submersible Platformanhchangleloi100% (2)
- ChalmetteDocument1 pageChalmetteanhchangleloiNo ratings yet
- Chemsorb Fitler GranulesDocument4 pagesChemsorb Fitler GranulesJeffersonNo ratings yet
- Gujarat Technological UniversityDocument1 pageGujarat Technological UniversityPatel DhruvilNo ratings yet
- Exercise On Order of ReactionDocument4 pagesExercise On Order of ReactionGopi KupuchittyNo ratings yet
- Inert GasDocument4 pagesInert GasCarlos BustamanteNo ratings yet
- The Technology of Tail Gases Purifying in Nitric ADocument27 pagesThe Technology of Tail Gases Purifying in Nitric AbalayogeshNo ratings yet
- Boiler RbiDocument65 pagesBoiler Rbidiaccessltd_17172961No ratings yet
- Adsorption Chromatography:: Presented By: Aarohi Thakur ADM. NO. H-2019-05-001Document15 pagesAdsorption Chromatography:: Presented By: Aarohi Thakur ADM. NO. H-2019-05-001Akshit KapilaNo ratings yet
- Trouble Shooting Compendium 2022-23Document142 pagesTrouble Shooting Compendium 2022-2300083583rfNo ratings yet
- POGIL Practice Substitution Nucleophilic Unimolecular SN1Document8 pagesPOGIL Practice Substitution Nucleophilic Unimolecular SN1DoctorNo ratings yet
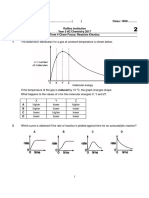
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- Influent WastewaterDocument8 pagesInfluent WastewaterunconformistNo ratings yet
- Master Data BaseDocument72 pagesMaster Data Basesubudhiprasanna100% (1)
- Chem Final Exa, MDocument14 pagesChem Final Exa, MOMARNo ratings yet
- Dokumen - Tips - 1 CNG Dealer Training 2 What Is CNG CNG Is Natural Gas Compressed To A Pressure of 200 250 KGCM G Why CNG Is Used in Vehicles Instead of NaturalDocument39 pagesDokumen - Tips - 1 CNG Dealer Training 2 What Is CNG CNG Is Natural Gas Compressed To A Pressure of 200 250 KGCM G Why CNG Is Used in Vehicles Instead of NaturalCandraNo ratings yet
- Part III - CRE II LecturesDocument59 pagesPart III - CRE II LecturesArunPThomas100% (1)
- CH6403Document7 pagesCH6403NikylNo ratings yet
- Thermochemistry Worksheet 1 and 2 KEYDocument4 pagesThermochemistry Worksheet 1 and 2 KEYhtyhongNo ratings yet
- Separation Processes Lab Manual (OBE 2018)Document78 pagesSeparation Processes Lab Manual (OBE 2018)Aasia FarrukhNo ratings yet
- Rate LawDocument3 pagesRate Lawnadia sykesNo ratings yet
- PSEAsia2013 54Document6 pagesPSEAsia2013 54Felipe MuñozNo ratings yet
- De PassionDocument6 pagesDe PassionAbdelali GuounainNo ratings yet
- SCIENCE 10 Q4 Module 8 Factors Affecting The Rate of Chemical ReactionDocument26 pagesSCIENCE 10 Q4 Module 8 Factors Affecting The Rate of Chemical ReactionRachel Ann GoteraNo ratings yet
- Me Lab 3Document71 pagesMe Lab 3JV Lopez0% (2)
- Reagents in Organic Synthesis - Unit 7 - Week 5: ORGANIC TRANSFORMATIONS-USING NON-TRANSITION METADocument1 pageReagents in Organic Synthesis - Unit 7 - Week 5: ORGANIC TRANSFORMATIONS-USING NON-TRANSITION METASamarjeet Kumar SinghNo ratings yet
- Cek 01, Part 1Document2 pagesCek 01, Part 1khairul89 TangguhNo ratings yet
- 2940-Prs-100-Ufd-121 - Fuel Gas & Fuel Oil SystemDocument1 page2940-Prs-100-Ufd-121 - Fuel Gas & Fuel Oil SystemasdfNo ratings yet
- Natural Gas EngineeringDocument8 pagesNatural Gas EngineeringProsper Dzidzeme AnumahNo ratings yet
- Razus PDFDocument10 pagesRazus PDFMohanad El-HarbawiNo ratings yet