Professional Documents
Culture Documents
Chemistry - 3rd Form2001
Chemistry - 3rd Form2001
Uploaded by
Robert EdwardsOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry - 3rd Form2001
Chemistry - 3rd Form2001
Uploaded by
Robert EdwardsCopyright:
Available Formats
1
GLENMUIR HIGH SCHOOL
EASTER TERMINAL EXAMINATIONS 2000
CHEMISTRY
FORM 3
TIME: 1 Hrs.
NAME: ___________________________________________________
INSTRUCTION:
This paper is divided into three sections. Answer ALL questions in Sections A and B
and ANY TWO questions from Section C.
SECTION A (20 Marks)
Each question has four possible answers labelled A, B,C and D. Answer ALL questions in this section by
circling the letter which corresponds to the answers you consider to be correct.
1.
The pollutant which irritates the lungs, damage plants and forms an acid which effect buildings refers
to:
A.
C.
2.
3.
a gain of electrons
A loss of hydrogen
II.
a gain in oxygen
A.
C.
I. II and III
II and III only
B.
D.
I and II only
II only
B.
D.
pink to blue
white to blue
Water vapour will turn cobalt chloride paper from
blue to pink
blue to white
The product from the combustion of a non-metallic element X, is shaken in water after which red and
blue litmus paper were added. Which of the following would you generally expect to observe.
Both red and blue litmus paper remained unchanged
Red litmus changed to blue whilst blue litmus remained unchanged
Blue litmus changed to red whilst red litmus remained unchanged
Both red and blue litmus paper changed colours
Which of the following is regarded as a component of pure dry air?
A.
6.
Hydrogen sulphide
Sulphur dioxide
I.
III.
A.
B.
C.
D.
5.
B.
D.
Oxidation can be defined as
A.
C.
4.
Carbon monoxide
Nitrogen dioxide
Methane gas
B.
Biogas
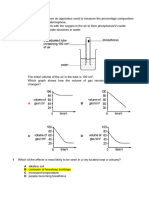
The figure below shows the water cycle
C.
Krypton
D.
Radium
Which of the following is a correct match of the processes involved at W, X, Y and Z?
A.
B.
C.
D.
7.
10.
Addition of sodium carbonate
Ion-exchange rasin
B.
D.
Boiling
Distillation
speeds up a chemical reaction
takes no part in a chemical reaction
alters the rate of a chemical reaction whilst remaining unchanged at the end
alters the rate of a chemical reaction and is changed into a new substance at the end
(i)
(ii)
(iii)
(iv)
it is an acidic gas
it is a reducing agent
it is slightly denser than air
it is virtually insoluble in water
A.
C.
(i), (ii), (iii) and (iv)
(i), (ii) and (iv) only
B.
D.
(i) and (iii) only
(ii) and (iv) only
All of the following elements burn in air to give a product which, when shaken with water forms a
solution with a pH value of 7 or greater EXCEPT.
Hydrogen
B.
Sulphur
C.
Magnesium
D.
Calcium
The compound mainly responsible for temporary hardness of water is
A.
C.
12.
Z
condensation
respiration
condensation
transpiration
Which of the following is/are properties of hydrogen?
A.
11.
Y
respiration
condensation
transpiration
evaporation
A catalyst is best defined as a substance which.
A.
B.
C.
D.
9.
X
transpiration
evaporation
respiration
condensation
All of the following will remove permanent hardness of water EXCEPT
A.
C.
8.
W
Evaporation
Transpiration
Evaporation
Respiration
Calcium carbonate
Calcium hydrogen carbonate
B.
D.
Calcium sulphate
Magnesium sulphate
B.
D.
a mixture of gases
a mixture of oxygen and impurities
The atmosphere is said to be
A.
C.
a layer of gases
a layer of dust particles
13.
The diagram above is used to produce gas X, using reagents A and B in the apparatus shown.
Which of the following is a correct match of X, A and B giving the function of B correctly.
A.
B.
X
Oxygen
Nitrogen
A
Hydrogen Perioxide
Magnesium oxide
B
Manganese dioxide
Nitric acid
Function of A
decomposer
decomposer
14.
C.
Oxygen
Manganese IV Oxide
Hydrogen peroxide
D.
Ammonia
Hydrogen
Nitrogen
Which of the following is NOT a property of Nitrogen?
A.
B.
C.
D.
15.
It is slightly denser than air
It is virtually insoluble in water
It is chemically inert under ordinary condition
It reacts with reactive metals at high temperature forming nitrates
Which of the following is a list of fossil fuels?
A.
B.
16.
catalyst
catalyst
Coal, crude oil, biogas
Coal, crude oil, natural gas
C.
D.
natural gas, biogas, methane
wind, hydroelectric, solar
Methods used to stop iron from rusting include
-
coating with tin
coating with zinc
connecting with magnesium rods
Which metal is most often used to protect iron in food containers, oil pipelines and roofing sheets?
A.
B.
C.
D.
17.
It dissolves in water
It burns with a blue flame
It rekindles a glowing splint
It forms a white precipitate with lime water
a hydrocarbon
B.
a carbohydrate
C.
carbon
D.
hydrogen
The gas uses for deep sea diving and to inflate blimps is
A.
20.
Roofing sheets
Magnesium
Tin
Zinc
Zinc
The burning of a substance X produces steam and energy only. The best conclusion as to what X
could be is.
A.
19.
Oil pipelines
Zinc
Magnesium
Tin
Magnesium
Which of the following is proof that a gas is oxygen?
A.
B.
C.
D.
18.
Food containers
Tin
Zinc
Magnesium
Tin
Argon
Xenon
B.
Helium
C.
Neon
D.
The process by which organisms obtain energy from food substances is
A.
combustion
B.
respiration
C.
digestion
D.
photosynthesis
SECTION B (20 Marks)
Answer ALL questions in this section in the space provided
1.
The element Magnesium is found in group II of the Periodic Table. Magnesium can be completely
burned in air to form an oxide.
(a)
Write a worded equation for the complete combustion of Magnesium.
_______________________________________________________________________ (2 marks)
(b)
What would you expect to observe if the product from the combustion of Magnesium was
shaken with water and reacted with the following;
(i)
red litmus paper _____________________________________________ (1 mark)
(ii)
blue litmus paper ____________________________________________ (1 mark)
(c)
What is the name of the type of oxide formed by Magnesium?
_______________________________________________________________________ (1 mark)
2.
The diagram below represents the nitrogen cycle.
(a)
(b)
3.
(a)
Identify the organisms labelled a, b, c.
(i)
Organism a _______________________________________________
(ii)
Organism b _______________________________________________
(iii)
Organism c ________________________________________________ (3 marks)
Identify the processes labelled 1, 2, 3
1.
____________________________________________________________
2.
____________________________________________________________
3.
____________________________________________________________ (3 marks)
Explain what is meant by the term rusting.
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
(2 marks)
(b)
State the chemical name for rust.
_________________________________________________________________________ (1 mark)
(c)
List two conditions that are necessary for rusting to occur.
________________________________________________________________________________
_________________________________________________________________________________
(1 mark)
(d)
4.
Write a worded equation for the corrosion of Chromium.
________________________________________________________________________ (2 marks)
State three (3) components of pure dry air and given their % by volume.
COMPONENTS
%BY VOLUME
1.
___________________________
_________________________________
2.
___________________________
_________________________________
3.
___________________________
(a)
With the aid of a table, identify three gases which polluted the air. For each gas identified
state
__________________________________
(3 marks)
SECTION C (20 Marks)
This section consists of FOUR questions, Answer ANY TWO of these questions on the answer sheet
provided.
1.
(i)
(ii)
2.
3.
4.
how they got into the air and
their environmental effects on both living and non-living things.
(3 marks)
(b)
Explain how acid rain is formed.
(2 marks)
(c)
Describe three kinds of damage which result from acid rain.
(3 marks)
(d)
Explain how the presence of Carbon dioxide and Sulphur dioxide in the air affects the pH
value of polluted and unpolluted rain water.
(2 marks)
Total 10 marks
(a)
If iron nails are covered in a sealed tube with tap water they rust. However if the water is first
boiled they do not rust. Give an explanation for these observations.
(3 marks)
(b)
Outline two METHODS that can be used to slow down or prevent the formation of rust.
(2 marks)
(c)
With the aid of worded equation, differentiate between complete and incomplete combustion.
(2 marks)
(d)
Compare the processes of respiration, photosynthesis and rusting in terms of the following
components oxygen, carbon dioxide, water and energy.
(3 marks)
Total 10 marks
(a)
Explain how permanent hardness of water is formed.
(2 marks)
(b)
Outline three methods of softening temporary hardness of water.
(3 marks)
(c)
Explain how hardness of water affects hot water pipes.
(2 marks)
(d)
Give three differences between soapy and soapless detergents.
Total 10 marks
(3 marks)
(a)
Yeast or Sodium hydrogen carbonate is usually added to dough during baking. Explain the
chemistry involved in this practice.
(4 marks)
(b)
With the aid of a diagram explain how animals, factories and plants recycle carbon dioxide in
nature.
(4 marks)
(d)
Outline two ways in which the physical properties of carbon dioxide affects its uses.
(2 marks)
Total 10 marks
You might also like
- Gracefully BrokenDocument4 pagesGracefully BrokenRobert Edwards50% (2)
- F2 IS Exam 1 (11-12)Document9 pagesF2 IS Exam 1 (11-12)羅天佑No ratings yet
- We Have The Victory HallelujahDocument2 pagesWe Have The Victory HallelujahRobert Edwards100% (1)
- 316 Stainless Steel PipeDocument4 pages316 Stainless Steel PipeUnisteel & engineeringNo ratings yet
- Heavy Metal Detox KlinghardftDocument34 pagesHeavy Metal Detox KlinghardftAlice Lee100% (12)
- CEH Marketing Reports - PigmentsDocument239 pagesCEH Marketing Reports - PigmentsnrkscribdacNo ratings yet
- Chemistry 1 1Document5 pagesChemistry 1 1youngtillionez99No ratings yet
- Baptist Lui Ming Choi Secondary School First Term Examination (2012-2013) Form 3 ChemistryDocument12 pagesBaptist Lui Ming Choi Secondary School First Term Examination (2012-2013) Form 3 ChemistryyuNo ratings yet
- Concept Chem TestDocument10 pagesConcept Chem TestJudith MartinezNo ratings yet
- Chemistry Worksheet 1 Year 11Document8 pagesChemistry Worksheet 1 Year 11fatma.darghouth2No ratings yet
- Chemistry Mock Exam ss3Document3 pagesChemistry Mock Exam ss3chrizyboyziNo ratings yet
- 0620 s03 QP 1Document20 pages0620 s03 QP 1Sana DiwanNo ratings yet
- Wed April 16 2014 Practice Multiple Choice Paper FULL 60 QuesDocument5 pagesWed April 16 2014 Practice Multiple Choice Paper FULL 60 QuesFrank MassiahNo ratings yet
- 5070 w12 QP 11Document16 pages5070 w12 QP 11mstudy123456No ratings yet
- Test Bank For Chemistry in Context Applying Chemistry To Society 7th Edition AcsDocument27 pagesTest Bank For Chemistry in Context Applying Chemistry To Society 7th Edition Acskyodile9s8cm5No ratings yet
- Chemistry SS IiDocument7 pagesChemistry SS IiAbba YakubuNo ratings yet
- Name - : St. Paul'S College F.4 Mid-Year Examination Sample Paper ChemistryDocument16 pagesName - : St. Paul'S College F.4 Mid-Year Examination Sample Paper ChemistryUniversityJCNo ratings yet
- ZIMSEC Form 2 End of Term 1 Exam Paper 1 and Paper 2 Questions Combined Science Set 1Document8 pagesZIMSEC Form 2 End of Term 1 Exam Paper 1 and Paper 2 Questions Combined Science Set 1Kudakwashe MusvaireNo ratings yet
- Chemistry Form Three AnnualDocument6 pagesChemistry Form Three Annualvecema1296No ratings yet
- Chemistry Year 12Document13 pagesChemistry Year 12chidubemonu89No ratings yet
- Chemistry Cambridge Grade 10Document15 pagesChemistry Cambridge Grade 10priyanto laksonoNo ratings yet
- HanksDocument20 pagesHanksRia MandasariNo ratings yet
- CHEMISTRYDocument41 pagesCHEMISTRYLindsayyNo ratings yet
- William Grade 10 Chemistry Paper (2020-21)Document21 pagesWilliam Grade 10 Chemistry Paper (2020-21)william.limmanjayaNo ratings yet
- Chemistry 0620:21 May:June 2017 SOLVEDDocument6 pagesChemistry 0620:21 May:June 2017 SOLVEDKani KhidhirNo ratings yet
- 596C06B5Document8 pages596C06B5L MyNo ratings yet
- 1 Nps - Itpl-2021/22/Term Ii-ChemDocument5 pages1 Nps - Itpl-2021/22/Term Ii-ChemMidhun JayachandranNo ratings yet
- Test Bank For Chemistry in Context 8th Edition American Chemical Society DownloadDocument42 pagesTest Bank For Chemistry in Context 8th Edition American Chemical Society DownloadHoward Walker100% (34)
- 5070 w12 QP 12Document16 pages5070 w12 QP 12mstudy123456No ratings yet
- SS2 Chem 2ndDocument2 pagesSS2 Chem 2ndGodspower OgbonnayaNo ratings yet
- Dha Senior School For Girls Online MOCK-Examinations: There Are FORTY Questions On This Paper. Answer All QuestionsDocument12 pagesDha Senior School For Girls Online MOCK-Examinations: There Are FORTY Questions On This Paper. Answer All QuestionsaNo ratings yet
- Air and AtmosphereDocument12 pagesAir and Atmospherebob leowNo ratings yet
- S.3chem 1Document8 pagesS.3chem 1Lakogaharry BillclintonNo ratings yet
- 5070 s09 QP 1 PDFDocument16 pages5070 s09 QP 1 PDFNeural Spark Physics CieNo ratings yet
- Chemistry 1996 Paper 2+ansDocument15 pagesChemistry 1996 Paper 2+ansapi-3824003No ratings yet
- 01 Subjective Test-02 X Science 25122022 QPDocument6 pages01 Subjective Test-02 X Science 25122022 QPViswa DharshanNo ratings yet
- Moshi ChemistryDocument4 pagesMoshi ChemistryJohn Hobela LuhendeNo ratings yet
- Full Download PDF of Test Bank For Chemistry in Context Applying Chemistry To Society, 7th Edition: ACS All ChapterDocument50 pagesFull Download PDF of Test Bank For Chemistry in Context Applying Chemistry To Society, 7th Edition: ACS All Chapterlunneytelkki100% (4)
- Summer 05Document16 pagesSummer 05Sana DiwanNo ratings yet
- Midterm Exam in General Chemistry1Document9 pagesMidterm Exam in General Chemistry1AnalynAsuncionAtaydeNo ratings yet
- 3na CHEM End-Of-year 09Document13 pages3na CHEM End-Of-year 09Francis Ho HoNo ratings yet
- F2 IS Exam 1 (13-14)Document9 pagesF2 IS Exam 1 (13-14)羅天佑No ratings yet
- Pei Hwa Chem Prelim P1 2012 QNDocument16 pagesPei Hwa Chem Prelim P1 2012 QNWonderfullyANo ratings yet
- 'O' Level Chemistry Paper 1 - 2012Document16 pages'O' Level Chemistry Paper 1 - 2012MayureshSoowamberNo ratings yet
- Topic 26 Air and WaterDocument1 pageTopic 26 Air and WaterCarolus WisnuNo ratings yet
- Uzair10 012940Document3 pagesUzair10 012940vanisahil23No ratings yet
- F6 Chemistry 2019 - CompressedDocument39 pagesF6 Chemistry 2019 - CompressedanyakahcNo ratings yet
- Chemistry Jamb Past Question 2013Document16 pagesChemistry Jamb Past Question 2013anyakahcNo ratings yet
- 5070 w09 QP 1Document16 pages5070 w09 QP 1mstudy123456No ratings yet
- Chemistry in Context 6th Edition American Chemical Society Acs Test BankDocument17 pagesChemistry in Context 6th Edition American Chemical Society Acs Test Bankjenniferrichardsonjrwfpzsdim100% (33)
- Diagnostic Test in General Chemistry 1Document13 pagesDiagnostic Test in General Chemistry 1Dearest Notes100% (1)
- Ebook Chemistry in Context 6Th Edition American Chemical Society Acs Test Bank Full Chapter PDFDocument48 pagesEbook Chemistry in Context 6Th Edition American Chemical Society Acs Test Bank Full Chapter PDFhaogwyneth050p96100% (15)
- 2019 Yearly Exam PaperDocument25 pages2019 Yearly Exam PaperYu-Tang LinNo ratings yet
- 5070 s11 QP 12Document16 pages5070 s11 QP 12mstudy123456No ratings yet
- s4 Chemistry Paper 1 Mock (2) - 1Document11 pagess4 Chemistry Paper 1 Mock (2) - 1Ndagire OliverNo ratings yet
- Week17 G9 Chapter1-4 Summary SignedDocument3 pagesWeek17 G9 Chapter1-4 Summary Signedkwokrenee827No ratings yet
- SA1 Chemistry MCQ PracticeDocument2 pagesSA1 Chemistry MCQ Practicechong56No ratings yet
- Chemistry Ssc-Ii: Answer Sheet No.Document7 pagesChemistry Ssc-Ii: Answer Sheet No.Kashif HussainNo ratings yet
- ChemistryDocument10 pagesChemistrykahgua0% (1)
- University of Cambridge International Examinations General Certificate of Education Ordinary LevelDocument16 pagesUniversity of Cambridge International Examinations General Certificate of Education Ordinary Levelmstudy123456No ratings yet
- QC - 2019-20 - Mock - S6 - Chem 1ADocument12 pagesQC - 2019-20 - Mock - S6 - Chem 1AOof GucciNo ratings yet
- Sources of Errors in Mechanics ExperimentsDocument5 pagesSources of Errors in Mechanics ExperimentsRobert EdwardsNo ratings yet
- Section6 Marketing NOTESDocument24 pagesSection6 Marketing NOTESRobert EdwardsNo ratings yet
- Section6 MarketingDocument24 pagesSection6 MarketingRobert EdwardsNo ratings yet
- 10,000 Reasons and What A Beautiful Name It IsDocument2 pages10,000 Reasons and What A Beautiful Name It IsRobert EdwardsNo ratings yet
- Types of Errors in PhysicsDocument3 pagesTypes of Errors in PhysicsRobert EdwardsNo ratings yet
- Rate of Reaction 1 QPDocument11 pagesRate of Reaction 1 QPRobert EdwardsNo ratings yet
- Chemistry Paper 1 2009Document7 pagesChemistry Paper 1 2009Robert EdwardsNo ratings yet
- Ps 1 SolDocument5 pagesPs 1 SolRobert EdwardsNo ratings yet
- Ps 6 SolDocument3 pagesPs 6 SolRobert EdwardsNo ratings yet
- Midterm Review Spring18 SolsDocument22 pagesMidterm Review Spring18 SolsRobert EdwardsNo ratings yet
- To Kill A Mocking BirdDocument12 pagesTo Kill A Mocking BirdRobert EdwardsNo ratings yet
- Ethylene Biosynthesis in Tropical FruitsDocument28 pagesEthylene Biosynthesis in Tropical FruitsRobert EdwardsNo ratings yet
- What Causes Algal Blooms?: NutrientsDocument2 pagesWhat Causes Algal Blooms?: NutrientsRobert EdwardsNo ratings yet
- Tuckshop DutyDocument1 pageTuckshop DutyRobert EdwardsNo ratings yet
- All We AskDocument2 pagesAll We AskRobert EdwardsNo ratings yet
- Angels We Have Heard On HighDocument2 pagesAngels We Have Heard On HighRobert EdwardsNo ratings yet
- PreviewDocument20 pagesPreviewalfredNo ratings yet
- Asme Section II A Sa-395Document12 pagesAsme Section II A Sa-395Anonymous GhPzn1xNo ratings yet
- ECCS-Behaviour and Design of Steel Plated StructuresDocument124 pagesECCS-Behaviour and Design of Steel Plated StructuresAlexandros GiNo ratings yet
- Hydrometallurgy: Arao J. Manhique, Walter W. Focke, Carvalho MadivateDocument7 pagesHydrometallurgy: Arao J. Manhique, Walter W. Focke, Carvalho MadivateYuliantari YuliantariNo ratings yet
- Worksheet 8 ClassDocument36 pagesWorksheet 8 ClassKerimberdiNo ratings yet
- Chemistry - Free Practice Exam PaperDocument18 pagesChemistry - Free Practice Exam PaperAsia SimpsonNo ratings yet
- Natural Organic Fert Program at Lake OBDocument3 pagesNatural Organic Fert Program at Lake OBShawn KamauNo ratings yet
- Chapter 3 MaterialDocument9 pagesChapter 3 Materialdawana samuelNo ratings yet
- Engineering Materials: (Hi) Wrought IronsDocument1 pageEngineering Materials: (Hi) Wrought Ironsyashvirsingh21No ratings yet
- Cast Iron - IspatGuruDocument14 pagesCast Iron - IspatGuruWalid Ben AmirNo ratings yet
- Water Quality Standards For Best Designated UsagesDocument7 pagesWater Quality Standards For Best Designated UsagesSagar ApteNo ratings yet
- Slag Fundamentals & Phase DiagramsDocument38 pagesSlag Fundamentals & Phase DiagramsNicole Altamirano Catalán100% (1)
- Diagrama TTT e Jominy Teórico: PL22, PL30, PL33, PL41, 4140 e 5135Document18 pagesDiagrama TTT e Jominy Teórico: PL22, PL30, PL33, PL41, 4140 e 5135DanielNo ratings yet
- GCSE AQA Chemistry Paper 1 (2021) - Practice PaperDocument32 pagesGCSE AQA Chemistry Paper 1 (2021) - Practice PaperPraiseNo ratings yet
- Esabbasicweldingfillarmetaltechnology 150312185120 Conversion Gate01Document301 pagesEsabbasicweldingfillarmetaltechnology 150312185120 Conversion Gate01arnoldbatista55No ratings yet
- General Awareness - General, Applied, Basic Chemistry Notes - Part 2 - TNPSC Question PapersDocument3 pagesGeneral Awareness - General, Applied, Basic Chemistry Notes - Part 2 - TNPSC Question Paperssiksac123No ratings yet
- Saep 1025Document50 pagesSaep 1025Aneesh JosephNo ratings yet
- Reducing Die Soldering in Die Casting - NADCADocument33 pagesReducing Die Soldering in Die Casting - NADCAAntonio MagañaNo ratings yet
- Chemical Cleaning of Fossil Power Station Steam GeneratorsDocument9 pagesChemical Cleaning of Fossil Power Station Steam Generatorssenthil031277100% (1)
- Silica-Carbonate HG Deposits (MODEL 27c Rytuba, 1986)Document5 pagesSilica-Carbonate HG Deposits (MODEL 27c Rytuba, 1986)Resa Rifal PraditiaNo ratings yet
- (EngineeringEBookspdf) Corrosion of Copper PDFDocument468 pages(EngineeringEBookspdf) Corrosion of Copper PDFFathi GabsiNo ratings yet
- Haematinics and Erythropoietin: By: DR Darakhshan RizviDocument51 pagesHaematinics and Erythropoietin: By: DR Darakhshan RizvianushkaNo ratings yet
- Investigation of Magnetite Nanoparticles Stability in Air by Thermal Analysis and FTIR SpectrosDocument14 pagesInvestigation of Magnetite Nanoparticles Stability in Air by Thermal Analysis and FTIR SpectrosMaría Pía Arancibia BravoNo ratings yet
- Engineering Failure Analysis: A. Goswami, S. KumarDocument7 pagesEngineering Failure Analysis: A. Goswami, S. KumarCHONKARN CHIABLAMNo ratings yet
- Klein GardDocument55 pagesKlein GardgpocobosNo ratings yet
- Analyzing The Performance of The Suppliers of AIW at MBA PROJECT REPORT MARKETINGDocument58 pagesAnalyzing The Performance of The Suppliers of AIW at MBA PROJECT REPORT MARKETINGBabasab Patil (Karrisatte)No ratings yet
- Niobium in Cast IronDocument13 pagesNiobium in Cast IronTayyab HussainNo ratings yet