Professional Documents
Culture Documents
First Observation of Photoinduced Nitrosyl Linkage Isomers of Iron Nitrosyl Porphyrins
First Observation of Photoinduced Nitrosyl Linkage Isomers of Iron Nitrosyl Porphyrins
Uploaded by
Sveti Jeronim0 ratings0% found this document useful (0 votes)
15 views2 pageschemistry
Original Title
07142
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentchemistry
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
15 views2 pagesFirst Observation of Photoinduced Nitrosyl Linkage Isomers of Iron Nitrosyl Porphyrins
First Observation of Photoinduced Nitrosyl Linkage Isomers of Iron Nitrosyl Porphyrins
Uploaded by
Sveti Jeronimchemistry
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
7142
J. Am. Chem. Soc. 2000, 122, 7142-7143
First Observation of Photoinduced Nitrosyl Linkage
Isomers of Iron Nitrosyl Porphyrins
Lin Cheng, Irina Novozhilova, Chris Kim,
Andrey Kovalevsky, Kimberly A. Bagley,*,
Philip Coppens,*, and George B. Richter-Addo*,
Department of Chemistry
State UniVersity of New York at Buffalo
Buffalo, New York 14260-3000
Department of Chemistry
State UniVersity College of New York at Buffalo
Buffalo, New York, 14222
Department of Chemistry and Biochemistry
UniVersity of Oklahoma, 620 Parrington OVal
Norman, Oklahoma, 73019
ReceiVed April 10, 2000
Knowledge of the binding modes of nitric oxide (NO) to
synthetic iron porphyrins is essential to an overall understanding
of the action of NO-heme-containing biomolecules, which play
a crucial role in several biologically important processes.1 While
much is known about the biological effects of NO, the detailed
mechanism of NO uptake and release by the heme group remains
to be elucidated. Kinetic studies of photochemically induced loss
and recombination of NO with hemoproteins2,3 and metalloporphyrins4,5 suggest that recombination after photolysis is a fast and
multistage process at room temperature.6-8
Known geometries of FeNO linkages in iron porphyrins and
heme have, to date, been limited to the Fe-N-O arrangement
in linear or bent forms.9 In general, iron nitrosyl porphyrins of
the ferric {FeNO}6 formulation have linear Fe-N-O groups,
whereas those of the ferrous {FeNO}7 formulation have bent
Fe-N-O groups. We have recently reported that photoexcitation
of {RuNO}6 ruthenium nitrosyl porphyrins results in the formation
of metastable O-bound (1-O; MS1) and side-on (2-N,O; MS2)
bound linkage isomers.10 We now report that metastable NO
linkage isomers of {FeNO}7 iron nitrosyl porphyrins are generated
upon illumination with light of 350 < < 550 nm wavelength
(300 W xenon arc lamp). While NO linkage isomers have been
observed for {MNO}6 (M ) Fe, Ru, Os)11-14 and for {MNO}10
Department of Chemistry, State University of New York at Buffalo,
Buffalo, New York 14260-3000.
Department of Chemistry, State University College of New York at
Buffalo, Buffalo, New York, 14222.
University of Oklahoma.
(1) Cheng, L.; Richter-Addo, G. B. Binding and Activation of Nitric Oxide
by Metalloporphyrins and Heme. In The Porphyrin Handbook; Kadish, K.
M., Smith, K. M., Guilard, R., Eds.; Academic Press: New York, 2000; Vol.
4 (Biochemistry and Binding: Activation of Small Molecules), pp 219-291.
(2) Walda, K. N.; Liu, X. Y.; Sharma, V. S.; Magde, D. Biochemistry 1994,
33, 2198-2209.
(3) Carlson, M. L.; Regan, R.; Elber, R.; Li, H.; Phillips, G. N.; Olson, J.
S.; Gibson, Q. H. Biochemistry 1994, 33, 10597-10606; Olson, J. S.; Phillips,
G. N. J. Biol. Chem. 1996, 271, 17593-17596.
(4) Zavarine, I. S.; Kini, A. D.; Morimoto, B. H.; Kubiak, C. P. J. Phys.
Chem. B 1998, 102, 7287-7292.
(5) Morlino, E. A.; Rodgers, M. A. J. Am. Chem. Soc. 1996, 118, 11798.
(6) Ford, P. C.; Bourassa, J.; Miranda, K.; Lee, B.; Lorkovic, I.; Boggs,
S.; Kudo, S.; Laverman, L. Coord. Chem. ReV. 1998, 171, 185-202.
(7) Hoshino, M.; Laverman, L.; Ford, P. C. Coord. Chem. ReV. 1999, 187,
75-102.
(8) Morlino, E. A.; Rodgers, M. A. J. Prog. React. Kinet. 1998, 23, 91115.
(9) For a listing of crystallographically characterized nitrosylmetalloporphyrins and nitrosyl hemes, see Table 6 in ref 1. See Tables 9 and 10 in ref
1 for a listing of known iron nitrosyl porphyrins.
(10) Fomitchev, D. V.; Coppens, P.; Li, T.; Bagley, K. A.; Chen, L.;
Richter-Addo, G. B. Chem. Commun. 1999, 2013-2014.
(11) Carducci, M. D.; Pressprich, M. R.; Coppens, P. J. Am. Chem. Soc.
1997, 119, 2669-2678.
(12) Fomitchev, D. V.; Coppens, P. Inorg. Chem. 1996, 35, 7021-7026.
complexes (M ) Ni),15 this is also the first observation of
photoinduced nitrosyl linkage isomerism for a bent nitrosyl
{MNO}7 complex of any metal.
Irradiation of the Fe(por)(NO) compounds16 (as KBr pellets)
at 25 K for 5-10 min results in the formation of new bands in
their IR spectra, downshifted from the parent NO stretching
frequencies. The observed frequencies of the photoinduced bands
for the 14N16O, 15N16O and 15N18O complexes of Fe(OEP) and
Fe(TTP) and their relative shifts are listed in the Supporting
Information. Warming the samples to 40 K results in the
disappearance of all new bands and the restoration of the
intensities of the parent NO bands. No free NO was observed in
the photolysis experiments.
In the case of Fe(TTP)(NO), photolysis at 25 K for 10 min
results in the appearance of a new band at 1532 cm-1 in the
difference IR spectrum (A in Figure 1, left), downshifted by 140
cm-1. Measurements on isotope-labeled Fe(TTP)(15N16O) and Fe(TTP)(15N18O) show the band to be due to the coordinated NO
group (Figure 1; Table S1 of the Supporting Information). A
second, much weaker, photoinduced band at 1502 cm-1 does not
change on isotopic substitution (Figure 1, left), and is hence
assigned as originating from another part of the complex. Because
of the rapid decay of the photoinduced IR features, samples were
irradiated continuously during the spectral measurements.
A similar IR band (A in Figure 1, right) is generated when
Fe(OEP)(NO) is irradiated under the same conditions. The use
of the 15N16O and 15N18O isotope labels again reveals the band to
be due to the coordinated NO group. However, a second new IR
band at 1520 cm-1 (labeled B) is also induced, together with an
isotope-substitution insensitive band at 1506 cm-1 (Figure 1,
right). While both the A and B bands decay at 25 K, the decay
of the B band is much slower, suggesting the formation of two
distinct photoproducts. The isotopic shifts of the IR bands are as
expected for Fe(OEP)(15N16O) and its photoproducts, and for the
Fe(OEP)(15N18O) derivative and its A photoproduct. The B band
of photolyzed Fe(OEP)(15N18O) appears as a doublet and the shift
of 95 cm-1 is larger than the expected value of 68 cm-1.
Vibrational coupling of this low-frequency band with other modes
of the complex may be responsible for the latter observation.
Conversion percentages are estimated at 6-10% for Fe(OEP)(NO), and 2-3% for Fe(TTP)(NO).
The observed shifts of NO for the Fe(por)(NO) photoproducts
are very similar to those recorded for other non-porphyrin Fe and
Ru complexes for which the identity of the photoinduced species
have been established by photocrystallographic experiments.11-14
The IR data thus suggests the formation of an MS1 (1-ON)
isomer upon irradiation. This assignment is supported by the DFT
calculations.
A series of theoretical calculations was performed on FeP(NO) (P ) porphine dianion) with the Amsterdam Density
Functional (ADF) package.17,18
The ground-state optimization of FeP(NO) reproduces the
structural distortions of the N4Fe(NO) core recently observed by
(13) Coppens, P.; Fomitchev, D. V.; Carducci, M. D.; Culp, K. J. Chem.
Soc., Dalton Trans. 1998, 865-872.
(14) Fomitchev, D. V.; Coppens, P. Comments Inorg. Chem. 1999, 21,
131-148.
(15) Fomitchev, D. V.; Furlani, T. R.; Coppens, P. Inorg. Chem. 1998, 37,
1519-1526.
(16) The five-coordinate nitrosyl iron porphyrins were prepared using
established procedures (ref 1) by reacting Fe(OEP)Cl and Fe(TPP)Cl (OEP
) octaethylporphyrinato dianion; TTP ) tetratolyporphyrinato dianion) with
NO gas in chloroform followed by addition of methanol.
(17) The Amsterdam Density Functional program package, v.1999.02; the
Vrije Universiteit: Amsterdam; the University of Calgary: Canada. Fonseca
Guerra, C.; Visser, O.; Snijders, J. G.; te Velde, G.; Baerends, E. J. In Methods
and Techniques for Computational Chemistry (METECC-5); Clementi, E.,
Corongiu, G., Eds.; STEF: Cagliari, 1995; pp 303-395.
10.1021/ja001243u CCC: $19.00 2000 American Chemical Society
Published on Web 07/07/2000
Communications to the Editor
J. Am. Chem. Soc., Vol. 122, No. 29, 2000 7143
Figure 2. The theoretical geometry of the metastable state FeP(ON),
viewed from three different directions.
Figure 1. Infrared difference spectra formed by subtracting the spectra
of the complex after illumination (at 25 K) from the infrared spectra of
the complex in the dark. (Left) Fe(TTP)(NO); (right) Fe(OEP)(NO).
Negative and positive features represent IR bands that are depleted, or
new or increased in intensity upon illumination, respectively. The
differential feature of the highest frequency band of Fe(OEP)(NO) is due
to a slight change in peak profile.
Ellison and Scheidt for Fe(OEP)(NO).19,20 As observed in their
X-ray diffraction experiment, the Fe-N(O) vector is tilted toward
the oxygen atom from the z-axis defined by the normal to the
plane through the four equatorial N atoms (by 8 according to
the optimization, 6-8 according to experiment), while the NO
group is staggered with respect to the equatorial bonds. The four
equatorial Fe-N distances divide in two groups with the bonds
closest to the direction of the NO tilt being shorter by 0.025 ,
as observed in the experiment and interpreted by Scheidt and
Ellison in terms of the dz2-NO * interaction and subsequent
tilting of the basal -orbitals.21
Full optimization of the staggered MS1 isonitrosyl structure
for FeP(ON) confirms that it corresponds to a local energy
minimum, with an energy 1.59 eV above that of the ground state.
As the B band formation is dependent on the ring substitution
(i.e., present for the OEP, but not for the TTP complex),
calculations on the ring-unsubstituted complex may not reveal a
second energy minimum. In the calculation of metastable linkage
isomers by Delley et al. of sodium nitroprusside,22 energy minima
for two related metastable conformations, eclipsed and staggered
(18) The core electrons of C, N, O, and Fe were treated by a frozen core
approximation. Basis sets were identical to those used in the Fe(II)P
calculations of Kozlowski et al. (Kozlowski, P. M.; Spiro, T. G.; Berces, A.;
Zgierski, M. Z. J. Phys. Chem. B 1998, 102, 2603-2608). The valence shells
of the H, C, N, and O atoms were described by a double- STO basis set,
extended with a polarization function (ADF database III). For the 3s, 3p, and
3d shells on Fe, a triple- STO basis set was employed (ADF database II).
All calculations were spin-unrestricted and based on the local density
approximation (LDA) in the parametrization of Vosko, Wilk, and Nusair
(VWN). Scalar relativistic effects on Fe were taken into account with the
Pauli formalism. In the geometry optimizations a convergence criterion for
gradients of 10-5 (au/) was used, while integrals were evaluated with an
accuracy of 8 significant digits.
(19) Ellison, M. K.; Scheidt, W. R. J. Am. Chem. Soc. 1997, 119, 74047405.
(20) Scheidt, W. R.; Duval, H. F.; Neal, T. J.; Ellison, M. K., J. Am. Chem.
Soc. 2000, 122, 4651-4659.
(21) Scheidt, W. R.; Ellison, M. K. Acc. Chem. Res. 1999, 32, 350-359.
with respect to the equatorial ligands, were found. However, in
our work the eclipsed isonitrosyl configuration for the model FeP(ON) compound invariably reverted to the staggered structure on
optimization. Attempts to optimize a side-on 2 MS2 geometry
invariably failed, the molecule returning to the ground-state
structure after a large number of cycles. This is in complete
agreement with the absence of an MS2 spectral feature in all our
light-on spectra. The theoretical optimized geometry of the FeON MS1 state is illustrated in Figure 2. The saddle shape of the
porphyrin macrocycle for both the optimized ground and metastable states geometries may be a property of the isolated molecule
and does not occur in the solid state.
In summary, we have provided the first spectroscopic and
theoretical evidence for nitrosyl linkage isomerism in iron
porphyrins, and the first observation of linkage isomers for any
five coordinate {MNO}7 complex. The results are relevant to the
overall study of NO binding, dissociation, and recombination
involving heme proteins. Chance and co-workers, for instance,
have characterized two photoproduct states of ferrous MbNO that
involve two (dissociated) NO conformations that rebind to the
iron center at different rates.23 Although isonitrosyls were not
observed in this latter study, the authors proposed that the fastrebinding state has the N-atom of the dissociated NO group closer
to the metal center, whereas in the slow-binding state the O-atom
is closer to the metal center, which would necessitate the flipping
of NO before rebinding.
We are pursuing similar studies on six-coordinate Fe-porphyrin
nitrosyl species.
Acknowledgment. Support of this work by the National Science
Foundation (CHE9981864, P.C.; CHE9625065, G.B.R.-A.; MCB9723828,
K.A.B.), the National Institutes of Health (R29GM53586, G.B.R.-A.),
and the Petroleum Research Fund administered by the American Chemical
Society (PRF28664AC3, P.C.) is gratefully acknowledged. All theoretical
calculations were performed at the Center for Computational Research
at SUNY at Buffalo, which is supported by Grant DBI9871132 from the
National Science Foundation. We thank Dr. W. R. Scheidt for a preprint
of reference 20.
Supporting Information Available: Table of IR frequencies and
isotope shifts (PDF). This material is available free of charge via the
Internet at http://pubs.acs.org.
JA001243U
(22) Delley, B.; Schefer, J.; Woike, T. J. Chem. Phys. 1997, 107, 1006710074.
(23) Miller, L. M.; Pedraza, A. J.; Chance, M. R. Biochemistry 1997, 36,
12199-12207.
You might also like
- L. C. O'Brien Et Al - Gas-Phase Inorganic Chemistry: Laser Spectroscopy of Calcium and Strontium MonocarboxylatesDocument5 pagesL. C. O'Brien Et Al - Gas-Phase Inorganic Chemistry: Laser Spectroscopy of Calcium and Strontium MonocarboxylatesDamxz5No ratings yet
- Theoretical Prediction of Noble Gas Containing AnionsDocument5 pagesTheoretical Prediction of Noble Gas Containing AnionsEnformableNo ratings yet
- Weinstock1960 PDFDocument6 pagesWeinstock1960 PDFAnonymous qnG8fsyCjlNo ratings yet
- Photophysical Effects of Metal-Carbon Bonds in Ortho-Metalated Complexes of Ir (111) and RH (111)Document7 pagesPhotophysical Effects of Metal-Carbon Bonds in Ortho-Metalated Complexes of Ir (111) and RH (111)Mera AhmadNo ratings yet
- Hassan 1Document3 pagesHassan 1Hasan HashemiNo ratings yet
- UV (Or: Gas-Phase Inorganic Chemistry: Laser Spectroscopy of Calcium and Strontium MonoformamidatesDocument3 pagesUV (Or: Gas-Phase Inorganic Chemistry: Laser Spectroscopy of Calcium and Strontium MonoformamidatesDamxz5No ratings yet
- Structures, Metal-Ligand Bond Strength, And Bonding Analysis Of Ferrocene Derivatives With Group-15 Heteroligands Fe (Η -E) And Fecp (Η -E) (E) N, P, As, Sb) - A Theoretical StudyDocument9 pagesStructures, Metal-Ligand Bond Strength, And Bonding Analysis Of Ferrocene Derivatives With Group-15 Heteroligands Fe (Η -E) And Fecp (Η -E) (E) N, P, As, Sb) - A Theoretical StudyFabian MelinaoNo ratings yet
- Enzyme Electrokinetics: Electrochemical Studies of The Anaerobic Interconversions Between Active and Inactive States of (Nife) - HydrogenaseDocument10 pagesEnzyme Electrokinetics: Electrochemical Studies of The Anaerobic Interconversions Between Active and Inactive States of (Nife) - HydrogenaseMichael PearsonNo ratings yet
- Stereoelectronic Effects On The Basicity and Nucleophilicity of Phosphites and Phosphates. Ab Initio Molecular Orbital Calculations and The A-EffectDocument7 pagesStereoelectronic Effects On The Basicity and Nucleophilicity of Phosphites and Phosphates. Ab Initio Molecular Orbital Calculations and The A-EffectvycttorNo ratings yet
- Insitu Trans Electron MicrosDocument21 pagesInsitu Trans Electron MicrosRabiNo ratings yet
- L. C. O'Brien and P. F. Bernath - Laser Spectroscopy of Calcium and Strontium Monoc y ClopentadienideDocument2 pagesL. C. O'Brien and P. F. Bernath - Laser Spectroscopy of Calcium and Strontium Monoc y Clopentadienidem4m4daNo ratings yet
- Ultrafast Time-Resolved Laser Spectroscopic Studies of - Bis (Ferrocene-Carboxyl-Ato) (Tetraphenyl-Porphyrinato) Tin (IV) : Intramolecular Electron-Transfer DynamicsDocument6 pagesUltrafast Time-Resolved Laser Spectroscopic Studies of - Bis (Ferrocene-Carboxyl-Ato) (Tetraphenyl-Porphyrinato) Tin (IV) : Intramolecular Electron-Transfer DynamicsgenesisalbaricoNo ratings yet
- N-Heterocyclic Carbene Gold (I) ComplexesDocument8 pagesN-Heterocyclic Carbene Gold (I) ComplexesgabrielaNo ratings yet
- Demonstration of A Phosphazirconocene As A Catalyst For The Ring Opening of Epoxides With TMSCLDocument3 pagesDemonstration of A Phosphazirconocene As A Catalyst For The Ring Opening of Epoxides With TMSCLRohan PrajapatiNo ratings yet
- Ion ChemistryDocument40 pagesIon ChemistryAlonsoNo ratings yet
- Nitrogen FixationDocument9 pagesNitrogen FixationQueenNo ratings yet
- Feng PRB La2nioso6Document9 pagesFeng PRB La2nioso6Hai FengNo ratings yet
- Paper FerrocenoDocument5 pagesPaper FerrocenoEmily Coromoto Gonzalez MarcanoNo ratings yet
- Electronic Structure Studies of The Spinel CoFe2O4 by X-Ray Photoelectron SpectrosDocument4 pagesElectronic Structure Studies of The Spinel CoFe2O4 by X-Ray Photoelectron SpectrosAlin DrucNo ratings yet
- Stereospecific Halogenation of P (O) - H Bonds With Copper (II) Chloride Affording Optically Active Z Z P (O) CLDocument4 pagesStereospecific Halogenation of P (O) - H Bonds With Copper (II) Chloride Affording Optically Active Z Z P (O) CLDiogomussumNo ratings yet
- 1c PDFDocument3 pages1c PDFJavier Vera MercadoNo ratings yet
- Doming Modes and Dynamics of Model Heme CompoundsDocument5 pagesDoming Modes and Dynamics of Model Heme Compoundsalinusha_7No ratings yet
- MC Clever Ty 1979Document24 pagesMC Clever Ty 1979Petru ApostolNo ratings yet
- Cationic Exchange in Nanosized Znfe O Spinel Revealed by Experimental and Simulated Near-Edge Absorption StructureDocument5 pagesCationic Exchange in Nanosized Znfe O Spinel Revealed by Experimental and Simulated Near-Edge Absorption StructureAna Paula FreitasNo ratings yet
- Shimoni Liv Ny 1998Document15 pagesShimoni Liv Ny 1998Octavin ExaudinaNo ratings yet
- Structure and Magnetic Properties of Trigonal Bipyramidal Iron Nitrosyl ComplexesDocument6 pagesStructure and Magnetic Properties of Trigonal Bipyramidal Iron Nitrosyl ComplexesArnau Dominguez ZoroaNo ratings yet
- Synthesis, NMR and Vibrational Spectroscopic Characterization, and Computational Study of The cis-IO F AnionDocument5 pagesSynthesis, NMR and Vibrational Spectroscopic Characterization, and Computational Study of The cis-IO F AnionDanishkhan BMCNo ratings yet
- Imposition of Polarity On A Centrosymmetric Zeolite Host: The Effect of Fluoride Ions On Template Ordering in Zeolite IFRDocument2 pagesImposition of Polarity On A Centrosymmetric Zeolite Host: The Effect of Fluoride Ions On Template Ordering in Zeolite IFRSveti JeronimNo ratings yet
- Synthesis of Novel BINOL-derived Chiral Bisphosphorus Ligands and Their Application in Catalytic Asymmetric HydrogenationDocument2 pagesSynthesis of Novel BINOL-derived Chiral Bisphosphorus Ligands and Their Application in Catalytic Asymmetric HydrogenationNguyễn Thanh TùngNo ratings yet
- Accepted Manuscript: Geochimica Et Cosmochimica ActaDocument36 pagesAccepted Manuscript: Geochimica Et Cosmochimica ActaMateo GutierrezNo ratings yet
- Adams 1986Document5 pagesAdams 1986Rasel MahfujNo ratings yet
- Journal of American Ceramic SocietyDocument8 pagesJournal of American Ceramic SocietyNeeraj PanwarNo ratings yet
- Abstract-Platinum ReviewDocument4 pagesAbstract-Platinum ReviewleonardoNo ratings yet
- Interaction of Poly (Dially1dimethylammonium Chloride) With Ferro-And Ferricyanide AnionsDocument5 pagesInteraction of Poly (Dially1dimethylammonium Chloride) With Ferro-And Ferricyanide AnionsPrabhat UpadhyayNo ratings yet
- H. Giefers Et Al - Phonon Spectroscopy of Oriented HCP IronDocument6 pagesH. Giefers Et Al - Phonon Spectroscopy of Oriented HCP IronMutrexczNo ratings yet
- Selectivity in The Reaction of Triplet Phenyl CationsDocument9 pagesSelectivity in The Reaction of Triplet Phenyl CationsSilvanaMedhatNo ratings yet
- Structure and Stability of Sodium IntercDocument4 pagesStructure and Stability of Sodium Intercdjqing2005No ratings yet
- Alexander M. Spokoyny Et Al - Carborane-Based Pincers: Synthesis and Structure of SeBSe and SBS PD (II) ComplexesDocument2 pagesAlexander M. Spokoyny Et Al - Carborane-Based Pincers: Synthesis and Structure of SeBSe and SBS PD (II) ComplexesGomsajNo ratings yet
- Hisao Kobayashi Et Al - Phonon Density of States and Compression Behavior in Iron Sulfide Under PressureDocument4 pagesHisao Kobayashi Et Al - Phonon Density of States and Compression Behavior in Iron Sulfide Under PressureDrebuioNo ratings yet
- Synthesis and Structure Rhodium Complexes Containing A Photolabile Q - Carbodiimlde Ligand. 1,3-Dipolar Cycloaddition of Phenyl Azide To TP'RH (CNR) P (TP' H Ydrotris (3,5-Dimethylpyrazolyi) Borate)Document10 pagesSynthesis and Structure Rhodium Complexes Containing A Photolabile Q - Carbodiimlde Ligand. 1,3-Dipolar Cycloaddition of Phenyl Azide To TP'RH (CNR) P (TP' H Ydrotris (3,5-Dimethylpyrazolyi) Borate)Nguyễn Thanh TùngNo ratings yet
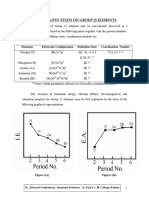
- Elements of Group VA or 15 (Elements of Nitrogen and Phosphorus Family, NsDocument12 pagesElements of Group VA or 15 (Elements of Nitrogen and Phosphorus Family, Nsar1434245No ratings yet
- Photoluminescence From Au Nanoparticles Embedded in Au:oxide Composite FilmsDocument4 pagesPhotoluminescence From Au Nanoparticles Embedded in Au:oxide Composite Filmsben0706No ratings yet
- Double PerovskiteDocument4 pagesDouble Perovskiterautsubhajit89No ratings yet
- Cy 5012-2024 Mingos Fusion FormalismDocument9 pagesCy 5012-2024 Mingos Fusion Formalism0066Ritul BhatiaNo ratings yet
- Effect of Substitution of MN With Fe or CR in Heusler Alloy of Co2MnsnDocument11 pagesEffect of Substitution of MN With Fe or CR in Heusler Alloy of Co2MnsnΙήήΘςέήτ βόγNo ratings yet
- Simulation of Point Defects in High-Density Luminescent Crystals: Oxygen in Barium FluorideDocument9 pagesSimulation of Point Defects in High-Density Luminescent Crystals: Oxygen in Barium FluorideDyra KesumaNo ratings yet
- Accepted Manuscript: 10.1016/j.jallcom.2017.02.199Document29 pagesAccepted Manuscript: 10.1016/j.jallcom.2017.02.199Jeena RoseNo ratings yet
- Synthesis of Nanoscale Ce Fe O Solid Solutions Via A Low-Temperature ApproachDocument2 pagesSynthesis of Nanoscale Ce Fe O Solid Solutions Via A Low-Temperature ApproachEmerson SilvaNo ratings yet
- 4104 A Study - Material 1 (Mossbauer Spectroscopy)Document21 pages4104 A Study - Material 1 (Mossbauer Spectroscopy)GuRi JassalNo ratings yet
- Synthesis and Reactivity of (M (η3-Allyl) (η2-Amidinato) (CO) 2 (Phosphonium Ylide) )Document6 pagesSynthesis and Reactivity of (M (η3-Allyl) (η2-Amidinato) (CO) 2 (Phosphonium Ylide) )cahz1307No ratings yet
- Vanpeteghem 2008Document12 pagesVanpeteghem 2008Jorge Álef Estevam Lau BomfimNo ratings yet
- Technical Papers: Biosynthesis of Penicillin. Role of Phenylacetic AcidDocument2 pagesTechnical Papers: Biosynthesis of Penicillin. Role of Phenylacetic AcidJuankNo ratings yet
- 1964 - SchmulbachHart - Molecular Addition Compounds of Amines and Iodine. Evidence For The Existence of A 2 To 1 Triethylamine-Iodine ComplexDocument5 pages1964 - SchmulbachHart - Molecular Addition Compounds of Amines and Iodine. Evidence For The Existence of A 2 To 1 Triethylamine-Iodine ComplexUAS TekimNo ratings yet
- Abs and Fluor of TPPH2Document11 pagesAbs and Fluor of TPPH2morikao21No ratings yet
- #12 Sulfur Containing Terpyridine Derivatives Synthesis, Coordination Properties, and Adsorption On The Gold SurfaceDocument17 pages#12 Sulfur Containing Terpyridine Derivatives Synthesis, Coordination Properties, and Adsorption On The Gold Surfacefabian cotacioNo ratings yet
- Comparative Study of Group 15 ElementsDocument20 pagesComparative Study of Group 15 ElementsUmesh JogeNo ratings yet
- 郭非凡EES FeCoNi的硼化物 SIDocument23 pages郭非凡EES FeCoNi的硼化物 SIorangewings翅橙No ratings yet
- #Ref-56 Do Artigo - 2019-Metal Complexes of Flavonoids...Document5 pages#Ref-56 Do Artigo - 2019-Metal Complexes of Flavonoids...las.chemicalNo ratings yet
- Lamansky 2001Document9 pagesLamansky 2001snehasis banerjeeNo ratings yet
- Air Conditioner MKA 2001 M: Art.-No.: 23.601.15 Bar Code: 4006825535778 Sales Unit: 1 PCDocument1 pageAir Conditioner MKA 2001 M: Art.-No.: 23.601.15 Bar Code: 4006825535778 Sales Unit: 1 PCSveti JeronimNo ratings yet
- Understanding Steam Sterilizer Physical Parameters: by Linda Clement and John BlileyDocument3 pagesUnderstanding Steam Sterilizer Physical Parameters: by Linda Clement and John BlileySveti Jeronim100% (1)
- Contents of Volume: Number 1Document1,055 pagesContents of Volume: Number 1Sveti JeronimNo ratings yet
- Additions and CorrectionsDocument1 pageAdditions and CorrectionsSveti JeronimNo ratings yet
- Creation of A Monomeric Ru Species On The Surface of Hydroxyapatite As An Efficient Heterogeneous Catalyst For Aerobic Alcohol OxidationDocument2 pagesCreation of A Monomeric Ru Species On The Surface of Hydroxyapatite As An Efficient Heterogeneous Catalyst For Aerobic Alcohol OxidationSveti JeronimNo ratings yet
- Imposition of Polarity On A Centrosymmetric Zeolite Host: The Effect of Fluoride Ions On Template Ordering in Zeolite IFRDocument2 pagesImposition of Polarity On A Centrosymmetric Zeolite Host: The Effect of Fluoride Ions On Template Ordering in Zeolite IFRSveti JeronimNo ratings yet
- Attenuating and Supplanting Nonclassical Stabilization: CR (CO) - Complexed Benzonorbornenyl CationsDocument2 pagesAttenuating and Supplanting Nonclassical Stabilization: CR (CO) - Complexed Benzonorbornenyl CationsSveti JeronimNo ratings yet
- Aromatic Heterocycles Containing Arsenic and Sulfur: The Synthesis of 1,2-And 1,3-Thiaarsole and The Gas-Phase Molecular Structure of 1,2-ThiaarsoleDocument5 pagesAromatic Heterocycles Containing Arsenic and Sulfur: The Synthesis of 1,2-And 1,3-Thiaarsole and The Gas-Phase Molecular Structure of 1,2-ThiaarsoleSveti JeronimNo ratings yet
- A Solid State NMR Study of Dynamics in A Hydrated Salivary Peptide Adsorbed To HydroxyapatiteDocument2 pagesA Solid State NMR Study of Dynamics in A Hydrated Salivary Peptide Adsorbed To HydroxyapatiteSveti JeronimNo ratings yet
- Spectroscopic Characterization of Spin-Labeled Magnetically Oriented Phospholipid Bilayers by EPR SpectrosDocument7 pagesSpectroscopic Characterization of Spin-Labeled Magnetically Oriented Phospholipid Bilayers by EPR SpectrosSveti JeronimNo ratings yet
- Daily Lesson Plan: in Science 6Document75 pagesDaily Lesson Plan: in Science 6Bryan Bangiban100% (2)
- Safety Data Sheet: Combiscreen® 11sysDocument6 pagesSafety Data Sheet: Combiscreen® 11sysCristian LaraNo ratings yet
- Biochimica Et Biophysica ActaDocument12 pagesBiochimica Et Biophysica Actaa1246913No ratings yet
- COA of Alpha ArbutinDocument1 pageCOA of Alpha ArbutinPan EmmaNo ratings yet
- Potential Energy SurfaceDocument21 pagesPotential Energy SurfaceSIDDHARTH MARATHANo ratings yet
- G333 ManualDocument24 pagesG333 ManualNico V.d. MerweNo ratings yet
- Eulers EquationDocument7 pagesEulers EquationShabana ferozNo ratings yet
- HarmattanDocument5 pagesHarmattanFixxy EfikaNo ratings yet
- 6-2 Stark EffectDocument44 pages6-2 Stark Effectcamzmila6No ratings yet
- Corrosion and Its Objective QuestionsDocument2 pagesCorrosion and Its Objective QuestionsSrinivasan Alagappan100% (4)
- Polycarbonate Chemical Compatibility ChartDocument14 pagesPolycarbonate Chemical Compatibility Chartteban09No ratings yet
- Unit 4 PuputDocument48 pagesUnit 4 PuputLinda ArifinNo ratings yet
- Lab Soil Testing StandardsDocument5 pagesLab Soil Testing Standardssamoonibrahim100% (1)
- Physics Project ReportDocument6 pagesPhysics Project ReportSad FateNo ratings yet
- Radiative Heat Transfer ProblemsDocument10 pagesRadiative Heat Transfer Problemseldwin_dj7216No ratings yet
- Basic Principle of ChemistryDocument31 pagesBasic Principle of ChemistrybybmaishanuNo ratings yet
- Module 4-Principles of Measurement and Laboratory Mathematics in Clinical ChemistryDocument6 pagesModule 4-Principles of Measurement and Laboratory Mathematics in Clinical ChemistryAllyah Ross DuqueNo ratings yet
- Hap HLC Cide 1220Document4 pagesHap HLC Cide 1220Laboratorium PT MASNo ratings yet
- 8951氟素油Document2 pages8951氟素油胡绪智No ratings yet
- Book of Design Water SystemDocument233 pagesBook of Design Water SystemAnn-Marie DeeganNo ratings yet
- Production: Ethers, Aliphatic)Document6 pagesProduction: Ethers, Aliphatic)Harsh ShahNo ratings yet
- OP AS31 Nozzle Cleaning Procedure 20211013 V1 MWADocument4 pagesOP AS31 Nozzle Cleaning Procedure 20211013 V1 MWAkldbin.farooq29No ratings yet
- Photocatalytic Removal of CR (VI) Using Three-Phase FluidizedDocument13 pagesPhotocatalytic Removal of CR (VI) Using Three-Phase Fluidizedshritik raj awadhiyaNo ratings yet
- Iec 60287-1-1Document44 pagesIec 60287-1-1Jose Giraldo100% (1)
- Chemical Kinetics - LectureDocument37 pagesChemical Kinetics - LectureEsmira Melić ŠutkovićNo ratings yet
- Free Radicals and The SkinDocument5 pagesFree Radicals and The SkinTamina PfeifferNo ratings yet
- Lube Oil SpecificationDocument24 pagesLube Oil SpecificationOrwah malkawiNo ratings yet
- Physics Revision ChecklistDocument24 pagesPhysics Revision ChecklistShifa RizwanNo ratings yet
- NotesDocument36 pagesNotesULTRA College of Engineering & Technology for WomenNo ratings yet
- K01587 - 20200217124343 - Chapter 4 - Natural and Synthetic RubberDocument27 pagesK01587 - 20200217124343 - Chapter 4 - Natural and Synthetic RubberAbdulRahim059No ratings yet