Professional Documents
Culture Documents
Reduction of Graphene Oxide Via - Ascorbic Acidw
Reduction of Graphene Oxide Via - Ascorbic Acidw
Uploaded by
MahmutTaşOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reduction of Graphene Oxide Via - Ascorbic Acidw
Reduction of Graphene Oxide Via - Ascorbic Acidw
Uploaded by
MahmutTaşCopyright:
Available Formats
View Article Online / Journal Homepage / Table of Contents for this issue
COMMUNICATION
www.rsc.org/chemcomm | ChemComm
Reduction of graphene oxide via L-ascorbic acidw
Jiali Zhang,a Haijun Yang,a Guangxia Shen,a Ping Cheng,a Jingyan Zhang*b and
Shouwu Guo*a
Downloaded by University of Arizona on 16 January 2013
Published on 24 December 2009 on http://pubs.rsc.org | doi:10.1039/B917705A
Received (in Cambridge, UK) 27th August 2009, Accepted 4th December 2009
First published as an Advance Article on the web 24th December 2009
DOI: 10.1039/b917705a
We demonstrated that the individual graphene oxide sheets can
be readily reduced under a mild condition using L-ascorbic acid
(L-AA). This simple approach should nd practical applications
in large scale production of water soluble graphene.
Ever since the discovery of the advantageous properties of
graphene, the challenge has been to produce individual
graphene sheets in a bulk quantity.15 In the last few years,
several approaches, such as micromechanical exfoliation of
graphite,1 chemical vapor deposition,26 and solution-based
chemical reduction of graphene oxide (GO) to graphene,710
have been developed. Among these methods, one may mention
the solution-based chemical reduction of GO because it has
advantages of low-cost and bulk-scale production. However,
the low solubilities of the chemically reduced graphene oxide
(CR-GO) sheets in water and most organic solvents, and the
strong pp stacking tendency between CR-GO sheets lead to
the formation of irreversible agglomerates unless the capping
reagents (polymers or surfactants) are used. On the other
hand, the capping reagents may aect the properties of the
graphene sheets, accordingly limited their practical applications.
Additionally, in the most successful cases, the chemical reduction
of the GO was conducted using hydrazine or hydrazine
hydrate as the reducing agent. However, because hydrazine
and hydrazine hydrate are highly poisonous and explosive,11
precautions must be taken when large quantities of hydrazine
or hydrazine hydrate are used. Consequently, a new approach
for eectively converting GO into stable graphene sheets
under mild conditions needs to be explored.12,13
L-Ascorbic acid (L-AA), having a mild reductive ability and
nontoxic property, is naturally employed as a reducing agent
in living things,1416 and has also been used as a primary
reductant in the laboratory.1720 Herein, we present a facile
approach for reducing GO sheets using L-AA as a reductant in
an aqueous solution at room temperature. As will be shown,
besides the pronounced reduction capability to GO, the
oxidized products of L-AA may also play a role as a capping
a
National Key Laboratory of Micro/Nano Fabrication Technology,
Key Laboratory for Thin Film and Microfabrication of the Ministry
of Education, Research Institute of Micro/Nano Science and
Technology, Shanghai Jiao Tong University, Shanghai 200240,
P.R. China. E-mail: swguo@sjtu.edu.cn
b
State Key Laboratory of Bioreactor Engineering, School of
Pharmacy, East China University of Science and Technology,
Shanghai 200237, P.R. China. E-mail: jyzhang@ecust.edu.cn
w Electronic supplementary information (ESI) available: Experimental
preparation and instrumentation, UV-vis spectra of the reduced GO,
XRD pattern of graphite and GO before and after the reduction, and
optical images of the thin papers of GO and reduced GO. See DOI:
10.1039/b917705a
1112 | Chem. Commun., 2010, 46, 11121114
reagent to stabilize as-reduced GO sheets simultaneously,
avoiding the usage of additional capping reagents.
More signicantly, in comparison with the conventional
reductants used in GO reduction, such as hydrazine and
hydrazine hydrate, L-AA itself and the oxidized products are
environmentally friendly.
The aqueous dispersion of GO sheets was prepared
following the literature procedure.21,22 Briey, the graphite
powder was rst oxidized into graphite oxide using
KMnO4/H2SO4, and then the graphite oxide was exfoliated
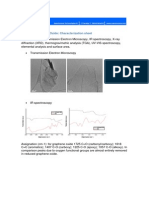
into GO sheets by ultrasonication in water. Fig. 1a and c
depict the typical atomic force microscopy (AFM) and
transmission electron microscopy (TEM) images of the GO sheets
we obtained. The thickness, measured from the height prole
of the AFM image, Fig. 1b, is about 1.2 nm, which is
consistent with the data reported in the literature, indicating
that the formation of the single layered GO.8 The reduction of
the GO using L-AA was performed in water at room temperature
(B23 1C). In a typical experiment, 50 mg of L-AA was added
to 50 mL (0.1 mg mL 1) of an aqueous dispersion of the GO
under vigorous stirring. The reduction progress was monitored
by UV-vis spectroscopy. As shown in Fig. 1d, with the
reduction progressing, the intensities of two UV-vis absorption
peaks centered at 230 and 300 nm, which are caused by the
GO, decayed gradually with the increase of reduction time,
and disappeared after 24 h. Meanwhile, a new absorption peak
showed up around 264 nm, and red-shifted with the reduction
time. The absorption intensity of the entire spectrum,
especially in the region above 300 nm, increased dramatically,
providing a rst hint that the GO might be reduced and the
aromatic structure might be restored gradually.7 The
reduction of the oxygen-containing groups in GO by L-AA
was also conrmed by FT-IR spectroscopy. As shown in
Fig. 2a, after 48 h reduction of the GO with L-AA, the
intensities of the FT-IR peaks corresponding to the oxygen
functionalities, such as the CQO stretching vibration peak at
1726 cm 1, the vibration and deformation peaks of OH
groups at 3395 cm 1 and 1410 cm 1, respectively, the CO
(epoxy) stretching vibration peak at 1226 cm 1, and the CO
(alkoxy) stretching peak at 1052 cm 1 decreased dramatically,
and some of them disappeared entirely. These observations
conrmed that most oxygen functionalities in the GO were
removed. The variation of the relative intensities of G (the E2g
mode of sp2 carbon atoms) and D (the symmetry A1g mode)
bands in the Raman spectra of the GO during the reduction
usually reveals the change of the electronic conjugation
state.9,23 As depicted in Fig. 2b, with the reduction, the ratio
of D/G increased signicantly. This agrees well with the
Raman spectrum of the GO reduced by hydrazine that was
This journal is
The Royal Society of Chemistry 2010
Downloaded by University of Arizona on 16 January 2013
Published on 24 December 2009 on http://pubs.rsc.org | doi:10.1039/B917705A
View Article Online
Fig. 3 XPS spectra of C 1s of GO before (a), and after (b) the
reduction with L-AA for 48 h. The peaks 1, 2, 3, and 4 correspond to
CQC/CC in aromatic rings, CO (epoxy and alkoxy), CQO, and
COOH groups, respectively.
Fig. 1 (a) A tapping mode AFM image of graphene oxide (GO)
sheets on mica surface, (b) the height prole of the AFM image,
(c) TEM image of the GO, and (d) UV-vis spectra of GO aqueous
dispersions before (1) and after being reduced via L-AA for 12 (2),
24 (3), and 48 (4) hours.
Fig. 2 FT-IR (a) and Raman (b) spectra of GO before (1) and after
being reduced via L-AA for 12 (2), 24 (3), and 48 (4) hours.
reported by Stankovich et al.,9 indicating the reduction did
take place.
The reduction of GO with L-AA was also characterized
using X-ray photoelectron spectroscopy (XPS) and X-ray
powder diraction (XRD). Fig. 3 shows the C 1s XPS spectra
of GO before and after the reduction with L-AA for 48 h.
Before reduction (Fig. 3a), four dierent peaks centered at
284.5, 286.4, 287.8, and 289.0 eV, corresponding to CQC/CC
in aromatic rings, CO (epoxy and alkoxy), CQO, and COOH
groups, respectively, were detected. After the reduction for
48 h (Fig. 3b), the intensities of all C 1s peaks of the carbons
binding to oxygen, especially the peak of CO (epoxy and
alkoxy), decreased dramatically, revealing that most oxygen
containing functional groups were removed after the
reduction. As shown in Fig. S1w, after the reduction, the sharp
XRD peak of GO (d-spacing 7.94 A at 2y = 11.11)
disappeared, but a new broad diraction peak (d-spacing 3.7 A
at 2y = 24.01) appeared, which is closer to the typical (002)
diraction peak of graphite (d-spacing 3.35 A at 2y = 26.61).
This result further supports the reduction of GO by L-AA.
Generally, electrical conductivity of reduced GO could
reect both the extent of the reduction and the restoration
This journal is
The Royal Society of Chemistry 2010
of the electronic conjugation state.10,24 To check the electrical
conductivity of the reduced GO, thin circular papers
(1 cm in diameter, 0.3 mm in thickness, see Fig. S2w) of the
reduced GO were prepared by ltration, and were dried under
vacuum at 80 1C for 12 h. To get reliable electrical conductivity
data, a four-point probe was carefully placed on the reduced
GO papers, and three dierent sites of each sample were
chosen to be measured. We found the average conductivity of
the reduced GO (reduced for 48 h with L-AA) is B800 S m 1,
which is comparable to the conductivity of the GO reduced
using hydrazine and other reductants.8 This result showed
unambiguously that the GO was reduced by L-AA, and the
electronic conjugation was re-established after the reduction.
To optimize the reduction capability of L-AA to GO, we
changed the ratio of L-AA to GO in the reduction experiments.
As expected, increasing the ratio of L-AA to GO can certainly
speed up the reduction. For instance, the UV-vis spectra,
Fig. S3w, showed that the decaying rate of the prominent UV
absorption peaks of the GO at 230 and 300 nm increased with
the increase of the ratio of L-AA to GO from 2 to 20 (by weight).
The formation of irreversible graphene agglomerates has
been a key problem in the preparation of individual graphene
sheets through aqueous solution based chemical reduction of
GO.7,8 To overcome this obstacle, appropriate polymers or
surfactants were usually added as capping reagents in most
cases.25,26 In this work, we found that the reduced GO sheets
can be suspended stably in water at room temperature for
several days without adding any capping reagent. Fig. 4a
shows the photograph of the aqueous suspensions
(0.1 mg mL 1) of the GO (left) and the L-AA reduced GO
(right) sheets that were kept at room temperature for four
days. The AFM image and the height prole, Fig. 4b and c,
illustrates that the individual sheet motif was still preserved.
The thickness of the L-AA reduced GO sheets ranges of B0.8 nm
(Fig. 4c). Fig. 4d depicts a typical high-resolution transmission
electron microscopy (HRTEM) image and a selected area
electron diraction (SAED) pattern (inset) of the L-AA
reduced GO sheet. The well-dened diraction spots in SAED
illustrates that the crystalline state of the reduced GO was
re-established, although the discontinued fringes in the
HRTEM image indicate that there are defects in the L-AA
reduced GO sheets. The stabilization mechanism of the
reduced GO aqueous suspension might originate from the
oxidized products of L-AA. It is well known that each L-AA
molecule can release two protons (deprotonation) to form
Chem. Commun., 2010, 46, 11121114 | 1113
View Article Online
and NSFC (No. 20774029) of China, which are gratefully
acknowledged.
Downloaded by University of Arizona on 16 January 2013
Published on 24 December 2009 on http://pubs.rsc.org | doi:10.1039/B917705A
Notes and references
Fig. 4 (a) Photographs of aqueous dispersions (0.1 mg mL 1) of GO
before (left) and after (right) the reduction with L-AA, which were kept
at room temperature over 4 days, (b) tapping mode AFM image of the
L-AA reduced GO sheets on mica substrate, (c) the height prole of the
AFM image, and (d) HRTEM image of the reduced GO sheets. The
inset in (d) is a typical SAED pattern of the reduced GO.
dehydroascorbic acid.16 The protons have commonly high
binding anity to the oxygen-containing groups, such as
hydroxyl and epoxide groups forming water molecules. The
dehydroascorbic acid can be converted further into oxalic and
guluronic acids.14,15 The reduced GO may also have some
residual oxygen functionalities, such as the periphery
carboxylic groups as shown in Fig. 2a. Thus, guluronic acid
or oxalic acids might form hydrogen bonds with the residual
oxygen functionalities on the reduced GO surfaces. Such
interactions can disrupt the pp stacking between the reduced
GO sheets, and further prevent the formation of the agglomerates.
In summary, we have demonstrated that the reduction of
GO with L-AA results in a substantial removal of oxygen
functionalities of the GO and the noteworthy restoration of
the electronic conjugation state of the reduced GO. This
method avoids the usage of harmful chemical reductants and
any additional capping agents (polymers or surfactants) that
are undesirable for most applications of graphene. Given these
advantages, the strategy described in this study can be developed
as a simple and environmentally friendly route to water
soluble graphene.
This work was supported by the National 863 (No.
2006AA04Z309) and 973 (No. 2007CB936000) programs,
1114 | Chem. Commun., 2010, 46, 11121114
1 K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang,
S. V. Dubonos, I. V. Crigorieva and A. A. Firsov, Science, 2004,
306, 666669.
2 C. Berger, Z. Song, T. Li, X. Li, A. Y. Ogbazghi, R. Feng, Z. Dai,
A. N. Marchenkov, E. H. Conrad, P. N. First and W. A. d. Heer,
J. Phys. Chem. B, 2004, 108, 1991219916.
3 A. Dato, V. Radmilovic, Z. Lee, J. Phillips and M. Frenklach,
Nano Lett., 2008, 8, 20122016.
4 K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim,
J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457,
706710.
5 A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic,
M. S. Dresselhaus and J. Kong, Nano Lett., 2009, 9, 3035.
6 P. W. Sutter, J. I. Flege and E. A. Sutter, Nat. Mater., 2008, 7,
406411.
7 D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat.
Nanotechnol., 2008, 3, 101105.
8 S. Park and R. S. Ruo, Nat. Nanotechnol., 2009, 4, 217224.
9 S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas,
A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruo,
Carbon, 2007, 45, 15581565.
10 V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner,
Nat. Nanotechnol., 2009, 4, 2529.
11 A. Furst, R. C. Berlo and S. Hooton, Chem. Rev., 1965, 65, 5168.
12 Y. Si and E. T. Samulski, Nano Lett., 2008, 8, 16791682.
13 G. Wang, J. Yang, J. Park, X. Gou, B. Wang, H. Liu and J. Yao,
J. Phys. Chem. C, 2008, 112, 81928195.
14 P. Karrer, Chem. Rev., 1934, 14, 1730.
15 H. J. Loeer and J. D. Ponting, Ind. Eng. Chem., Anal. Ed., 1942,
14, 846849.
16 Vitamin C: Its Chemistry and Biochemistry, ed. M. B. Davies,
J. Austin and D. A. Partridge, Royal Society of Chemistry, UK,
1991.
17 M. Ambrosi, E. Fratini, V. Alfredsson, B. W. Ninham, R. Giorgi,
P. L. Nostro and P. Baglioni, J. Am. Chem. Soc., 2006, 128,
72097214.
18 L. Lu, A. Kobayashi, K. Tawa and Y. Ozaki, Chem. Mater., 2006,
18, 48944901.
19 G. S. Metraux, Y. C. Cao, R. Jin and C. A. Mirkin, Nano Lett.,
2003, 3, 519522.
20 Y. Wang, P. H. C. Camargo, S. E. Skrabalak, H. Gu and Y. Xia,
Langmuir, 2008, 24, 1204212046.
21 W. S. Hummers and R. E. Oerman, J. Am. Chem. Soc., 1958, 80,
13391339.
22 S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas,
E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and
R. S. Ruo, Nature, 2006, 442, 282286.
23 F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 11261130.
24 S. Gilje, S. Han, M. Wang, K. L. Wang and R. B. Kaner,
Nano Lett., 2007, 7, 33943398.
25 S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams,
M. A. Hamon and R. C. Haddon, J. Am. Chem. Soc., 2006, 128,
77207721.
26 S. Stankovich, R. D. Piner, X. Chen, N. Wu, S. T. Nguyen and
R. S. Ruo, J. Mater. Chem., 2006, 16, 155159.
This journal is
The Royal Society of Chemistry 2010
You might also like
- Chemistry Unit 1 P2 2017 PDFDocument18 pagesChemistry Unit 1 P2 2017 PDFNalini Gangaram33% (6)
- PsaDocument5 pagesPsaSamanta De Jesus Ferreira100% (1)
- An Improved Hummers Method For Eco-Friendly Synthesis of Graphene OxideDocument5 pagesAn Improved Hummers Method For Eco-Friendly Synthesis of Graphene OxideFamiloni LayoNo ratings yet
- Quantitative Determination of Citric and Ascorbic Acid WDocument3 pagesQuantitative Determination of Citric and Ascorbic Acid Wblackjiriu100% (1)
- Improve Systhesis Graphene OxideDocument6 pagesImprove Systhesis Graphene OxideĐương VũNo ratings yet
- Amino-Functionalized Reduced Graphene-Oxide-Copper (I) Oxide Composite: A Prospective Catalyst For Photo-Reduction of CODocument6 pagesAmino-Functionalized Reduced Graphene-Oxide-Copper (I) Oxide Composite: A Prospective Catalyst For Photo-Reduction of COrafelNo ratings yet
- Restoring The Electrical Conductivity of Graphene Oxide Films by UV Light Induced Oxygen DesorptionDocument11 pagesRestoring The Electrical Conductivity of Graphene Oxide Films by UV Light Induced Oxygen DesorptionFamiloni LayoNo ratings yet
- tmp8C6C TMPDocument10 pagestmp8C6C TMPFrontiersNo ratings yet
- Adma 201204530 SM SupplDocument11 pagesAdma 201204530 SM SupplJulio RiosNo ratings yet
- An Environment-Friendly Route To Graphene Oxide by Concentrated Ozone With Improved Catalytic PerformanceDocument12 pagesAn Environment-Friendly Route To Graphene Oxide by Concentrated Ozone With Improved Catalytic PerformanceRodrigo Gabriel BuenoNo ratings yet
- Li ACS Catal 2016Document6 pagesLi ACS Catal 2016Mary BNo ratings yet
- Synthesis of Visible-Light Responsive Graphene Oxide-TiO2 Composites With P-N HeterojunctionDocument8 pagesSynthesis of Visible-Light Responsive Graphene Oxide-TiO2 Composites With P-N HeterojunctionFamiloni LayoNo ratings yet
- Uv Radiation GO in WaterDocument10 pagesUv Radiation GO in Waterreza zeinNo ratings yet
- 1 s2.0 S0167577X22002178 MainDocument4 pages1 s2.0 S0167577X22002178 MainKhaoula El AsameNo ratings yet
- Photo Electro CatalystDocument19 pagesPhoto Electro CatalystHijab HaiderNo ratings yet
- The Effects of Annealing Temperature On The Permittivity and Electromagnetic Attenuation Performance of Reduced Graphene OxideDocument6 pagesThe Effects of Annealing Temperature On The Permittivity and Electromagnetic Attenuation Performance of Reduced Graphene OxideAna-Maria DucuNo ratings yet
- Hydrogenolysis of Glycerol To 1,2-PropanediolDocument5 pagesHydrogenolysis of Glycerol To 1,2-PropanediolKesavan GovinathanNo ratings yet
- Sulfur-Doped Graphene As An EfficientDocument7 pagesSulfur-Doped Graphene As An EfficientJorge VazquezNo ratings yet
- The Mechanism of The Oxidation of Propene To AcroleinDocument9 pagesThe Mechanism of The Oxidation of Propene To AcroleinLeonardo BrunelliNo ratings yet
- C5nj02314a1 PDFDocument11 pagesC5nj02314a1 PDFWalter Ricardo BritoNo ratings yet
- Se Doped Bi2o3Document11 pagesSe Doped Bi2o3Mudassir NazarNo ratings yet
- Ojpstmp Stamppdf D 05T10 18 JAPIAU 111 7 074904 1Document9 pagesOjpstmp Stamppdf D 05T10 18 JAPIAU 111 7 074904 1Noureddine BarkaNo ratings yet
- (I) The Hummers MethodDocument23 pages(I) The Hummers MethodFamiloni LayoNo ratings yet
- Water SplitingDocument8 pagesWater SplitingAKASHNo ratings yet
- Loryuenyong 2013Document6 pagesLoryuenyong 2013RahulNo ratings yet
- Ange2012 ChienDocument5 pagesAnge2012 ChienanantaNo ratings yet
- Pan ACS SustChemEnggDocument10 pagesPan ACS SustChemEnggTrần ChứcNo ratings yet
- Spe 738 PaDocument6 pagesSpe 738 PaAliNo ratings yet
- Chemical Dehydrogenation of Hydrogenated Single Layer Graphene For Reversible Electrical ConductivityDocument4 pagesChemical Dehydrogenation of Hydrogenated Single Layer Graphene For Reversible Electrical ConductivityTania ChatterjeeNo ratings yet
- Direct CO Electroreduction From CarbonateDocument13 pagesDirect CO Electroreduction From Carbonateferonica chungNo ratings yet
- ASB PyrolisisDocument4 pagesASB PyrolisisJaffimJaffimNo ratings yet
- View Free ArticleDocument5 pagesView Free ArticlesadhuNo ratings yet
- Growth and Optical Properties of Organic GOA CrystalsDocument7 pagesGrowth and Optical Properties of Organic GOA CrystalsAJER JOURNALNo ratings yet
- Heon-Yul Ryu, Palwasha Jalalzai, Nagendra Prasad Yerriboina, Tae-Gon Kim, Satomi Hamada, and Jin-Goo ParkDocument5 pagesHeon-Yul Ryu, Palwasha Jalalzai, Nagendra Prasad Yerriboina, Tae-Gon Kim, Satomi Hamada, and Jin-Goo Parksumit kumarNo ratings yet
- Infrared Spectrum of Carbon Dioxide in Aqueous Solution 1Document4 pagesInfrared Spectrum of Carbon Dioxide in Aqueous Solution 1sm_carvalhoNo ratings yet
- 1 s2.0 S0254058418306035 MainDocument11 pages1 s2.0 S0254058418306035 Maincupkake4tressNo ratings yet
- Efficiency of Nitrogen Desorption From Lix Zeolite by Rapid Oxygen Purge in A Pancake AdsorberDocument4 pagesEfficiency of Nitrogen Desorption From Lix Zeolite by Rapid Oxygen Purge in A Pancake AdsorberRobert Solano MontoyaNo ratings yet
- h2 O2 ProductionDocument9 pagesh2 O2 ProductionVaibhav ChaudharyNo ratings yet
- Electrochemical Reduction of Co TO Methane in Methanol at Low TemperatureDocument2 pagesElectrochemical Reduction of Co TO Methane in Methanol at Low TemperatureHassaanNo ratings yet
- Improvement of NO Removal From Wastewater by Using Batch Electrocoagulation Unit With Vertical Monopolar Aluminum ElectrodesDocument9 pagesImprovement of NO Removal From Wastewater by Using Batch Electrocoagulation Unit With Vertical Monopolar Aluminum Electrodesnishu thathsaraniNo ratings yet
- D.S. Sutar Et al-TFL-2012Document6 pagesD.S. Sutar Et al-TFL-2012divakar botchaNo ratings yet
- Fractal Approach To Adsorption Isotherms of Water VaporDocument6 pagesFractal Approach To Adsorption Isotherms of Water VaporSara Juanita Prada RubioNo ratings yet
- Radiolytic Dechlorination of Pcbs in Presence of Active Carbon, Solid Oxides, Bentonite and ZeoliteDocument6 pagesRadiolytic Dechlorination of Pcbs in Presence of Active Carbon, Solid Oxides, Bentonite and ZeolitePham PhuNo ratings yet
- Reduced Graphene Oxide: Characterization Sheet: Nanoinnova Technologies SL C/Faraday 7, 28049 MadridDocument3 pagesReduced Graphene Oxide: Characterization Sheet: Nanoinnova Technologies SL C/Faraday 7, 28049 Madrideid elsayedNo ratings yet
- Mafra 2023Document8 pagesMafra 2023marciomafraNo ratings yet
- Electrochemical DBS Wastewater Treatment ResearchDocument4 pagesElectrochemical DBS Wastewater Treatment ResearchvahidNo ratings yet
- Investigation of Thermal Conductivity, Viscosity, and Electrical Conductivity of Graphene Based NanofluidsDocument9 pagesInvestigation of Thermal Conductivity, Viscosity, and Electrical Conductivity of Graphene Based Nanofluidsvuongcoi102No ratings yet
- A New Method For Preparing Hydrophobic Nano-Copper PowdersDocument5 pagesA New Method For Preparing Hydrophobic Nano-Copper PowdersbacNo ratings yet
- Improvement of Microalgal Nox Removal in Bubble Column and Airlift ReactorsDocument3 pagesImprovement of Microalgal Nox Removal in Bubble Column and Airlift ReactorsSacra PsyntergiaNo ratings yet
- B9NR00186GDocument4 pagesB9NR00186Gcnu5322No ratings yet
- 3ftfbsdi "Sujdmf 4zouiftjt Boe $ibsbdufsj (Bujpo PG (Sbqifof 5ijo 'JMNT CZ $ifnjdbm 3fevdujpo PG &ygpmjbufe Boe Oufsdbmbufe (Sbqijuf 0yjefDocument7 pages3ftfbsdi "Sujdmf 4zouiftjt Boe $ibsbdufsj (Bujpo PG (Sbqifof 5ijo 'JMNT CZ $ifnjdbm 3fevdujpo PG &ygpmjbufe Boe Oufsdbmbufe (Sbqijuf 0yjefXana RodriguesNo ratings yet
- Spectrophotometric Determination of Carbonate Ion Concentrations: Elimination of Instrument-Dependent Offsets and Calculation of in Situ Saturation StatesDocument37 pagesSpectrophotometric Determination of Carbonate Ion Concentrations: Elimination of Instrument-Dependent Offsets and Calculation of in Situ Saturation StatesJesús PieroNo ratings yet
- Journal Pre-Proofs: Inorganic Chemistry CommunicationsDocument19 pagesJournal Pre-Proofs: Inorganic Chemistry CommunicationsYusup SetiawanNo ratings yet
- Silver Modified Porous 3D Nitrogen-Doped Graphene Aerogel Highly Efficient Oxygen Reduction Electrocatalyst For ZN Air BatteryDocument38 pagesSilver Modified Porous 3D Nitrogen-Doped Graphene Aerogel Highly Efficient Oxygen Reduction Electrocatalyst For ZN Air Batterysiti khotijahNo ratings yet
- Carbon 50 (2012) 5351 - 5358Document8 pagesCarbon 50 (2012) 5351 - 5358Khanh NhiNo ratings yet
- 10.1515 htmp.2009.28.3.141Document6 pages10.1515 htmp.2009.28.3.141Oualid HamdaouiعععNo ratings yet
- Jurnal Perancangan AlatDocument6 pagesJurnal Perancangan AlatFreeQueenNo ratings yet
- Jurnal Reduksi ElektrolitDocument7 pagesJurnal Reduksi ElektrolitIqbal SaharaNo ratings yet
- Environmental-Friendly Synthesis of Reduced Graphene Oxide (rGO) Using Gamma IrradiationDocument9 pagesEnvironmental-Friendly Synthesis of Reduced Graphene Oxide (rGO) Using Gamma IrradiationLuis Alejandro Pedreros MartinezNo ratings yet
- CT Dientu Va HD QXTDocument5 pagesCT Dientu Va HD QXTNguyen Thi Thuy TienNo ratings yet
- Paul-Srinivasan2020 Article MechanisticAnalysisOfAnodicDisDocument14 pagesPaul-Srinivasan2020 Article MechanisticAnalysisOfAnodicDisTwinkle PaulNo ratings yet
- Graphene Oxide: Fundamentals and ApplicationsFrom EverandGraphene Oxide: Fundamentals and ApplicationsAyrat M. DimievNo ratings yet
- November 2013 (v3) QP - Paper 1 CIE Chemistry IGCSEDocument20 pagesNovember 2013 (v3) QP - Paper 1 CIE Chemistry IGCSEMaira. BalsaNo ratings yet
- Physical Chem Form 5 and 6Document402 pagesPhysical Chem Form 5 and 6khayrikhan0No ratings yet
- Unit 2 Study GuideDocument9 pagesUnit 2 Study GuideAjeet SinghNo ratings yet
- Deva Glide ManualDocument20 pagesDeva Glide ManualshibumbNo ratings yet
- How To Make MMS - Jim Humble - MMS - Master Mineral SolutionDocument9 pagesHow To Make MMS - Jim Humble - MMS - Master Mineral Solutionoscar rios100% (3)
- BDocument4 pagesBAnonymous JwAC4yPNo ratings yet
- Exam1 With AnswerADocument14 pagesExam1 With AnswerAKimberly JinNo ratings yet
- LAB MANUAL General Chemistry 105 v2.0Document58 pagesLAB MANUAL General Chemistry 105 v2.0student blogNo ratings yet
- Aluminum BronzeDocument37 pagesAluminum Bronzepipedown456100% (1)
- Dairy ScienceDocument1,383 pagesDairy ScienceStev D'BulletNo ratings yet
- Selangor SPMC Skema Kimia Kertas 3 (Set 1)Document13 pagesSelangor SPMC Skema Kimia Kertas 3 (Set 1)Hafizoh HarunNo ratings yet
- Ionic EquilibriumDocument4 pagesIonic EquilibriumFu HongNo ratings yet
- Edexcel Chemistry Unit 4 Exams QuestionsDocument302 pagesEdexcel Chemistry Unit 4 Exams Questionskeyur10050% (2)
- Ch19 Lessons19 - 4Document41 pagesCh19 Lessons19 - 4Denzel Perdon NicdaoNo ratings yet
- Identification of Sodium AcetateDocument2 pagesIdentification of Sodium AcetateDarlene Faith BeroñaNo ratings yet
- Sample Paper (Class - 11) : EYSE (AY 2020 - 2021) PhysicsDocument19 pagesSample Paper (Class - 11) : EYSE (AY 2020 - 2021) PhysicsPrinceNo ratings yet
- Titration ExerciseDocument2 pagesTitration ExerciseYemima KurniaNo ratings yet
- Aqa Chem1 QP Jan13 PDFDocument16 pagesAqa Chem1 QP Jan13 PDFMazlinNo ratings yet
- SCH Exam Review 2011Document9 pagesSCH Exam Review 2011Dami SogbesanNo ratings yet
- How Does The Concentration of Hydrochloric Acid Affect The Rate of Reaction With MagnesiumDocument4 pagesHow Does The Concentration of Hydrochloric Acid Affect The Rate of Reaction With MagnesiumshalomtseNo ratings yet
- Chemistry Coursework - Chapter 2.0 MethodologyDocument2 pagesChemistry Coursework - Chapter 2.0 MethodologyXuan Hua LaiNo ratings yet
- Multiple Choice QuestionsDocument7 pagesMultiple Choice QuestionsGiorgi GobronidzeNo ratings yet
- Chemistry The Molecular Nature of Matter Jespersen 7th Edition Solutions ManualDocument14 pagesChemistry The Molecular Nature of Matter Jespersen 7th Edition Solutions ManualWilliam Williams100% (37)
- Et ChantsDocument5 pagesEt ChantsVenkatakrishna.ANo ratings yet
- Acidizing Corrosion Inhibitors A ReviewDocument12 pagesAcidizing Corrosion Inhibitors A ReviewwjawichNo ratings yet
- QUIMICAmarkscheme HL Paper2Document22 pagesQUIMICAmarkscheme HL Paper2Fiona DonovanNo ratings yet
- Kertas 1 Pat 2023Document15 pagesKertas 1 Pat 2023Nazirah binti HarunNo ratings yet
- Equilibrium NotesDocument10 pagesEquilibrium NotesAlana SebyNo ratings yet