Professional Documents
Culture Documents
Carnot Cycle, Rankine Cycle, Otto Cycle, Diesel Cycle and Dual Combustion Cycle
Carnot Cycle, Rankine Cycle, Otto Cycle, Diesel Cycle and Dual Combustion Cycle
Uploaded by
Zahra JafarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carnot Cycle, Rankine Cycle, Otto Cycle, Diesel Cycle and Dual Combustion Cycle
Carnot Cycle, Rankine Cycle, Otto Cycle, Diesel Cycle and Dual Combustion Cycle
Uploaded by
Zahra JafarCopyright:
Available Formats
Assalam-u-alaikum and Good afternoon fellow students and respected maam.
I am Sana Jafar, and my other team members are Zoha Navaid, Qazi Saud and Qaisar Amin.
We are here to present five basic thermodynamic cycles i.e Carnot cycle, Rankine cycle, Otto
cycle, Diesel Cycle and dual combustion cycle.
Lets get started.
We begin by answering this simple question that:
What is a thermodynamic cycle?
A thermodynamic cycle is a series of processes where the properties of the system are the same after the
cycle as they were prior. Three main properties temperature, pressure, and specific volume are
tracked when a system undergoes a set of processes. To be considered a cycle, all three properties need
to be the same at their initial state and at the end. One property could remain the same throughout any
one of processes; the cycle is considered isothermal if temperature is constant, isobaric if pressure is
constant, and isochoric or isometric if specific volume is constant. The most efficient type of cycle is one
that has only reversible processes, such as the Carnot cycle, which is made up of four reversible
processes.
A thermodynamic cycle is a series of thermodynamic processes which returns a system to its initial
state. Properties depend only on the thermodynamic state and thus do not change over a cycle.
Variables such as heat and work are not zero over a cycle, but rather depend on the process.
The first law of thermodynamics dictates that the net heat input is equal to the net work output over
any cycle. The repeating nature of the process path allows for continuous operation, making the
cycle an important concept in thermodynamics.
Example of P-V diagram of a thermodynamic cycle.
If the cyclic process moves clockwise around the loop, then it represents a heat engine, and W will
be positive. If it moves counterclockwise then it represents a heat pump, and W will be negative.
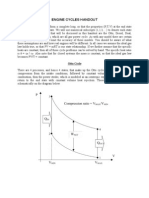
The Dual Combustion Cycle
Modern oil engines known also as diesel engine, use solid injection of the fuel.
The ideal cycle which is used as a basis for comparison is called the dual combustion
cycle or the mixed cycle, and is shown on a p-v diagram in Figure. 3.1 In this cycle, heat
is supplied in two parts; the first part at constant volume and the second in constant
pressure. Hence the name dual combustion.
P
P3=p4
p.v conts
2
5
1
V2=V3
V1=V5
Figure 3.1 : p-v diagram
3.2
The dual combustion cycle process.
Process 1 to 2 is isentropic compression.
Process 2 to 3 is reversible constant volume heating.
Process 3 to 4 is reversible constant pressure heating.
Process 4 to 5 is isentropic expansion.
Process 5 to 1 is reversible constant volume cooling. (rejection of heat)
In order to fix the thermal efficiency completely three factors are necessary.
There are the ratio of compression, r v = v1/ v2 ; the ratio of pressure, r p = p3/p2 and the
ratio of volume and cut, rc = v4/v3 .
1
rv
Then it can be shown that
rp rc 1
rp 1 rp rc 1
The efficiency of dual combustion cycle depends not only on the compression
ratio but also on the relative amount of heat supplied at constant volume and at constant
pressure. The best method of calculating thermal efficiency is to evaluate each
temperature throughout the cycle and then use this equation,
1 Q2 / Q1
. The heat
supplied, Q1, is calculated using the equation,
Q1 cv T3 T2 c p T4 T3
The heat rejected, Q2 , is calculated by
Q2 c v T5 T1
The dual cycle is a better approximation to the modern high speed compression
ignition engine than either the Diesel cycle or the Otto cycle.
You might also like
- Astrology Ebooks PDFDocument15 pagesAstrology Ebooks PDFsankarjv75% (4)
- Rankine CycleDocument7 pagesRankine Cycledeepakkesri260% (1)
- Effectiveness of Distribution Channel'Document56 pagesEffectiveness of Distribution Channel'Keyur Bhojak100% (3)
- Study PlanDocument2 pagesStudy Planirfan100% (1)
- DC - Sop - EenDocument8 pagesDC - Sop - EenBilal KhalidNo ratings yet
- Air Standard CycleDocument93 pagesAir Standard CycleJonathan CerdanNo ratings yet
- Cyclic Process Second Law EnginesDocument18 pagesCyclic Process Second Law EnginesM Khaidiz RafiNo ratings yet
- Module 1 NotesDocument62 pagesModule 1 NotesmanojNo ratings yet
- Power CyclesDocument10 pagesPower CyclesSNo ratings yet
- Thermodynamic CyclesDocument9 pagesThermodynamic CyclesPilar Guzmán MartínezNo ratings yet
- ME6404 UwDocument92 pagesME6404 UwAnonymous Zx7EG1PaNo ratings yet
- Thermodynamic Cycles: Wesleyan University-Philippines Mabini Extension, Cabanatuan CityDocument9 pagesThermodynamic Cycles: Wesleyan University-Philippines Mabini Extension, Cabanatuan CityRoss Sonny CruzNo ratings yet
- Thermo CyclesDocument3 pagesThermo CyclesRhandi MuliaNo ratings yet
- Lec9 - Air Cycle Refrigeration SystemsDocument38 pagesLec9 - Air Cycle Refrigeration SystemsSantanu DattaNo ratings yet
- Air Standed Cycles Lec-7Document20 pagesAir Standed Cycles Lec-7pleasename1No ratings yet
- Rankine CycleDocument6 pagesRankine CycleGeçmiş GölgelerNo ratings yet
- SME1207Document201 pagesSME1207OM TAPARENo ratings yet
- Lec9 PDFDocument34 pagesLec9 PDFshravanNo ratings yet
- Air Standard Cycles - BasicsDocument19 pagesAir Standard Cycles - Basicsexpressive87No ratings yet
- TD Power CyclesDocument6 pagesTD Power CyclesKiran KumarNo ratings yet
- UNIT I Gas Power Cycles FinalDocument75 pagesUNIT I Gas Power Cycles FinalMAYUR BHOSALENo ratings yet
- Lec18 RefrigerationDocument56 pagesLec18 RefrigerationArvin Loui BasconNo ratings yet
- Rankine Cycle SumDocument76 pagesRankine Cycle Sumمحمد تانزيم ابراهيم100% (1)
- Air Standard Cycles - BasicsDocument34 pagesAir Standard Cycles - Basicsrazvan66m100% (1)
- Thermodynamic CyclesDocument13 pagesThermodynamic CyclesAshish KumarNo ratings yet
- 3Document8 pages3SHREYAS MSNo ratings yet
- Gas Power Cycles Sivakumar.E VITDocument47 pagesGas Power Cycles Sivakumar.E VITmohan govindasamyNo ratings yet
- Basic Thermodynamics Prof. S.K. Som Department of Mechanical Engineering Indian Institute of Technology, KharagpurDocument23 pagesBasic Thermodynamics Prof. S.K. Som Department of Mechanical Engineering Indian Institute of Technology, KharagpurAshutosh ShuklaNo ratings yet
- Thermodynamic Air Standard CycleDocument34 pagesThermodynamic Air Standard CycleMahadi HasanNo ratings yet
- Air StandardDocument53 pagesAir StandardMary RobinsonNo ratings yet
- Applications of TDsDocument15 pagesApplications of TDsReddyvari VenugopalNo ratings yet
- Lecture 1 - Rankine Power CyclesDocument5 pagesLecture 1 - Rankine Power CyclesMuhammad Alam Zaib KhanNo ratings yet
- Marine Power Plant I DieselDocument6 pagesMarine Power Plant I DieselRich Samuel AlmazarNo ratings yet
- M 363 ContentDocument115 pagesM 363 Contentn.hartonoNo ratings yet
- UNIT 8 ThermodynamicsDocument9 pagesUNIT 8 ThermodynamicsHimadhar SaduNo ratings yet
- Thermo 2Document23 pagesThermo 2SHek Hxni100% (1)
- Carnot CycleDocument26 pagesCarnot CycleNafisa AnikaNo ratings yet
- Rankine CycleDocument5 pagesRankine CycleRohit JainNo ratings yet
- CollectionDocument7 pagesCollectionRohan RustagiNo ratings yet
- Air Cycle Refrigeration SystemDocument30 pagesAir Cycle Refrigeration SystemtsegayNo ratings yet
- Turbine Engine CalculationDocument112 pagesTurbine Engine CalculationSusmita SivasankaranNo ratings yet
- M363contentDocument112 pagesM363contenthanythekingNo ratings yet
- Rudolf Diesel, A German Inventor, Patented His Internal Combustion Engine, WhichDocument6 pagesRudolf Diesel, A German Inventor, Patented His Internal Combustion Engine, WhichAsraNo ratings yet
- Chapter 2 Power CycleDocument44 pagesChapter 2 Power CycleBese Mat100% (1)
- Diesel Cycle Presentation LecDocument26 pagesDiesel Cycle Presentation LecMeroNaruto0% (1)
- Thermo 3 Cyclic ProcessesDocument13 pagesThermo 3 Cyclic ProcessesFebrian RomanNo ratings yet
- Thermodynamics: Diesel Cylce: The Ideal Cycle For Compression-Ignation Engines Stirling and Ericsson CyclesDocument35 pagesThermodynamics: Diesel Cylce: The Ideal Cycle For Compression-Ignation Engines Stirling and Ericsson Cyclesaji wiranegaraNo ratings yet
- Rankine Cycle - Wikipedia, The Free EncyclopediaDocument7 pagesRankine Cycle - Wikipedia, The Free EncyclopediaGanguli Tutor RajNo ratings yet
- Rankine CycleDocument6 pagesRankine Cyclerr1819No ratings yet
- Applied ThermodynamicsDocument48 pagesApplied Thermodynamicsdavesh jadonNo ratings yet
- Rankine Cycle:: (Citation Needed)Document11 pagesRankine Cycle:: (Citation Needed)bnida99No ratings yet
- Description of A Pistonprop EngineDocument5 pagesDescription of A Pistonprop EngineNaman PatidarNo ratings yet
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyDocument68 pagesGas Power Cycles Study Guide in Powerpoint: To AccompanyAbraham HutomoNo ratings yet
- Carnot Cycle NotesDocument7 pagesCarnot Cycle NotesDigvijay JadejaNo ratings yet
- Air Cycle Refrigeration SystemsDocument8 pagesAir Cycle Refrigeration Systemsmesfn derbNo ratings yet
- Gas Power CyclesDocument48 pagesGas Power CyclesIsmailNo ratings yet
- Power CyclesDocument14 pagesPower CyclesA ZNo ratings yet
- CycleDocument6 pagesCycleMohamed NaserNo ratings yet
- Air Cycle Refrigeration Systems (Indian Institute of Technology Kharagpur)Document15 pagesAir Cycle Refrigeration Systems (Indian Institute of Technology Kharagpur)emawz21No ratings yet
- Finite Physical Dimensions Optimal Thermodynamics 1: FundamentalsFrom EverandFinite Physical Dimensions Optimal Thermodynamics 1: FundamentalsNo ratings yet
- S S+Lab+Report+TemplateDocument4 pagesS S+Lab+Report+TemplateZahra JafarNo ratings yet
- Sections: A, B, C, D (Spring 2016) : EE223: S & SDocument2 pagesSections: A, B, C, D (Spring 2016) : EE223: S & SZahra JafarNo ratings yet
- Me 101: Basic Mechanical Engineering: Chapter # 3 Equilibrium in Two DimensionDocument8 pagesMe 101: Basic Mechanical Engineering: Chapter # 3 Equilibrium in Two DimensionZahra JafarNo ratings yet
- Chapter 2 Boolean Algebra Logic GatesDocument29 pagesChapter 2 Boolean Algebra Logic GatesZahra JafarNo ratings yet
- Common-Emitter Characteristics: Transistor Operating in Active Region Large-Signal or DCDocument10 pagesCommon-Emitter Characteristics: Transistor Operating in Active Region Large-Signal or DCZahra JafarNo ratings yet
- Basic Mechanical Engineering: Assignment # 1Document1 pageBasic Mechanical Engineering: Assignment # 1Zahra JafarNo ratings yet
- Physics 02 Introduction To Scalars and Vectors 27-08-2014Document4 pagesPhysics 02 Introduction To Scalars and Vectors 27-08-2014Zahra JafarNo ratings yet
- Work Study and ErgonomicsDocument4 pagesWork Study and ErgonomicsVikas VarmaNo ratings yet
- 2080 Um002 - en e PDFDocument270 pages2080 Um002 - en e PDFΔημητρηςΣαρακυρουNo ratings yet
- Spjat Sample Paper 1Document14 pagesSpjat Sample Paper 1Pritish NayakNo ratings yet
- FM-CID-021 Technical Assistance Plan GEMDocument3 pagesFM-CID-021 Technical Assistance Plan GEMMa Rk AntonioNo ratings yet
- Primary Homework Help Moon FactsDocument7 pagesPrimary Homework Help Moon Factsh3tdfrzv100% (1)
- Assessment Jenny3Document29 pagesAssessment Jenny3Kim SeokjinNo ratings yet
- Acrylic: Products ﻚﻴﻠﻳﺮﻛﻻا تﺎﺠﺘﻨﻣDocument113 pagesAcrylic: Products ﻚﻴﻠﻳﺮﻛﻻا تﺎﺠﺘﻨﻣamrgamal81085No ratings yet
- Capitalizing On The Shifting Consumer Food Value EquationDocument1 pageCapitalizing On The Shifting Consumer Food Value EquationIman Nurakhmad FajarNo ratings yet
- Unfairbnb - How Online Rental Platforms Use The EU To Defeat Cities' Affordable Housing MeasuresDocument28 pagesUnfairbnb - How Online Rental Platforms Use The EU To Defeat Cities' Affordable Housing MeasuresTatiana100% (1)
- EWS Editor 320 EngDocument11 pagesEWS Editor 320 EngStefan AslamNo ratings yet
- Chapter 1 Successive DifferentiationDocument15 pagesChapter 1 Successive Differentiationharvinder ahluwaliaNo ratings yet
- Registered Electrical Engineer Licensure ExaminationDocument35 pagesRegistered Electrical Engineer Licensure ExaminationAileen BobadillaNo ratings yet
- DWM 700Document16 pagesDWM 700Tetelo VincentNo ratings yet
- Test PDFDocument5 pagesTest PDFOkekporo JoshuaNo ratings yet
- Pipe Line Girth WeldDocument15 pagesPipe Line Girth WeldLương Hồ Vũ100% (1)
- Schwartz Marin E Et Al - Psychedelic Research and Its Biocolonial Legacies - LS - Pulse - 50 - Dec 2021Document2 pagesSchwartz Marin E Et Al - Psychedelic Research and Its Biocolonial Legacies - LS - Pulse - 50 - Dec 2021patymurrietaNo ratings yet
- Umat FaqDocument15 pagesUmat FaqEren SevinceNo ratings yet
- How To Configure Networking in KDE PlasmaDocument9 pagesHow To Configure Networking in KDE Plasmasoftware supportNo ratings yet
- Chapter IIDocument10 pagesChapter IIeswariNo ratings yet
- Developing Leaders at Southwest AirlinesDocument6 pagesDeveloping Leaders at Southwest Airlinesapi-356490685No ratings yet
- Tips On Oral AdvocacyDocument6 pagesTips On Oral AdvocacyRafely GatdulaNo ratings yet
- Wieviorka - Violence and The SubjectDocument10 pagesWieviorka - Violence and The SubjectjuanpejoloteNo ratings yet
- 411-5221-955.08.08 SGSN User GuideDocument622 pages411-5221-955.08.08 SGSN User Guideav8r_txNo ratings yet
- SCORING RUBRIC For Ballroom DanceDocument1 pageSCORING RUBRIC For Ballroom DanceRemuel CadalzoNo ratings yet
- MHR 300 Live - Course Syllabus Fall 2010 - FINAL5-XDocument24 pagesMHR 300 Live - Course Syllabus Fall 2010 - FINAL5-XBen WilliamsNo ratings yet
- Mann Kendall Statistic and COVDocument11 pagesMann Kendall Statistic and COVMayankNo ratings yet