Professional Documents
Culture Documents
Ni TLR4 and CHS Paper
Ni TLR4 and CHS Paper
Uploaded by
Vivek GoudCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ni TLR4 and CHS Paper
Ni TLR4 and CHS Paper
Uploaded by
Vivek GoudCopyright:
Available Formats
news and views
2010 Nature America, Inc. All rights reserved.
are unstable and can give rise at least to TH1
cells. Members of the Stockinger laboratory
recently expanded these observations with
the help of a mouse expressing Cre recombinase under the control of Il17a regulatory elements. In vivo lineage fate mapping
revealed that under certain inflammatory
conditions TH17 cells give rise to TH1 cells
(M.V. et al., unpublished data). Interestingly,
these ex-TH17-TH1 cells are distinct from
de novogenerated TH1 cells in that they
retain some of their TH17 progenitor program, including the expression of AhR. This
offers the intriguing possibility that under
inflammatory conditions during which a
TH17 celldominated response is reshaped
into one dominated by TH1 cells, sustained
AhR activation can result in immune suppression and tolerance (Fig. 1).
The high toxicity and carcinogenicity associated with TCDD and many other AhR ligands
would make them unsuitable for any direct
therapeutic use. In addition, AhR ligands that
are metabolized by the AhR-initiated system
may provide only a transient stimulus and
furthermore, if present at the onset of immune
activation, are associated with increased onset
and severity of autoimmunity7,8. This system of
stimulatory effects on several immune and nonimmune cell types, along with negative feedback
mechanisms, is highly complex and will require
much exploration before it is fully understood.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
1. Apetoh, L. et al. Nat. Immunol. 11, 854861 (2010).
2. Gandhi, R. et al. Nat. Immunol. 11, 846853 (2010).
3. Stumhofer, J.S. et al. Nat. Immunol. 7, 937945
(2006).
4. Saraiva, M. & OGarra, A. Nat. Rev. Immunol. 10,
170181 (2010).
5. Anderson, C.F., Oukka, M., Kuchroo, V.J. & Sacks, D.
J. Exp. Med. 204, 285297 (2007).
6. Jankovic, D. et al. J. Exp. Med. 204, 273283
(2007).
7. Quintana, F.J. et al. Nature 453, 6571 (2008).
8. Veldhoen, M. et al. Nature 453, 106109 (2008).
9. Funatake, C .J. et al. J. Immunotoxicol. 6, 194203
(2009).
10. Koch, M.A. et al. Nat. Immunol. 10, 595602 (2009).
11. Zheng, Y. et al. Nature 458, 351356 (2009).
12. Veldhoen, M., Hirota, K., Christensen, J., OGarra, A.
& Stockinger, B. J. Exp. Med. 206, 4349 (2009).
Innate sensing of nickel
Marc E Rothenberg
Although nickel allergy is very common, the specific receptor for nickel has not been identified. TLR4 is now shown
to bind nickel and cause inflammation, an interaction that is specific to humans.
ontact sensitivity is an immunological
hypersensitivity response in the skin that is
an annoying problemcausing burning, itching,
redness, swelling and even blistersto millions
of people worldwide1. Interestingly, the number
of substances that trigger this response is limited
compared with the myriad to which the skin is
exposed. For example, among plants, poison ivy
is most notorious for inducing contact dermatitis. Among the heavy metals, nickel stands out
as the chief driver of contact allergy, with up to
30% of the population being affected2. This is
most often manifested by allergic skin reactions
to nickel-containing jewelry (most commonly
rings and earrings) and, more recently, cellular telephones3 (Fig. 1). Furthermore, nickel
is of clinical relevance in the context of the
biocompatibility of cardiovascular stents and
orthopedic and dental implants.
An important outstanding question is
why some substances evoke contact sensitivity whereas most do not. The term nickel
allergy is really a misnomer, as nickel is not
a classic allergen that directly induces IgE,
but rather a hapten that binds to proteins and
induces delayed-type hypersensitivity cellular responses. Previous work has shown that
Marc E. Rothenberg is in the Department of
Pediatrics, Division of Allergy and Immunology,
Cincinnati Childrens Hospital Medical Center,
University of Cincinnati College of Medicine,
Cincinnati, Ohio, USA.
e-mail: Rothenberg@cchmc.org
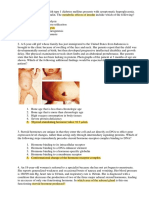
TLR4
TLR4
MD2
MD2
Ni2+
Ni2+
IRF3
Type I
IFNs
NF-B
Proinflammatory
molecules
DTH
Contact
allergy
Nickel-haptenated proteins
Figure 1 Contact allergy, shown as erythema in this figure, is commonly induced by nickel ions
present in nickel-containing jewelry such as rings and earrings, as well as in nickel-containing cellular
telephones. Nickel ion (Ni2+) is shown to bind directly to TLR4, particularly at histidine residues located
in the region of interaction where TLR4-MD2 molecules form homodimers with one another. This
binding is sufficient for activation of TLR4 (in the absence of LPS) and the subsequent transcription
of IRF3 and NF-B. The later production of type I interferons (IFN) and proinflammatory molecules
provides a necessary and cooperative signal that synergizes with delayed-type hypersensitivity (DTH)
responses triggered by nickel-haptenated proteins, leading to contact allergy.
nickel (in the form of nickel salt or ion) is a
potent stimulator of cellular activation, capable
of inducing signal transduction in several cell
types including dendritic cells and endothelial
cells4. Notably, nickel induces expression of
proinflammatory genes in endothelial cells,
a process dependent on induction of NF-B
activation. This suggests that nickel may
interact with Toll-like receptor (TLR) signal
nature immunology volume 11 number 9 SEPTEMBER 2010
transduction, a critical pathway involved in
the sensing of pathogen-associated molecular
patterns and the production of proinflammatory mediators. Progress toward understanding
nickel allergy has been hindered by the lack of
mouse modelsas mice do not readily develop
nickel hypersensitivityas well as by the fact
that the specific binding protein (or receptor) for nickel has not been identified. These
781
2010 Nature America, Inc. All rights reserved.
news and views
hindrances may now be erased by the groundbreaking article by Schmidt et al. in this issue
of Nature Immunology5. The authors have not
only identified TLR4 as the nickel receptor but
also elucidated the nickel binding site, and they
present a model whereby nickel is capable of
activating TLR4 homodimer formation, leading to signal transduction and production of
proinflammatory cytokines.
Using primary human endothelial cells,
Schmidt et al. first demonstrated that nickel
indeed induces expression of NF-kB target
genes such as interleukin 8 (IL-8) by a mechanism dependent upon MyD88 and interleukin 1
(IL-1) receptorassociated kinase-1 (IRAK1),
a critical adaptor and signaling molecule,
respectively linked to receptors of the IL-1
TLR family. Furthermore, using transfected
cell lines, the authors demonstrated that
specific expression of human TLR4 (and
its co-receptor MD2), but not other human
TLRs or mouse TLR4, was sufficient for
responsiveness to nickel (independent of
lipopolysaccharide (LPS)). Mechanistically,
the authors identified the specific region of
human TLR4 that is responsible for nickel
responses (amino acids 369616). Taking into
consideration the previously published crystal
structures of the human TLR4-MD2 complex, which forms a homodimer in the presence of the LPS ligand, the authors focused
on two nonconserved histidine residues present in this region. On the basis of the known
role of histidine in binding to charged ions,
and structural modeling of putative metalbinding sites indicating that the imidazole
groups of two histidines in this region (His456
782
and His458) were at optimal distances to interact with nickel ions, the authors proved that
nickel specifically interacted with these amino
acids. Mutagenesis studies demonstrated that
these two histidines (which are not conserved
in mouse TLR4) are together required for
nickel-induced NF-B activation. Notably,
LPS-induced signaling was unaltered by these
histidine mutations, showing that nickel and
LPS use distinct binding sites to TLR4. Further
elegant experiments showed that the introduction of these two histidine residues into mouse
TLR4 is sufficient to produce nickel responsiveness. To prove the in vivo relevance of their
findings, the authors transgenically expressed
human TLR4 in Tlr4/ mice and demonstrated
that bone marrowderived macrophages from
the transgenic mice gained responsiveness to
nickel. Additionally, these human TLR4
transgenic mice were readily susceptible to
induction of experimental allergic contact
hypersensitivity induced by nickel.
These results clearly identify nickel as an
inorganic activator of the TLR system. Whereas
other contact allergens (such as 2,4,6-trinitrochlorobenzene and oxazolone) seem to interact
indirectly with TLR2 and TLR4 signaling6, these
results are the first to show direct triggering
of pathogen recognition receptors by contact
allergens. They also explain why current mouse
models of nickel-induced contact allergy require
second signals, such as adjuvants. Structurally,
these findings suggest that nickel may directly
bridge two adjacent TLR4 molecules, allowing
a dimer formation similar to that induced by
LPS, which is sufficient for signal transduction. The finding that nickel binding to TLR4
occurs at a site distinct from the LPS binding
site offers a strategy for potentially interfering
with nickel hypersensitivity while preserving
TLR4-dependent host responses.
There are few critical comments to be made
about these elegant experiments; instead,
myriad scientific questions and directions now
emerge. First, what is the interactive relationship between nickel and other TLR4 ligands,
such as LPS? Second, and related, do other,
more natural ions known to bind to histidines,
including protons themselves, influence TLR4
signaling and/or nickel responses? Do mutations affecting the skin barrier protein filaggrin, which are known to induce susceptibility
to nickel contact dermatitis7, interact with the
identified pathway? Does combustion-source
particulate matter, rich in nickel but negligible
in LPS content8, mediate lung inflammation by
nickel-induced TLR4 activation? And finally,
does my Blackberry contain nickel, or should
I just get an iPhone?
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
1. Fonacier, L.S., Dreskin, S.C. & Leung, D.Y. J. Allergy
Clin. Immunol. 125, S138S149 (2010).
2. Thyssen, J.P. & Menne, T. Chem. Res. Toxicol. 23,
309318 (2010).
3. Moennich, J.N., Zirwas, M. & Jacob, S.E. Cutis 84,
199200 (2009).
4. Viemann, D. et al. J. Immunol. 178, 31983207
(2007).
5. Schmidt, M. et al. Nat. Immunol. 11, 814819 (2010).
6. Martin, S.F. et al. J. Exp. Med. 205, 21512162
(2008).
7. Carlsen, B.C. et al. Contact Dermatitis 63, 8995
(2010).
8. Gilmour, P.S., Schladweiler, M.C., Richards, J.H.,
Ledbetter, A.D. & Kodavanti, U.P. Inhal. Toxicol. 16
(Suppl. 1), 518 (2004).
volume 11 number 9 SEPTEMBER 2010 nature immunology
You might also like
- MCQs in Developmental BiologyDocument6 pagesMCQs in Developmental BiologySundaralingam Raj62% (13)
- Notes From Langman's Medical EmbryologyDocument13 pagesNotes From Langman's Medical EmbryologyJeane Irish Paller Egot92% (12)
- Iso 14971Document1 pageIso 14971Vivek Goud0% (1)
- Roitt - Roitt's Essential Immunology 10th EdDocument494 pagesRoitt - Roitt's Essential Immunology 10th EdSetio Hartomo100% (7)
- Role of Tlr2, Tlr4, and Myd88 in Murine Ozone-Induced Airway Hyperresponsiveness and NeutrophiliaDocument7 pagesRole of Tlr2, Tlr4, and Myd88 in Murine Ozone-Induced Airway Hyperresponsiveness and NeutrophiliaArkhan HanafiNo ratings yet
- Review: Toll-Like Receptors and The Control of ImmunityDocument23 pagesReview: Toll-Like Receptors and The Control of ImmunityIrma NeyraNo ratings yet
- Toll-Like Receptors: The Swiss Army Knife of Immunity and Vaccine DevelopmentDocument10 pagesToll-Like Receptors: The Swiss Army Knife of Immunity and Vaccine DevelopmentWilson Barros LuizNo ratings yet
- Pathogen Recognition With Toll-Like Receptors: Taro Kawai and Shizuo AkiraDocument7 pagesPathogen Recognition With Toll-Like Receptors: Taro Kawai and Shizuo Akiraetik ainun rohmahNo ratings yet
- Innate Immune Functions of Astrocytes Are Dependent Upon Tumor Necrosis Factor-AlphaDocument15 pagesInnate Immune Functions of Astrocytes Are Dependent Upon Tumor Necrosis Factor-AlphaCony GSNo ratings yet
- TLR NeutrofilsDocument6 pagesTLR NeutrofilsMariaNo ratings yet
- Maroso2010 PDFDocument8 pagesMaroso2010 PDFAleksandra RasicNo ratings yet
- TLR4 Activation Mediates Kidney Ischemia/reperfusion Injury: The Journal of Clinical Investigation November 2007Document14 pagesTLR4 Activation Mediates Kidney Ischemia/reperfusion Injury: The Journal of Clinical Investigation November 2007stephen AlexanderNo ratings yet
- BAHAN Fitzpatrick 13 - 2Document27 pagesBAHAN Fitzpatrick 13 - 2S FznsNo ratings yet
- Toll-Like Receptors and Skin: JeadvDocument10 pagesToll-Like Receptors and Skin: JeadvBenor Amri MustaqimNo ratings yet
- Natural Killer Cells and Innate Immunity To Protozoan PathogensDocument12 pagesNatural Killer Cells and Innate Immunity To Protozoan PathogensthehayNo ratings yet
- Nat Rev Immunol 2011 WestDocument15 pagesNat Rev Immunol 2011 WestGabriel CamarenaNo ratings yet
- Comprehensive Database On Toll Like Receptors: LiteratureDocument6 pagesComprehensive Database On Toll Like Receptors: Literaturepatialokkumar4465No ratings yet
- The Role of Pattern-Recognition Receptors in Innate Immunity: Update On Toll-Like ReceptorsDocument12 pagesThe Role of Pattern-Recognition Receptors in Innate Immunity: Update On Toll-Like ReceptorslilianapradaNo ratings yet
- Interleukins (From IL-1 To IL-38), Interferons, Transforming Growth Factor B, and TNF-a: Receptors, Functions, and Roles in DiseasesDocument27 pagesInterleukins (From IL-1 To IL-38), Interferons, Transforming Growth Factor B, and TNF-a: Receptors, Functions, and Roles in DiseasesAryantoNo ratings yet
- Protective Roles of Mast Cells Against Enterobacterial Infection Are Mediated by Toll-Like Receptor 4Document7 pagesProtective Roles of Mast Cells Against Enterobacterial Infection Are Mediated by Toll-Like Receptor 4harumiaikoNo ratings yet
- TLR Wikki 4Document2 pagesTLR Wikki 4jebabalajiiiNo ratings yet
- Toll Like Receptor 4Document10 pagesToll Like Receptor 4NajeebNo ratings yet
- Gervaziev 2010Document8 pagesGervaziev 2010Ludmila OleninaNo ratings yet
- Veterinary Immunology and ImmunopathologyDocument6 pagesVeterinary Immunology and ImmunopathologyCristhian Felipe Salinas GuerreroNo ratings yet
- 1 s2.0 S0021925820870148 MainDocument11 pages1 s2.0 S0021925820870148 MainAina SarahNo ratings yet
- Tollip: A Multitasking Protein in Innate Immunity and Protein TraffickingDocument8 pagesTollip: A Multitasking Protein in Innate Immunity and Protein TraffickingFadhil AhsanNo ratings yet
- Lund 2006Document17 pagesLund 2006marialuizacdasilvaNo ratings yet
- Immunity To Protozoa Relative Role of B and T Cells in Immunity To ProtozoaDocument9 pagesImmunity To Protozoa Relative Role of B and T Cells in Immunity To ProtozoaSri WahyuniNo ratings yet
- Inflammation and Immunopathogenesis of Tuberculosis ProgressionDocument25 pagesInflammation and Immunopathogenesis of Tuberculosis ProgressionicaeeNo ratings yet
- Fimmu 05 00386Document13 pagesFimmu 05 00386Dani PazNo ratings yet
- 1974 - Journal of Allergy and Clinical ImmunologyDocument13 pages1974 - Journal of Allergy and Clinical ImmunologyijposadaaNo ratings yet
- Critical Review: Signaling Through Toll-Like Receptor 4 and Mast Cell-Dependent Innate Immunity ResponsesDocument8 pagesCritical Review: Signaling Through Toll-Like Receptor 4 and Mast Cell-Dependent Innate Immunity ResponsesLara SantosNo ratings yet
- TLR DrosophilaDocument8 pagesTLR DrosophilaSaraTorresNo ratings yet
- Immunological Mechanisms in Allergic Contact Dermatitis: Abstract and IntroductionDocument11 pagesImmunological Mechanisms in Allergic Contact Dermatitis: Abstract and IntroductionMarcellRaymondNo ratings yet
- Dapus No 6Document10 pagesDapus No 6tri erdiansyahNo ratings yet
- 14th ICI Abstract BookDocument4 pages14th ICI Abstract BookMuhammadGagasSasongkoNo ratings yet
- An Overview of The Non-Canonical Inflammasome 2020Document13 pagesAn Overview of The Non-Canonical Inflammasome 2020Muhammad AthamnaNo ratings yet
- Ijms 24 14884 v3Document16 pagesIjms 24 14884 v3Naga MuthuNo ratings yet
- Stark and Darnell JAK-STAT at TwentyDocument12 pagesStark and Darnell JAK-STAT at TwentyNilabh RanjanNo ratings yet
- Mechanisms of Allergic Immunity: Crah1@le - Ac.ukDocument98 pagesMechanisms of Allergic Immunity: Crah1@le - Ac.ukNaba ManalNo ratings yet
- Keestra Et Al 2010 tlr21Document9 pagesKeestra Et Al 2010 tlr21misilpiyapiyoNo ratings yet
- Fimmu 13 812774Document22 pagesFimmu 13 812774nutritionist1234567No ratings yet
- Basophils As Antigen Presenting Cells: Mohan S. Maddur, Srini V. Kaveri and Jagadeesh BayryDocument4 pagesBasophils As Antigen Presenting Cells: Mohan S. Maddur, Srini V. Kaveri and Jagadeesh BayryJuan RodriguezNo ratings yet
- Type Reaction, Neuritis and Disability in Leprosy.: What Is The Current Epideiniological Situation?Document25 pagesType Reaction, Neuritis and Disability in Leprosy.: What Is The Current Epideiniological Situation?Sadam_fasterNo ratings yet
- Tumor Necrosis FactorDocument77 pagesTumor Necrosis FactorRahul SinghNo ratings yet
- 2010 Mineo - TLR2Document9 pages2010 Mineo - TLR2Ywlliane KitNo ratings yet
- Toll-Like Receptors (TLRS) and Nod-Like Receptors (NLRS) in InflammatoryDocument12 pagesToll-Like Receptors (TLRS) and Nod-Like Receptors (NLRS) in InflammatoryLarissa SilvaNo ratings yet
- Review Article: Holding The Inflammatory System in Check: Tlrs and Their Targeted Therapy in AsthmaDocument9 pagesReview Article: Holding The Inflammatory System in Check: Tlrs and Their Targeted Therapy in AsthmaArianRizkiAmaliaNo ratings yet
- Fimmu 11 01964Document8 pagesFimmu 11 01964wellst1No ratings yet
- Kulit Kulit KulitDocument15 pagesKulit Kulit KulitFarahnisa MappasissiNo ratings yet
- The TH1/TH2 Paradigm in Allergy.: Th1 and Th2 Responses: What Are They?Document4 pagesThe TH1/TH2 Paradigm in Allergy.: Th1 and Th2 Responses: What Are They?Venus HullanaNo ratings yet
- Lei 2011Document10 pagesLei 2011AycaNo ratings yet
- Toll-Like Receptors As Key Mediators in Innate Antifungal ImmunityDocument14 pagesToll-Like Receptors As Key Mediators in Innate Antifungal ImmunityKlaus Ramirez SuarezNo ratings yet
- MTB TLR Autophagy Apoptosis HDTDocument14 pagesMTB TLR Autophagy Apoptosis HDTamwayindia2024100% (1)
- Toll Like Receptors and Their LigandsDocument21 pagesToll Like Receptors and Their Ligandsakash_81087No ratings yet
- Neutrophils in Response To German TLR2-Mediated Activation ofDocument9 pagesNeutrophils in Response To German TLR2-Mediated Activation ofpatialokkumarNo ratings yet
- Texto Tesis DefinitivoDocument173 pagesTexto Tesis DefinitivoPEREZ BLAS MAGALI MONSERRATNo ratings yet
- Activation of Innate Immune Responses Through Toll-Like Receptor 3 Causes A Rapid Loss of Salivary Gland FunctionDocument6 pagesActivation of Innate Immune Responses Through Toll-Like Receptor 3 Causes A Rapid Loss of Salivary Gland FunctionBimalKrishnaNo ratings yet
- Modulate Endothelial Function and Coagulation Bacterial Lipoprotein TLR2 Agonists BroadlyDocument13 pagesModulate Endothelial Function and Coagulation Bacterial Lipoprotein TLR2 Agonists Broadlynandhus2227No ratings yet
- TMP 1 B46Document8 pagesTMP 1 B46FrontiersNo ratings yet
- Allergy - 2019 - MaedaDocument15 pagesAllergy - 2019 - MaedaLUAN SILVANo ratings yet
- Co-signal Molecules in T Cell Activation: Immune Regulation in Health and DiseaseFrom EverandCo-signal Molecules in T Cell Activation: Immune Regulation in Health and DiseaseMiyuki AzumaNo ratings yet
- Metal Allergy: From Dermatitis to Implant and Device FailureFrom EverandMetal Allergy: From Dermatitis to Implant and Device FailureJennifer K ChenNo ratings yet
- 20180929063016Document1 page20180929063016Vivek GoudNo ratings yet
- CounterfeitDocument5 pagesCounterfeitVivek GoudNo ratings yet
- 32 CDV 13Document1 page32 CDV 13Vivek GoudNo ratings yet
- CIRCOR Aerospace Plan For Counterfeit Parts PB140Document7 pagesCIRCOR Aerospace Plan For Counterfeit Parts PB140Vivek GoudNo ratings yet
- Habco Manuf Counterfit ProcedureDocument6 pagesHabco Manuf Counterfit ProcedureVivek GoudNo ratings yet
- Qa00932017 00 Counterfeit Parts Protection PlanDocument2 pagesQa00932017 00 Counterfeit Parts Protection PlanVivek GoudNo ratings yet
- UK Manufacturer of Steel Strip - Ben Bennett JR - Spring SteelDocument2 pagesUK Manufacturer of Steel Strip - Ben Bennett JR - Spring SteelVivek GoudNo ratings yet
- Icf Vendor ManualDocument16 pagesIcf Vendor ManualVivek GoudNo ratings yet
- AK9ch - АК9ч Aluminium Casting Alloys gost standardDocument2 pagesAK9ch - АК9ч Aluminium Casting Alloys gost standardVivek GoudNo ratings yet
- 1 - 3 August Month Current Affairs MCQ: Android App For MobileDocument11 pages1 - 3 August Month Current Affairs MCQ: Android App For MobileVivek GoudNo ratings yet
- High Capacity Steganography Using Diamond Encoding: Bhavana Kore Rishi GinjupalliDocument14 pagesHigh Capacity Steganography Using Diamond Encoding: Bhavana Kore Rishi GinjupalliVivek GoudNo ratings yet
- Clear Close CLC Syms Syms: % Assignment 8Document1 pageClear Close CLC Syms Syms: % Assignment 8Vivek GoudNo ratings yet
- MARKETING-Taladvance Slide GB Sept 2017Document27 pagesMARKETING-Taladvance Slide GB Sept 2017ahmetgezer34No ratings yet
- Acute Phase ReactionDocument3 pagesAcute Phase Reactionstefan0236No ratings yet
- General Types of Hormone Receptors:: Receptors Function and Signal TransductionDocument12 pagesGeneral Types of Hormone Receptors:: Receptors Function and Signal Transductionmamoun mufarraqNo ratings yet
- Growth Factors in Periodontal Regeneration - PeriobasicsDocument16 pagesGrowth Factors in Periodontal Regeneration - Periobasicsdileep9002392100% (1)
- Iups Programa 25072017 BXDocument76 pagesIups Programa 25072017 BXSonja BuvinicNo ratings yet
- Bnab 014Document38 pagesBnab 014RodrigoNo ratings yet
- Exam 19 Endocrine SystemDocument6 pagesExam 19 Endocrine SystemPurwa RaneNo ratings yet
- Mindfulness Based Stress Reduction: An Integrated View of Physiological and Molecular Aspects of Mindfulness Intervention On Stress ManagementDocument14 pagesMindfulness Based Stress Reduction: An Integrated View of Physiological and Molecular Aspects of Mindfulness Intervention On Stress ManagementajmrdNo ratings yet
- Hematopoiesis: Makio Ogawa, MD, PHDDocument6 pagesHematopoiesis: Makio Ogawa, MD, PHDVredy LesnarNo ratings yet
- Albanese - Imaging of Prosthetic Joints - A Combined Radiological and Clinical Perspective PDFDocument185 pagesAlbanese - Imaging of Prosthetic Joints - A Combined Radiological and Clinical Perspective PDFSergio Gordillo Tovar100% (1)
- Franco 2017Document12 pagesFranco 2017ReshmaaRajendranNo ratings yet
- Caler, 2000Document9 pagesCaler, 2000samannosheenNo ratings yet
- Intercellular CommunicationDocument8 pagesIntercellular CommunicationDina MaulidaNo ratings yet
- Icsa Usmle Courses ContentDocument18 pagesIcsa Usmle Courses ContentdostoviskyNo ratings yet
- Hormones As Signals, Classification of HormonesDocument22 pagesHormones As Signals, Classification of HormonesHarini BalasubramanianNo ratings yet
- Genetic and Biological Hallmarks of Colorectal CancerDocument34 pagesGenetic and Biological Hallmarks of Colorectal CancerFredNo ratings yet
- 001192187GS01Document33 pages001192187GS01radhoinezerellyNo ratings yet
- Association Between Cortisol, Insulin Resistance and ZincDocument8 pagesAssociation Between Cortisol, Insulin Resistance and ZincEzard DavidNo ratings yet
- Nino Gachechiladze ImunitetiDocument268 pagesNino Gachechiladze ImunitetiSaba GabadzeNo ratings yet
- Gpcrs and CancervistaDocument45 pagesGpcrs and CancervistapriyaaNo ratings yet
- UntitledDocument21 pagesUntitledPravinNo ratings yet
- Insulin Like Growth Factor-1 IGF-1 A TarDocument7 pagesInsulin Like Growth Factor-1 IGF-1 A TarDiana Hanifiyah SutipnoNo ratings yet
- Neurochemical Characteristics of AmisulprideDocument15 pagesNeurochemical Characteristics of AmisulprideamonzicilinaNo ratings yet
- Signal Transduction in Bacterial Chemotaxis: Lengeler Et Al. Pp. 514-523Document16 pagesSignal Transduction in Bacterial Chemotaxis: Lengeler Et Al. Pp. 514-523lobnaNo ratings yet
- Ex - Effect of Physostigmine and Atropine On DRCDocument9 pagesEx - Effect of Physostigmine and Atropine On DRCAryan Pisat100% (1)
- Foundations in Biology Exam 2 NotesDocument2 pagesFoundations in Biology Exam 2 NotesMatt ParkNo ratings yet
- 7.6. Enhancing Secondary Metabolite Production in Plants Exploring Traditional - CsDocument14 pages7.6. Enhancing Secondary Metabolite Production in Plants Exploring Traditional - CsFarida RahayuNo ratings yet
- British J Pharmacology - 2011 - Alexander - Guide To Receptors and Channels GRAC 5th EditionDocument326 pagesBritish J Pharmacology - 2011 - Alexander - Guide To Receptors and Channels GRAC 5th EditionMichell AlmeidaNo ratings yet