Professional Documents
Culture Documents
Activated Carbons From Lignocellulosic
Activated Carbons From Lignocellulosic
Uploaded by
vinodOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Activated Carbons From Lignocellulosic
Activated Carbons From Lignocellulosic
Uploaded by
vinodCopyright:
Available Formats
Carbon. Vol. 30, No. 7. pp. I I I I- I I 18.
1992
Printed in Great Britain.
Copyright
0008.6223/92
$5.00 + .OO
0 1992 Pergamon
Press Ltd.
ACTIVATED CARBONS FROM LIGNOCELLULOSIC
MATERIALS BY CHEMICAL AND/OR PHYSICAL
ACTIVATION: AN OVERVIEW
Departamento
F. RODRIGUEZ-REINOSO
and M. MOLINA-SABIO
de Quimica Inorgnica e lngenieria Quimica, Universidad de Alicante, Alicante, Spain
(Received 4 February 1992; accepted in revisedform 5 March 1992)
Abstract-Four
series of activated carbons prepared from lignocellulosic materials (almond shells and
olive and peach stones) by either physical activation-gasification
(uncatalysed and iron catalysed) in CO*
or in a water-nitrogen mixture-of
chars or direct chemical activation with ZnClz of the precursor were
selected to show the comparative behavior of the activation procedures. Activation with CO2 opens and
widens the microporosity of the char with even a shift to meso- and macroporosity, the ablation of the
exterior of the particle being very important at high burn-OF, the final activated carbon has a well developed micro- and macroporosity, with a relatively small contribution of mesoporosity. The iron catalysed
COz gasification and gasification with water-nitrogen mixture produce carbons with a well developed
macroporosity, although the latter has the advantage of maintaining a well developed micro- and mesoporosity. Direct chemical activation of the precursor with ZnClz produces, in only one step, a larger yield
of activated carbon having microporosity as well developed as in the CO2 gasification of the char, with the
advantage of producing a much larger mesopore volume.
Key Words-Activated
carbon, physical activation, chemical activation, pore development.
1. INTRODUCHON
of activated carbon from lignocellulosic materials by physical or chemical activation is
very important from the industrial point of view.
Many of these materials (coconut shells, fruit stones,
etc.) on carbonization
produce non-graphitizable,
high-purity chars of appropriate hardness and bulk
density, which are very adequate as precursors for activated carbons of high quality, useful in adsorption
of both gases and solutes from aqueous solution[ 11.
On the other hand, these materials permit the preparation ofactivated carbons with a variety ofpore size
distributions by modifying the preparation conditions of either physical or chemical activation procedures. In both methods there is a reaction of the precursor with the activating agent to develop the
porosity, but they differ not only in the practical procedure, but also in the mechanism by which the activating agent develops such porosity.
In a physical activation process the lignocellulosic
precursor is carbonized under an inert atmosphere,
and the resulting char-with
a small adsorption capacity-is
subjected to a partial and controlled gasification at high temperature with steam, carbon dioxide, air, or a mixture of these[2]. This gasification
selectively eliminates first the more reactive carbon
atoms ofthe structure generating the porosity; further
gasification will produce the final carbon with the
pore structure sought. In a chemical activation process the lignocellulosic precursor is mixed with a
chemical restricting the formation of tars (ZnC&,
HJP04, etc.) and, after kneading, carbonized and
washed to produce the final activated carbon. The
chemical incorporated to the interior ofthe precursor
particle reacts with the products resulting from the
The preparation
CAR 30:7-G
III1
thermal decomposition ofthe precursor, reducing the
evolution ofvolatile matter and inhibiting the shrinking of the particle; in this way, the conversion of the
precursor to carbon is high, and once the chemical is
eliminated after the heat treatment, a large amount of
porosity is formed[2].
Although the general mechanism for the physical
activation is better understood today because of the
much larger number of publications in the open literature, both methods are used by the producers of
activated carbon in an attempt to prepare carbons for
various applications. In any case, the number of variables of an activation process is large and knowledge
of them is very important to develop the porosity
sought for a given application. In this sense, this
paper presents the evolution in porosity of several series of activated carbons prepared by physical and
chemical activation of lignocellulosic materials in an
attempt to show the comparative behavior ofboth activation procedures and to clarify some mechanistic
aspects. Activated carbons prepared from almond
shells and olive and peach stones by partial gasification of the corresponding chars with CO, (uncatalysed and catalysed by iron) or a water-nitrogen mixture are compared with carbons prepared by
impregnation with ZnCl, followed by a simple heat
treatment.
2. CARBONIZATION
Carbonization
of lignocellulosic
materials such as
coconut shells, olive stones, etc., in an inert atmosphere produces a nongraphitizable
char[ 31. Pyroly-
sis of the polymeric cellulose or lignin during carbonization liberates most of the non-carbon elements-
1112
F. RODRIGUEZ-REINOSO
and M. MOLINA-SABIO
mainly hydrogen, oxygen, and nitrogen-in
the form
of gases and tars, leaving behind a rigid carbon skeleton formed by aromatic sheets and strips, often bent,
resembling a mixture ofwood shavings and crumpled
paper[4]. The gaps between these elementary graphitic crystallites constitute the porosity of the char.
Figure 1 shows the evolution of weight loss during
carbonization
in nitrogen (heating rate SK/min;
soaking time, 1 h) of three different lignocellulosic
precursors, almond shells, and olive and peach
stones. The evolution of bulk density for the latter is
also included in the same figure. The results for the
three materials are very similar and in agreement
with previous results for other similar materials[2,5].
There are three clear stages in the carbonization process: (a) loss of water in the 300-470K range; (b) primary pyrolysis in the 470-770K range, with evolution of most gases and tars and formation of the basic
structure of the char; and (c) consolidation of char
structure at 770-l 120K, with a very small weight
loss. The small reduction in bulk density with increasing carbonization
temperature, coupled with
the large reduction in weight (especially in the second
stage) implies a contraction of the precursor particle;
such contraction continues above 770K, since the
density slightly increases in the temperature range
770- 1120K.
The porosity of the char is not always accessible,
because it becomes filled or blocked by disorganized
carbon resulting from deposition and decomposition
of tars. It is often found that the surface area of this
type of char as measured by adsorption of N2 at 77K
is low, around 100 m/g, but 500-600 m2/g for N2 at
90K and around 600 m2/g for CO2 at 273K[6-I].
These results are interpreted in terms of activated diffusion of nitrogen molecules through constrictions in
the entrance of the micropores caused by the tarry
substances; at the low temperature of measurement
(77K), the entrance of the molecules is a rate-controlled process with a positive temperature coefficient
and an increase in the adsorption temperature (e.g.,
to 90K); or the use of a molecule of similar dimensions at much higher temperature (CO, at 273K) will
increase the rate of diffusion of the molecule through
WeiQht IOSS (%I
the constrictions and the amount adsorbed. In any
case, the resulting char has a small adsorption capacity, and the way to make this porosity available is by
activation of the char by partial gasification or by carrying out the carbonization process after addition of
substances that restrict the formation of tar.
3. PHYSICAL (OR THERMAL)
ACTIVATION
The so-called physical activation is the partial gasification of the char with steam, carbon dioxide, and
air, or a combination
of these, at temperatures
around 11OO-1250K, the disorganized material is removed first-with
the subsequent increase in pore
volume-and
by this the elementary crystallites become exposed to the action of the activating agent for
further development
of porosity with increasing
burn-off. Previous work[8-1 I] has shown that the
most important variables in the gasification process
from the point of view of porosity development are
the activating agent, the final bum-off reached, and
the presence of inorganic impurities that catalyze or
inhibit the gasification reaction.
To study the effect of these variables, three series
of activated carbons prepared from olive stones and
almond shells were selected. Series D has been prepared by activation at 1098K in a flow of CO2 of the
char obtained by carbonization of the olive stones in
nitrogen at 1123K[ 121. Series AV has been prepared[ 131 by activation of the same char at 1073K
with a steam-nitrogen mixture (partial pressure of
H20, 13kPa). Series AFe was prepared by direct activation in CO2 at 1073K of almond shells loaded
with 0.4% Fe (by impregnating the precursor with a
5% solution of iron nitrate) to catalyze the gasification reaction[ 141; the use of direct activation in CO2
was based in previous studies showing that this process was equivalent to the conventional carbonization followed by gasification[ 151.
Figure 2 includes the evolution of bum-off as a
function of reaction time for the three different gasification processes. Since the tendency of the three
plots of gasification rate are different, the point is to
see whether this will affect the development of the po-
d (Q/cm31
- 0.9
100'
300
600
900
1*00d3
T (K)
Fig. 1. Weight loss on carbonization of (A) almond shells,
(Cl) olive stones, and (0) peach stones and evolution ofbulk
density (0) for peach stones.
20
40
60
60
100
Time (h)
Fig 2. Variation of bum-off with reaction time for uncatalysed (0, series D) and Fe catalysed (A, series AFe) gasification in CO2 and gasification in steam (0, series AV).
Activated carbons from &mcellulosic
materials
1113
o,3V
V (cm3/g)
(cm3/g Icharl)
1.2
AV-74
0.2
l.O0.1
D-80
0.8 -
-0
20
40
60
60
100
20
Burn-off (%)
AV-53
D-52
AFe-67
iYig
AFe-17
0.2-
o.o0.2
0.4
0.6
0.8
1.0
P/p,
Fig. 3. N2 (77K) adsorption isotherms on some carbons of
series D, AV, and AFe. Burn-off is included in the nomenclature of carbons.
rosity during activation. The gasification rate for series D is almost constant throughout the whole bumoff range studied, and the Nz (77K) adsorption isotherms (Fig. 3) show a continuous increase in adsorptive capacity and a widening of the porosity (as detected by the change in isotherm shape). The volume
of micropores-deduced
from the application of the
Dubinin-Radushkevich
(DR) equation[ 16]-and
the volume of mesopores (calculated by subtracting
the micropore volume from the volume adsorbed at
P/P,,
= 0.95)
have been plotted in Fig. 4 as a function
of burn-off. All data are expressed as liquid volume
per unit weight of activated carbon in Fig. 4a and per
unit weight of initial char in Fig. 4b. Figure 4a shows
a continuous increase in pore volume with increasing
burn-off, especially the micropore volume. On the
other hand, the evolution of macropore volume measured by mercury porosimetry (Fig. 5a) is also similar, the absolute values of micro and macropore volume becoming almost coincident for the carbons
with larger burn-off.
Although from the industrial point of view the
data of Figs. 4a and Sa are the more important, when
the same data are expressed per unit weight of starting
char (Figs. 4b and 5b), one can get a better idea of
what is occurring during the gasification of the
char[ 17,181. There is a development of all ranges of
porosity (although small for the mesoporosity) up to
about 40%-50% burn-off followed by a clear decrease
at higher burn-offs, as described for other carbonaceous materials[ 19-2 11. Only an 8% burn-off in CO*
eliminates the disorganized material (constrictions
40
60
60
100
Burn-off (%)
Fig. 4. Variation of micro- (open symbols) and mesopore
(closed symbols) volume with bum-off for carbons of series
D (0) AV (0) and AFe (n).
caused by tar deposition), causing an increase in micropore volume and especially in micropore width,
since the adsorption of N2 at 77K is not activated as
in the char (the volume given for the char was obtained from the adsorption of N2 at 90K, since it was
too small when measured at 77K). As gasification
proceeds, there is a further increase in micropore volume and in micropore width (as denoted by the opening of the knee of the adsorption isotherm, Fig. 3)
and even a shift to meso and even macropores; but
after 40% burn-off there is a phenomenon more important than the widening of the porosity-the
ablation of the exterior of the carbon particle, causing an
important decrease in pore volume.
In series AV, in which the same char is gasified
with a steam-nitrogen mixture, both the gasification
and the evolution of porosity are different from series
D. The activation conditions were selected in such a
way as to produce a gasification rate similar to that of
series D. As shown in Fig. 2, this was achieved up to
a 30% burn-off, gasification being faster for series AV
thereafter. The isotherms of Fig. 3 show that for low
burn-off the adsorption isotherms for both series are
similar, but for higher bum-off they clearly differentiate, those of series AV exhibiting a more open knee
at low relative pressure and a steeper branch at high
relative pressure; both indicate a wider micropore
size distribution and a larger contribution of mesoporosity in carbons of series AV.
The micropore volume develops as in series D,
and even the absolute values are very similar for a
b)
o.oi-0
j
20
40
jade
60
80
Burn-off (%)
O.O_~
0
20
40
Burn-off
60
80
(%)
Fig. 5. Variation of macropore volume (mercury porosimetry) for carbons of series D (0), AV (Cl), and AFe (A).
1114
F. RODRiGUEZ-REINOSOand M. MOLINA-SABIO
given bum-off, as shown in Fig. 4a. However, the evolution of mesopore volume is very different in both
series, larger in series AV, the difference increasing
with bum-off. Gasification with steam produces a
more noticeable widening of microporosity to mesoporosity, as shown in Fig. 4b, the volume of mesopores increasing continuously
with increasing
burn-OR, since at the same time the evolution of microporosity shown in the same figure is similar to series D, one has to deduce that gasification with steam
produces a large development of microporosity but
simultaneously a large widening of this microporosity, this process being more important than the destruction of large porosity by the external ablation of
the carbon particle. This is also clearly shown by the
evolution of macropore volume in series AV with increasing gasification, completely different from series
D (Figs. 5 and 6).
Gasification with CO, produces a larger volume of
large macropores, whereas steam produces a larger
volume of mesopores (see Fig. 6 for the cumulative
plots of pore volume measured by mercury porosimetry). When the data are expressed per unit weight
of starting char (Fig. 5b) it is seen that CO2 produces
a relatively large increase in macropore volume up to
about 50% bum-off, followed by a considerable decrease at larger bum-offs, whereas a continuous increase takes place in gasificaion by steam. Since the
development ofmicroporosity is very similar for both
activating agents, with a considerable decrease after
about 40% burn-off (Fig. 4b), it is clear that for steam
gasification there is a continuous enlargement of
small pores in all ranges of burn-of, this means that
the ablation of the exterior of the carbon particles in
CO, at high burn-off is much more important than in
steam.
This difference in behavior found for the gasification of the same char with CO2 or water-nitrogen
mixture could be due to fact that the lower partial
pressure of water in the conditions used here would
produce a more selective attack of the carbon structure and a more uniform widening ofporosity, the attack by the much larger concentration of CO, being
less selective. This effect is, of course, coupled with
the different extent of inhibition by reaction products
taking place in both reactions under the experimental
conditions used[ 18,2 1,221.
When the precursor contains inorganic impurities
that may catalyze the gasification reaction with CO*,
both the gasification rate and the porosity will be different. As shown in Fig. 2, the gasification rate for series AFe is initially very fast even though the gasification temperature (1073K) is lower than in series D
(1098K), and it decreases with increasing burn-off,
becoming similar to the uncatalysed reaction at high
burn-offs. The shape of the adsorption isotherms
(Fig. 3 includes, as typical examples, the isotherms
corresponding to the carbons obtained after 2 and 8
h gasification) indicate that the carbons are essentially microporous, similar to those of low burn-off in
series D, although the development of microporosity
and mesoporosity during gasification is very small, as
shown in Fig. 4a; the absolute values of meso- and
micropore volume are in general smaller than in series D and AV. When these values are expressed per
unit weight of starting char (Fig. 4b), there is a systematic loss of both micro- and mesopore volumes
with increasing gasification, indicating that gasification produces either an external burning of the particle or a development of larger size pores not detected by the adsorption of N2 at 77K. The mercury
porosimetry results (Fig. 5a) show that the latter is the
most important factor at low degrees of burn-off and
that the external ablation of the particle (Fig. 5b) becomes important at high levels of burn-off. This type
of evolution of porosity upon gasification has been
described for other non-lignocellulosic
precursors,
and is a function of the nature of the catalyst introduced prior to the gasification[ 11,191. In general
terms, the evolution can be explained by assuming
that the C-CO, reaction is catalysed only in the vicinity of the catalyst particles, producing large pores; this
process continues until the catalyst becomes inactive,
when the gasification rate becomes similar to that of
the uncatalysed reaction. Whereas the catalyst is active, the amount of carbon gasified by the uncatalysed reaction is small and the subsequent development of microporosity is also very small.
4. CHEMICAL
log r (nm)
Fig. 6. Cumulative pore volume plots (mercury porosimetry) for some carbons of series D and AV.
ACTIVATION (WITH ZnQ)
Chemical activation of lignocellulosic materials
with ZnClz leads to the production of activated carbons with a good yield and a well developed porosity
in only one step. The precursor is impregnated with
a concentrated ZnClz solution, and after kneading (7
h at 358K was selected here as the contact time between the precursor and the activating solution, followed by evaporation of the solution), the material is
carbonized in an inert atmosphere and thoroughly
washed to extract the ZnCl,.
The variables with a direct incidence in the development of the porosity are the amount of ZnClz in-
Activated carbons from lign~ellulosic
corporated in the precursor and the temperature of
heat treatment. It is common to carry out the carbonization at around 700K or higher, since at this temperature the primary carbonziation is finished (Fig.
I), and it is assumed that &Cl, loses the water (taken
from the precursor in the first stages of thermal degradation) at around 700K[ 231.
The experiments carried out for carbonization in
the 773-1073K range with lignocellulosic materials
impregnated with different concentrations of ZnCll
indicate that increasing carbonization temperatures
produces a slight weight loss (similar to that observed
in Fig. 1 for the same range of tem~rature),
reorganization of the carbon structure, and partical shrinking[24]. These changes lead to an increase in bulk
density and to a considerable decrease of the porosity
of the carbon. The n-butane (27310 adsorption isotherms on samples impregnated with a fixed concentration of &Cl, (expressed here as Xz,, grams of Zn
per gram of stone)-Xz,
= 0.96-carbonized
at 773
and 1073K illustrate this behavior (compare isotherms D and C in Fig. 7). Is to be noted that the
range of small pores is the one more affected, since
carbon C has a na~ower pore size dist~bution (as denoted by the knee of the isotherm), and the amount
adsorbed decreases considerabty in respect to carbon
0. However, the possibilities of preparing carbons
with different porosity are considerably increased
when changing the amount of chemical incorporated
to the precursor.
Figure 8 shows the evolution of micropore volume
(obtained by application of the DR equation to the nbutane adsorption data), the mesopore volume (calculated by subtracting the micropore volume from
the amount adsorbed at P/P0 = 0.95), and the yield
of the process of activation as a function of the Zn
isoconcentration,
Xz,; the n-butane adso~tion
therms for some of the carbons (carbons A, B, and D
had X,, values of 0.24, 0.48, and 0.96, respectively,
and were heat treated at 773K) are included in Fig. 7.
V (cm3/g)
1.2 -
0.6
0.2
._L_L__
0t4
0.6
P/P,
0.8
I
1.0
Fig. 7. Adsowtion isotherms of n-butane at 273K on ZnC1,
aciivated cardons. A:Xz, = 0.24. B: Xz, = 0.48. D: X,, =
0.96 (carbonization temperature = 773K). C: X,, = 0.96
(carbonization temperature = 1073K).
0.8 ,-
materials
1115
Yield (%I
bn3/g)
0.4
0.8
1.2
25
XZn (g Znlg precursor 1
Fig. 8. Effect of Zn content of the precursor X,,) on micro(0) and mesopore volume (0) and yield (A).
There are three clear tendencies of evolution of porosity as X,, increases. For Xz, < 0.3 there is predominance of microporosity creation[25,26], this microporosity being rather uniform (e.g., carbon A in Fig.
7), the yield increasing with increasing Zn concentration. For the X,, range 0.3- i .Othere is a competition
among creation and widening of microporosity, so
that the micropore volume does not increase so fast
and there is an appreciable increase in mesoporosity.
There is a simultaneous decrease of the yield, very
marked up to X,, = 0.5, as a consequence of a larger
evolution of volatiles during impregnation[24]. Further increase in Zn concentration above X,, = 1.0
produces an even larger widening of the porosity, decreasing both the volume of micro- and mesopores,
especially the former (Fig. 8).
On the other hand, Fig. 9 indicates that for carbons prepared with X,, < 0.3, with a pore volume
(volume of n-butane adsorbed at P/P,= 0.95)
up to
0.5 cm3/g, the volume of ZnC& (a~uming that the
density of solid Z&l, is 2.9 g/cm) incorporated to
the precursor is similar to the pore volume, whereas
for larger values of X,, (pore volume larger than 0.7
cm,/g) the adsorbed volume is lower than the volume
of ZnCl, in the precursor. All these results suggest the
following activation mechanism for ZnCl,. During
the impregnation step, under the conditions selected
here, the chemical reaches the interior of the precursor particle and produces some hydrolysis reactions-as
shown by the weight loss, exit of volatiles,
weakening of the structure, increase in elasticity-
1116
F.
RODRiGUEZ-REINOSO
V of n-butane hn3/g)
Fig. 9. Volume of n-butane adsorbed at P/PO = 0.95 as a
function of volume of ZnCI* retained by precursor on impregnation.
and a swelling of the particle. Both phenomena are
more marked as the concentration of the ZnCl, solution becomes greater. In contrast with this, during
the heat treatment, the ZnClz prevents the formation
of tars, increasing the yield of the process. The minimum weight loss produced in the impregnation and
carbonization processes occurs in the 0.2-0.3 range
of Xz,. Since this is a relatively small proportion of
ZnCl,, the compound can be uniformly distributed
with a large dispersion throughout the interior of the
particle, producing a carbon (after intensive washing)
with an uniform microporosity, having a total volume coincident with the volume of ZnCl, incorporated in the precursor. The macroporosity of these
carbons is small, 0.04 cm3/g, as described for carbons
prepared from coconut shells[27].
For Xz, values larger than 0.3 the hydrolysis and
swelling become accentuated. The hydrolysis produces a larger weight loss during carbon preparation,
and even at values of Xz, larger than 1.O it produces
a destruction of the porous texture after heat treatment. Although the incorporation of larger amounts
of ZnClz produces a larger swelling of the particles,
such large amounts cannot be distributed uniformly
and M.
MOLINA-SABIO
throughout the interior of the particle, and consequently, although the pore volume increases, the pore
size distribution will be more heterogeneous. Mercury porosimetry measurements
in these carbons
confirm the development of macroporosity.
Activated carbons prepared by activation with
ZnClz are very different from those prepared by physical activation with CO,. The comparison of results
for both methods can be made on the basis of the values of micropore and mesopore volume calculated as
before and plotted as a function of the yield of the activation process (two stages in the case of activation
with COJ. Figure 10 includes such plots for the values expressed in terms of unit weight of activated carbon ( 1Oa)or per unit weight ofstarting lignocellulosic
precursor (lob). Figure 10a indicates that in the first
stages of the activation process ZnC& develops the
porosity to a larger extent than CO*, and furthermore
the yield of the process is always much larger for the
former. In other words, the same micropore volume
is reached with ZnCl, activation at a much larger
yield. The plots of Fig. lob show more clearly the differences among the initial stages of the two activation
procedures, since one has to consider that the activation with CO? implies a previous carbonization
(with about 74% weight loss), and consequently when
the data are referred to the weight of the initial precursor, the development of porosity, especially microporosity, is much lower than in the activation with
ZnCl*.
When the degree ofactivation is larger (by increasing the degree of gasification in CO2 or by increasing
the ZnCl, concentration, X,.) the differences in porosity development become more drastic. Not only
the development of micropore volume is larger in
chemical activation, but in addition the mesopore
volume increases considerably (Fig. lob). As a consequence, the precursor develops the porosity more
in chemical activation, and the final result is that it
leads to carbons (Fig. 10a) having similar micropore
volume, but a much larger mesopore volume for a
larger yield of the process. Only at very high ZnCll
loadings (X,, larger than 0.96) is there a partial destruction of the internal structure of the particle.
o 8V (cm3/g)
II5
a)
0.6
0.4
0.2
O_
l0
20
Yield cz-
40
Fig. 10. Variation in micro- (open symbols) and mesopore (closed symbols) volume with overall yield for
CO? (0) and ZnCll (0) activated carbons.
50
1117
Activated carbons from lignocellulosic materials
5. CONCLUSION
As shown above, both physical and chemical activation of lignocellulosic materials can be used to
prepare activated carbons with a variety of pore size
distributions, but the activation mechanisms and the
flexibility to produce different pore size distributions
are very different. In physical activation with CO2 or
steam there is previously a carbonization of the precursor which is almost finished at a temperature
lower than the one used in the activation[ 151. This
means that the carbonization would take place before
the gasification reaction would start, even if the precursor is subjected directly to activation, and consequently in the first stages of the gasification, one
would start with a not so well developed porosity in
the carbon. Activation with CO, opens and widens
the microporosity, with even a shift to meso- and macroporosity, the ablation ofthe exterior of the particle
being very important at high burn-offs. In any case,
the final activated carbon has a well developed microporosity with a relatively small contribution of mesoporosity and, in general, a well developed macroporosity (the extent of which would be a function of
the macroporosity related to the cellular structure of
the particular precursor).
In order to obtain activated carbons with different
pore size distributions,
gasification has to be restricted to selected areas of the surface. This is the case
for the CO, activation catalysed by iron and the activation with nitrogen-water mixtures. The latter has
the advantage of maintaining a well developed microporosity and mesoporosity, which is not the case
for the catalysed reaction, although both produce carbons with a well developed macroporosity.
In any case, the preparation of activated carbons
with a well developed porosity in all ranges of pore
size by physical activation requires the elimination of
a large part of the internal mass of the carbon, and
this means necessarily a low yield and in most cases
low mechanical properties of the final carbon.
In chemical activation, the chemical is introduced
into the precursor, where it produces physical and
chemical changes modifying the thermal degradation
process. As a consequence, the temperature of the
process does not need to be high. During impregnation with the ZnCl, solution, and especially during
evaporation, there is a weakening of the precursor
structure, hydrolysis reactions (with loss of volatile
matter), an increase in elasticity, and a swelling of the
particle. These phenomena are intensified with increasing amounts of ZnCl, introduced into the precursor. Upon carbonization, ZnCl, restricts the formation of tars with the subsequent formation of solid
carbon, and this prevents somewhat the contraction
of the particle. After carbonization most of the ZnCl?
is still in the particle, and the intense washing to eliminate it produces the porosity. This means that the
amount and distribution ofthe ZnCl, incorporated in
the precursor govern the porosity of the carbon, thus
making this activation method very flexible for the
preparation of activated carbons with different pore
size distributions. Of course, the further gasification
ofthese carbons with CO, or steam opens further possibilities, as shown elsewhere[24]. A further possibility of modifying the pore size distribution ofactivated
carbon is to use a different chemical (e.g., H3P04,
KOH, etc.) able to produce a more or less pronounced change in the thermal degradation of the
precursor than ZnCl,.
Acknowledgements-Financial
support from DGICYT
(Project No. PB86/279) and Caja de Ahorros Provincial de
Alicante is gratefully acknowledged.
REFERENCES
1. R. C. Bansal, J. B. Donnet and F. Stoeckli, Active carbon. Marcel Dekker. New York (1988).
2. M. Smisek and S. Ce.rny, Activecarbdn. Manufacture,
properties and applications. Elsevier, Amsterdam
(1970).
3. H. Marsh, In Carbon and Coal GasiJication (Edited by
J. L. Figueriredo and J. A. Moulijn) p. 27. Martinus
Nijhoff, Dordrecht, Netherlands (1986).
4. H. F. Stoeckli, Carbon 28, 1 (1990).
5. B. McEnanev and K. J. Master. Termochimica Acta 82.
81 (1984). .
6. A. Linares-Solano, J. D. Lbpez-Gonzilez, M. MolinaSabio and F. Rodrieuez-Reinoso. J. Chem. Tech. Biotechnol. 30,65
(1985).
7. F. Rodriguez-Reinoso,
J. D. L6pez-GonzBlez and C.
Berenguei, Carbon 20, 5 I3 ( 1982); 22, 13 (1984).
F. Martinez-Vilchez and F. Rod8. J. D. L&z-Gonzaez.
riguez-geinoso, Carbbn 18,4 13 (1980).
9. F. Rodriguez-Reinoso
and A. Linares-Solano, In
Chemistry and Physics of Carbon (Edited by P. A.
Thrower) Vol. 21, p. 1. Marcel Dekker, New York
(1988).
10. F. Rodriguez-Reinoso, J. M. Martin-Martinez, M. Molina-Sabio, I. Perez-Lledb and C. Prado-Burguete, Carbon 23, 19 (1985).
Il. J. Holmes and P. H. Emmett, J. Phys. Colloid Chem.
57, 1276 (1947).
12. F. Rodriguez-Reinoso, J. M. Martin-Martinez, M. Molina-Sabio, R. Torregrosa and J. Ganido, J. Colloid Znterface Sci. 106,3 15 ( 1985).
13. F. Rodriguez-Reinoso, I. Ptrez-Lledb and A. LinaresSolano (submitted for publication).
A. Linares14. F. Rodriguez-Reinoso, M. Almela-Alar&,
Solano and C. Salinas-Martinez de Lecea. Proc. 17th
Biennial Conference on Carbon, Lexington, KY, p. 237
(1985).
15. F. Rodriguez-Reinoso, A. Linares-Solano, M. MolinaSabio and J. D. L6pez-Gonzllez. Ads. Sci. & Tech. 1,
2 I I (1984).
16. M. M. Dubinin, In Progress in Surface and Membrane
Science (Edited by D. A. Cadenhead), Vol. 9, p. 1. Academic Press, New York (1975).
17. F. Rodriguez-Reinoso, In Fundamental Issues in Control of Carbon Gasification Reactivity (Edited by J. Lahaye and P. Ehrburger) Vol. 192, p. 533. NATO, AS1
series. Kluwer Academic Publishers. Dordrecht. The
Netherlands (199 1).
18. T. Wigmans, In Carbon and Coal Gasification (Edited
by J. L. Figueiredo and J. A. Moulijn), p. 559. Martinus
Niihoff. Dordrecht, The Netherlands ( 1986).
19. H.-Marsh and B. Rand, Carbon 9,47,.63,79
(197 I).
20. P. L. Walker, Jr., In Carbon and Coal Gasification (Edited by J. L. Figueiredo and J. A Moulijn), p. 1. Martinus Nijhoff, Dordrecht, The Netherlands (I 986).
1118
F. RODRIGUEZ-REINOSOand M. MOLINA-SABIO
2 1. T. Wigmans, Carbon 27, 13 ( 1989).
22. T. Tomkov, T. Siemieniewska, F. Czechowski and A.
Jankowska, Fuel 56, 12 1 ( 1977).
23. United Kingdom Patent 27834 (1934).
24. F. Caturla, M. Molina-Sabio and F. Rodriguez-Reinoso, Carbon 29,999 (1991).
25. F. Ruiz Bevia, D. Prats Rico and F. A. Marcilla Gomis,
Ind. Eng. Prod. Res. Dev. 23,266,269 (1984).
26. F. Rodriguez-Reinoso, M. Mohna-Sabio, G. Y. Buss
and F. Caturla, European Patent EP 032925 I (1989).
27. 0. Kadlec, A. Varhanikova and A. Zukal, Carbon 8,
321 (1970).
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Modeling of Ammonia Removal From Wastewater Using Air Stripping/ Modified Clinoptilolite: Reusability, Optimization, Isotherm, Kinetic, and Equilibrium StudiesDocument22 pagesModeling of Ammonia Removal From Wastewater Using Air Stripping/ Modified Clinoptilolite: Reusability, Optimization, Isotherm, Kinetic, and Equilibrium StudiesvinodNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Final Theses Sunny M.Ed.Document101 pagesFinal Theses Sunny M.Ed.vinodNo ratings yet
- Gokul - 8.7.16Document8 pagesGokul - 8.7.16vinodNo ratings yet
- Water Spray Reactor For Ammonia Removal Via Air Stripping: An Evaluation On Mass Transfer and Process EfficiencyDocument8 pagesWater Spray Reactor For Ammonia Removal Via Air Stripping: An Evaluation On Mass Transfer and Process EfficiencyvinodNo ratings yet
- Fateh Enviro Engineers SchemeDocument1 pageFateh Enviro Engineers SchemevinodNo ratings yet
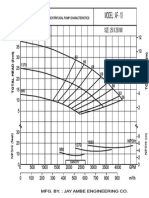
- Jay Ambe AF - 16 CurveDocument1 pageJay Ambe AF - 16 CurvevinodNo ratings yet
- Jay Ambe Axial Pump AF - 10 CurveDocument1 pageJay Ambe Axial Pump AF - 10 CurvevinodNo ratings yet
- Kriloskar KSMB Pump SeriesDocument1 pageKriloskar KSMB Pump SeriesvinodNo ratings yet
- Stripper ColumnDocument1 pageStripper ColumnvinodNo ratings yet
- Operation Characteristic of A Mechanical Vapor Recompression Heat Pump Driven by A Centrifugal FanDocument8 pagesOperation Characteristic of A Mechanical Vapor Recompression Heat Pump Driven by A Centrifugal FanvinodNo ratings yet
- Artificial IntelligenceDocument14 pagesArtificial IntelligencevinodNo ratings yet
- 0.5 MLD PlantDocument1 page0.5 MLD PlantvinodNo ratings yet
- Img 0006-1Document1 pageImg 0006-1vinodNo ratings yet
- PT Mass BalanceDocument3 pagesPT Mass BalancevinodNo ratings yet
- Process Biochemistry: Beatriz Veleirinho, J.A. Lopes-da-SilvaDocument4 pagesProcess Biochemistry: Beatriz Veleirinho, J.A. Lopes-da-SilvavinodNo ratings yet
- Manish KumarDocument4 pagesManish KumarvinodNo ratings yet
- Use of Steam and Co2 As Activating AgentsDocument9 pagesUse of Steam and Co2 As Activating AgentsvinodNo ratings yet
- Chemical For NeutralizationDocument2 pagesChemical For NeutralizationvinodNo ratings yet
- Shylaja 2019Document9 pagesShylaja 2019G G HegdeNo ratings yet
- Concrete Mix Design Mixorder C15-20 - 011743 PDFDocument1 pageConcrete Mix Design Mixorder C15-20 - 011743 PDFkarume ndirituNo ratings yet
- QB 5 - Basic NDT - LT QBDocument5 pagesQB 5 - Basic NDT - LT QBprabhakaran.SNo ratings yet
- Physico-Mechanical Properties and Automotive Fuel Resistance of Epdm-Enr Blends With Hybrid FillersDocument7 pagesPhysico-Mechanical Properties and Automotive Fuel Resistance of Epdm-Enr Blends With Hybrid FillersArjun Satheesh KumarNo ratings yet
- Astm D 3174-04Document5 pagesAstm D 3174-04Servando LozanoNo ratings yet
- TS 1 To 6Document11 pagesTS 1 To 6Anshul Gautam100% (1)
- Liquid CrystalsDocument33 pagesLiquid Crystalsvarundasjh80% (10)
- 5 Accuracy HWDocument2 pages5 Accuracy HWLÂM VŨ THÙYNo ratings yet
- 5.111 Principles of Chemical Science: Mit OpencoursewareDocument6 pages5.111 Principles of Chemical Science: Mit OpencoursewaresarjitgaurNo ratings yet
- Analysis of TriglyceridesDocument8 pagesAnalysis of Triglyceridesdstar13No ratings yet
- Fosroc Nitoflor FC150: Constructive SolutionsDocument4 pagesFosroc Nitoflor FC150: Constructive SolutionsABHI MITRANo ratings yet
- 1 Maxwell's Equations in Matter (Integrate With Next Section)Document2 pages1 Maxwell's Equations in Matter (Integrate With Next Section)iordache100% (1)
- 17 97Document1 page17 97Luis EstradaNo ratings yet
- General Chapters - 711 - DISSOLUTIONDocument11 pagesGeneral Chapters - 711 - DISSOLUTIONFitri WahyuningsihNo ratings yet
- Frt11 BetzDocument25 pagesFrt11 BetzArindam DasNo ratings yet
- 9701 s07 QP 2Document12 pages9701 s07 QP 2Hubbak KhanNo ratings yet
- Magnetism in SSDocument12 pagesMagnetism in SSSusheel WankhedeNo ratings yet
- 6 Single Phase AC Voltage Controller With R and RL LoadsDocument6 pages6 Single Phase AC Voltage Controller With R and RL LoadsSeminars BRECWNo ratings yet
- Gas Sweetening Simulation and Its Optimization by Two Typical AmineDocument8 pagesGas Sweetening Simulation and Its Optimization by Two Typical AmineYogesh PatilNo ratings yet
- Drafting of Heat ExchangerDocument1 pageDrafting of Heat ExchangerHeru YulindoNo ratings yet
- Gagani2017 PDFDocument15 pagesGagani2017 PDFGautamNo ratings yet
- Wastewater DN80 To DN600 PDFDocument36 pagesWastewater DN80 To DN600 PDFMR seaNo ratings yet
- 0 Material Safety Data Sheet: Benzene MSDSDocument6 pages0 Material Safety Data Sheet: Benzene MSDSAli RazaNo ratings yet
- Lee's DiscDocument3 pagesLee's DiscRocker RanjithNo ratings yet
- REFFIPLANT Training CourseDocument76 pagesREFFIPLANT Training CourseKESAVARAPU UMA SAI MAHESHNo ratings yet
- Chemical Bonding 05 Class Notes PDFDocument19 pagesChemical Bonding 05 Class Notes PDFmodel photo copyNo ratings yet
- HMT16 MarksDocument12 pagesHMT16 MarkstagoreboopathyNo ratings yet
- Equation of State of TitaniumDocument6 pagesEquation of State of TitaniumKing OfheartsNo ratings yet
- Fresh and Hardened Properties of 3D Printable - Paul2018Document9 pagesFresh and Hardened Properties of 3D Printable - Paul2018AliReza ZiaratiNo ratings yet