Professional Documents
Culture Documents
Column and TLC
Column and TLC
Uploaded by
jeniccax17Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Column and TLC
Column and TLC
Uploaded by
jeniccax17Copyright:
Available Formats
Column and Thin Layer Chromatography
Mary Coleen A. David, Eunice Mae D. del Valle, Sean Romeo B. Desagon, Maria Cauline M. Fang, Jenicca Pamela Y. Go,
Jan Chrtien M. Guillo
Group 3, 2F-Pharmacy, Organic Chemistry Laboratory, Faculty of Pharmacy, University of Santo Tomas
ABSTRACT
Chromatography is a modern and sophisticated method of separating mixtures, wherein the
physical separation of different adsorbed materials is accomplished in a single adsorbent. It can be based
on the selective distribution of chemicals between a stationary and a mobile phase. The mobile phase is
allowed to pass through the system of the separated mixture while the a well-defined spot or band is
placed as a stationary phase. There are different types of chromatography and each has its own
advantages and disadvantages. In this experiment, hexane-acetone was used to extract the different
pigments of malunggay leaves. The colored components of malunggay leaves or the extract were
separated further using column chromatography. The collected compounds then underwent Thin Layer
Chromatography (TLC). TLC was used to determine the purity and for measuring the Rf values of the
colored components.
INTRODUCTION
Chromatography is a preparative
technique
that
separates
and
isolates
components for further studies. It is a technique
in which compounds in a mixture are separated
based on differing affinities between a mobile
phase and a stationary phase. Each component
of a mixture has a different partition coefficient
between the two phases and will move through a
system at a different rate. Mobile phase is a
medium used in chromatography which moves
through the stationary phase. In TLC and
column chromatography, the mobile phase is an
organic liquid. Stationary phase is a material
used in chromatography which does not move.
The mobile phase passes through the stationary
phase. The stationary phase is either a pure solid
substance such as alumina or silica or a thin
coating of liquid on a solid support or a gel.
During column chromatography,
compounds of mixtures are separated by having
it pass through a column packed with silica gel.
Silica gel was used because compounds will
usually adhere to the silica to different extents.
Xantophyll, Chlorophyll a, and chlorophyll b
are the three colored components of the eluate
of malunggay leaves.
Thin Layer Chromatography was used
for rapid analysis of organic mixtures. TLC can
be used to help determine the number of
components in a mixture, the identity of
compounds, and the purity of a compound. In
TLC the stationary phase is a spread in a thin
layer on an inert support, usually a plastic sheet
or a glass plate. The mobile phase moves
upward by capillary action. TLC method was
also used to measure the Retention or
Retardation factor (Rf value).
Separation of the colored components of
the malunggay leaves using column
chromatography, determination of the purity of
the components using TLC, and the
measurement of the Rf values of the colored
components in the TLC were the objectives met
in this experiment.
MATERIALS AND METHODS
MATERIALS
The materials used in this experiment for
malunggay leaves are the mortar and pestle, for
grinding purposes, Pasteur pipette, for accurate
measurement, iron stand and iron clamp, to
support the column, beaker, watch glass, test
tubes, to collect the different eluates, capillary
tubes, to apply the spots, precoated TLC plates,
malunggay leaves, silica gel, cotton, and
hexane-acetone (7:3).
A column was made using a Pasteur
pipette plugged with cotton and uniformly
packed with silica gel. The column was
assembled with an iron clamp and iron stand in
METHOD
The pigments were extracted by
crushing malunggay leaves with hexane-acetone
in a mortar with a pestle.
a column chromatography set up. 0.5 mL of the
extract was placed on top of the column using a
Pasteur pipette for accurate measurement. 5.0
mL of each solvent systems (hexane-acetone,
acetone, and acetone-methanol) was introduced
successively to the set up. Solvent systems were
added to separate the colored components at
different rates.
The eluates, in different colors, were
collected in different test tubes as shown below.
(Colorless eluates were discarded) Counting the
number of drops of each eluate that are collected
in each tube was done cautiously for the
experiment.
The eluates were applied on a TLC plate,
having a measurement of 5 cm x 8 cm, and
spotted ten times using a capillary tube. Hexaneacetone, a developing chamber, was then added
in the beaker and filter paper lining the inner
wall of the beaker. The beaker was covered
using a watch glass for it to equilibrate.
The solvent system was allowed to rise up
to 1 cm from the upper end before removing the
TLC plate. The components was visualized
using a UV lamp and the distance traveled was
measured in order to compute for the Rf value.
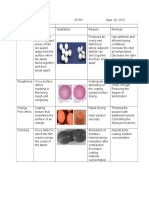
Thin Layer Chromatography
Color
of Distance
of Rf
Component
component
Value
from origin in
cm
Yellow
4.5 cm
0.75
Yellow green
2.5 cm
0.42
Green
2.6 cm
0.43
Light green
2.7 cm
0.45
The solvent front for the TLC plate is 6 cm.
distance travelled by the component
Rf = origin
distance travelled by solvent
RESULTS AND DISCUSSION
Column Chromatography
Four colors were yielded from the
extraction of the colored components of
malunggay
leaves
using
Column
Chromatography. The number of drops of each
eluate are shown in the table below.
Color of Component
Volume
(drops)
of
Yellow
15
Yellow green
54
Green
21
Light green
76
eluate
Rf value of yellow component:
Rf=
4.5
=0.75
6
Rf value of yellow green component:
Rf=
2.5
=0.42
6
Rf value of green component:
Rf=
2.6
=0.43
6
Rf value of light green component:
Xantophylls reflect yellow components
and chlorophyll reflects green components.
Chlorophyll a reflects the darker shade of green
while lighter green or yellow green for
chlorophyll b. Xantophyll is a non-polar
compound, which was confirmed using column
chromatography. Hexane-acetone is a non-polar
compound and yellow was the first eluate to
come out. Since like dissolves like, this proves
that Xantophyll is a non-polar compound.
Rf=
2.7
=0.45
6
The distance travelled by the yellow
component is the farthest in the TLC plate
compared to the other colored components. It
travelled the farthest because it is a non-polar
compound. Polar compounds stick easier to the
TLC plates. The yellow component eventually
disappeared, leaving the green components
behind.
REFERENCES:
1. Thin Layer Chromatography. Retrieved from
http://www.chem.umass.edu/~samal/269/tlc.pdf
2. Thin Layer and Chromatography. Retrieved
from
http://www.xula.edu/chemistry/documents/orgle
clab/12TLCCol.pdf
3. Bell, C. Jr., Clark, A., & Taber, D. (2001).
Organic Chemistry Laboratory. USA:
Brooks/Cole.
4. Pavla, D. Lampman, G., Kriz, G., & Engel, R.
(1998). Introduction to organic laboratory
techniques: a microscale approach. 3rd Ed.
USA: Saunders Publishing Company.
You might also like
- Business and Society Stakeholders Ethics Public Policy 17Th Edition Anne Lawrence Full ChapterDocument67 pagesBusiness and Society Stakeholders Ethics Public Policy 17Th Edition Anne Lawrence Full Chaptermichael.lynch15578% (9)
- GXT SeriesDocument8 pagesGXT SeriesCésar S. Silva100% (2)
- 38t GEI41047 PDocument28 pages38t GEI41047 PJorge DiazNo ratings yet
- ChromatographyDocument23 pagesChromatographysatish pradhanNo ratings yet
- Manicure and PedicureDocument38 pagesManicure and PedicureDesiree Clarisse B. DelaCruz100% (4)
- Thin Layer Chromatography and Column Chromatography Results and DiscussionDocument2 pagesThin Layer Chromatography and Column Chromatography Results and DiscussionJennifer Heredia67% (3)
- Paper Chromatography Formal Report ORG ChemDocument5 pagesPaper Chromatography Formal Report ORG ChemCheng BauzonNo ratings yet
- Chromatography LabDocument9 pagesChromatography Labjtrumpeter224100% (1)
- Column Chromatography (Separation of Lycopene and β-carotene)Document7 pagesColumn Chromatography (Separation of Lycopene and β-carotene)Bea A.No ratings yet
- Exp 7 ChromatographyDocument10 pagesExp 7 ChromatographyNur AidaNo ratings yet
- Pasig RiverDocument34 pagesPasig RiverNhilo ReginoNo ratings yet
- Computer Project 1: Assignment 1.1Document10 pagesComputer Project 1: Assignment 1.1Nelu TurcanuNo ratings yet
- Formal Report On Thin Layer ChromatographyDocument2 pagesFormal Report On Thin Layer ChromatographyAthena OcampoNo ratings yet
- 5.column and Thin Layer ChromatographyDocument3 pages5.column and Thin Layer ChromatographyroseannequyoNo ratings yet
- Formal Report ChromatographyDocument4 pagesFormal Report ChromatographyCalvin BautistaNo ratings yet
- Formal Report Expt 5Document6 pagesFormal Report Expt 5AnonymouscatNo ratings yet
- Column and Thin Layer ChromatographyDocument3 pagesColumn and Thin Layer ChromatographyAileen Delos SantosNo ratings yet
- ChromatographyDocument10 pagesChromatographyJohn Henrick G. UyNo ratings yet
- Results and Discussion: Malunggay Leaves. On The Other Hand, Thin LayerDocument2 pagesResults and Discussion: Malunggay Leaves. On The Other Hand, Thin LayerJennifer HerediaNo ratings yet
- Separating and Determining The Purity of The Colored Pigments Present in Siling Labuyo Through ChromatographyDocument5 pagesSeparating and Determining The Purity of The Colored Pigments Present in Siling Labuyo Through Chromatographyrica_pinpinNo ratings yet
- Column and Thin Layer ChromatographyDocument5 pagesColumn and Thin Layer ChromatographyChamzelle100% (1)
- Experiment 5 ChromatographyDocument3 pagesExperiment 5 ChromatographyJames Quan100% (2)
- Column ChromatographyDocument2 pagesColumn ChromatographyKerwin MañezNo ratings yet
- Column and Thin Layer ChromatographyDocument3 pagesColumn and Thin Layer ChromatographyChristine Evan HoNo ratings yet
- Separating Pigments of Chile Pepper Using Column Chromatography and Thin Layer ChromatographyDocument5 pagesSeparating Pigments of Chile Pepper Using Column Chromatography and Thin Layer ChromatographyKyleBernalÜNo ratings yet
- Column and Thin Layer ChromatographyDocument3 pagesColumn and Thin Layer ChromatographyAileen Delos SantosNo ratings yet
- EXPERIMENT 5 - Chroamtorgraphy GRP9 RevDocument2 pagesEXPERIMENT 5 - Chroamtorgraphy GRP9 RevMic100% (2)
- Formal ReportDocument3 pagesFormal ReportTacttoNo ratings yet
- TLC Formal ReportDocument3 pagesTLC Formal ReportMagat AlexNo ratings yet
- Paper Chromatography Formal Report ORG ChemDocument5 pagesPaper Chromatography Formal Report ORG ChemRachel Anne Barlao100% (2)
- Formal Report On ChromatographyDocument4 pagesFormal Report On ChromatographyLanceNo ratings yet
- CHEM 2020 Lab Manual (Introduction, Safety, Exp.1)Document6 pagesCHEM 2020 Lab Manual (Introduction, Safety, Exp.1)Kennedy Avery Morgan Jr.No ratings yet
- ChromatographyDocument88 pagesChromatographyMohammad Sabir HussainNo ratings yet
- Chromatographic Methods FDocument10 pagesChromatographic Methods FMunna PatelNo ratings yet
- Experiment 2 TLCDocument6 pagesExperiment 2 TLCAnonymous 75TDy2yNo ratings yet
- Chromatography P1eeaoqbpea91bc5e2b1cc84Document91 pagesChromatography P1eeaoqbpea91bc5e2b1cc84Asif AliNo ratings yet
- Lab ReportDocument6 pagesLab ReportMarivic Bencio RacaNo ratings yet
- TLC Practical 2Document6 pagesTLC Practical 2kumanmichael986No ratings yet
- Paper ChromatographyDocument6 pagesPaper ChromatographyMuslimah Anggun100% (1)
- Written Exp 4Document5 pagesWritten Exp 4Ayshee CapuchinoNo ratings yet
- 5 ChromatographyDocument7 pages5 ChromatographyAntonio CharismaNo ratings yet
- Separation and Identification of Plant Pigments by TLC MainDocument5 pagesSeparation and Identification of Plant Pigments by TLC MainnaomiNo ratings yet
- Red Siling Labuyo ChromatographyDocument5 pagesRed Siling Labuyo ChromatographyLesley Bernadette Gomez100% (1)
- ChromatographyDocument38 pagesChromatographyelizabeth merzyNo ratings yet
- Chromatography of Amino Acids Lab ReportDocument2 pagesChromatography of Amino Acids Lab ReportAjagwu EustaceNo ratings yet
- Exercise 4 (Chromatography)Document6 pagesExercise 4 (Chromatography)fangirlton0% (1)
- 355073814 Column Chromatography Separation of Lycopene and β caroteneDocument7 pages355073814 Column Chromatography Separation of Lycopene and β carotenePL CarpenteroNo ratings yet
- Advanced Chemistry Final Lab ReportDocument14 pagesAdvanced Chemistry Final Lab Reportapi-644259218No ratings yet
- Term Paper Column ChromatographyDocument22 pagesTerm Paper Column ChromatographySuhail Khan100% (1)
- Experiment 8 BiochemDocument10 pagesExperiment 8 BiochemMsfaeza HanafiNo ratings yet
- CHEM1042 Expt C ManualDocument7 pagesCHEM1042 Expt C ManualGIGI LAUNo ratings yet
- Chromatographic TechniquesDocument42 pagesChromatographic TechniquesRaina JainNo ratings yet
- Experiment 2 ChromatographyDocument3 pagesExperiment 2 ChromatographyChacha Mercado0% (1)
- Applied Chemistry ProjectDocument23 pagesApplied Chemistry ProjectSimarjot SinghNo ratings yet
- Separating Pigments of Chile Pepper Using Column Chromatography and Thin Layer ChromatographyDocument4 pagesSeparating Pigments of Chile Pepper Using Column Chromatography and Thin Layer Chromatographyeneganiron100% (1)
- Chromatography For UploadDocument15 pagesChromatography For UploadZion LivingstoneNo ratings yet
- Qualitative Analysis of Amino Acid in Unknown Sample Through Paper Chromatography Techniques With Eluent of N Butanol and Phenol PDFDocument6 pagesQualitative Analysis of Amino Acid in Unknown Sample Through Paper Chromatography Techniques With Eluent of N Butanol and Phenol PDFAnggraini Nugroho PNo ratings yet
- Types of Chroma To Grap GyDocument75 pagesTypes of Chroma To Grap GyMohammad RehanNo ratings yet
- Column and Thin Layer ChromatographyDocument3 pagesColumn and Thin Layer ChromatographyDiana Marie de LeonNo ratings yet
- DocumentDocument5 pagesDocumentadibshanto115No ratings yet
- CHEM 43.1 Exercise 4Document7 pagesCHEM 43.1 Exercise 4paradoxcomplexNo ratings yet
- ChromatographyDocument49 pagesChromatographyanadelia eser jose100% (3)
- What Is Column ChromatographyDocument5 pagesWhat Is Column ChromatographyAngelNo ratings yet
- Light and Colour Theories, and their relation to light and colour standardizationFrom EverandLight and Colour Theories, and their relation to light and colour standardizationNo ratings yet
- Practical Handbook of Pharmaceutical Chemistry for M.PharmFrom EverandPractical Handbook of Pharmaceutical Chemistry for M.PharmNo ratings yet
- Analytical Characterization of BiotherapeuticsFrom EverandAnalytical Characterization of BiotherapeuticsJennie R. LillNo ratings yet
- Group 2 CaseDocument3 pagesGroup 2 Casejeniccax17No ratings yet
- DrugsDocument1 pageDrugsjeniccax17No ratings yet
- Phar Chem Finals - Chapt 1-4 ExercisesDocument7 pagesPhar Chem Finals - Chapt 1-4 Exercisesjeniccax17100% (1)
- For Kids:: Aardvark RelayDocument4 pagesFor Kids:: Aardvark Relayjeniccax17No ratings yet
- Chapter 9 - Health EconomicsDocument33 pagesChapter 9 - Health Economicsjeniccax17No ratings yet
- Tablet DefectsDocument3 pagesTablet Defectsjeniccax17No ratings yet
- Label Exp. 9 and 10Document1 pageLabel Exp. 9 and 10jeniccax17No ratings yet
- Synthesis of Acetylsalicylic AcidDocument7 pagesSynthesis of Acetylsalicylic Acidjeniccax17No ratings yet
- Nclex Boot CampDocument24 pagesNclex Boot CampMariekris Sangalang100% (12)
- 320 Lecture 27Document8 pages320 Lecture 27Gathy BrayohNo ratings yet
- Metasploit FrameworkDocument1 pageMetasploit FrameworkPAUL VINCENT FAJARDONo ratings yet
- SyllogismDocument25 pagesSyllogismSunil GahlotNo ratings yet
- Fliqlo - Mobile AppDocument1 pageFliqlo - Mobile AppJon BaerNo ratings yet
- 19 - Bearing Capacity - Eccentric LoadingDocument5 pages19 - Bearing Capacity - Eccentric LoadinghiyeonNo ratings yet
- Sherlock Holmes Script - Dialogue TranscriptDocument83 pagesSherlock Holmes Script - Dialogue TranscriptLocustaNo ratings yet
- EW74Ëó+ Á+ ÚDocument9 pagesEW74Ëó+ Á+ Úundibal rivasNo ratings yet
- Solubility Equilibrium HomeworkDocument2 pagesSolubility Equilibrium HomeworkEyayu ZewduNo ratings yet
- Week 1: Introduction: NM NM Ev Ev E DT T P EDocument9 pagesWeek 1: Introduction: NM NM Ev Ev E DT T P EInstituto Centro de Desenvolvimento da GestãoNo ratings yet
- Staedtler Digitalpen 2.0 enDocument132 pagesStaedtler Digitalpen 2.0 enceciardittoNo ratings yet
- Applications of DEsDocument37 pagesApplications of DEsjomgir09No ratings yet
- Full Download Strategies For Teaching Learners With Special Needs 11th Edition Polloway Test BankDocument36 pagesFull Download Strategies For Teaching Learners With Special Needs 11th Edition Polloway Test Banklevidelpnrr100% (31)
- Design of Partition PlateDocument5 pagesDesign of Partition Platepippo2378793No ratings yet
- Controllers and Controller StationsDocument65 pagesControllers and Controller StationsLucian ChorusNo ratings yet
- 2021 - 1 - Alternative Sampling Arrangement For Chemical Composition Tests For General Acceptance (GA) ApplicationDocument5 pages2021 - 1 - Alternative Sampling Arrangement For Chemical Composition Tests For General Acceptance (GA) Applicationkumshing88cwNo ratings yet
- Pathway - Skoliosis GROUPDocument12 pagesPathway - Skoliosis GROUPAnonymous NZTQVgjaNo ratings yet
- Elphos Erald: Police Chief Clarifies Child's ID Kit UsageDocument10 pagesElphos Erald: Police Chief Clarifies Child's ID Kit UsageThe Delphos HeraldNo ratings yet
- Endovac BrochureDocument8 pagesEndovac BrochureGeorge MK100% (1)
- Semi Soft Coking Coal and PCI CoalDocument2 pagesSemi Soft Coking Coal and PCI CoalYusuff QuadrilateralNo ratings yet
- Scienco AP-30: Air Piston PumpDocument2 pagesScienco AP-30: Air Piston PumpSergio SalgadoNo ratings yet
- Dorothy E. Johnson: Behavioral System ModelDocument15 pagesDorothy E. Johnson: Behavioral System Modelwickwax100% (1)
- Construction Terms - English-FilipinoDocument1 pageConstruction Terms - English-FilipinoRhomayne Triz LapuzNo ratings yet
- SAMPLE of Moon Time by Lucy H. Pearce, Womancraft PublishingDocument33 pagesSAMPLE of Moon Time by Lucy H. Pearce, Womancraft PublishingWomancraft PublishingNo ratings yet