Professional Documents
Culture Documents
Efectos Aleantes Nodular
Efectos Aleantes Nodular
Uploaded by
jose.figueroa@foseco.com0 ratings0% found this document useful (0 votes)
19 views2 pagesThis document discusses the effects of various alloying elements on the microstructure and properties of ductile iron. It explains that alloying affects the diffusion rate of carbon within the matrix and can result in different matrix microstructures depending on cooling rate. Graphite morphology also influences the ease of forming ferrite. Certain elements like phosphorus and chromium strongly segregate between graphite nodules. The ability to form pearlite, which is important for hardenability, depends on cooling rate, nodule count, and alloy content. Elements like copper, tin, and antimony promote pearlite formation, while molybdenum and manganese stabilize pearlite. Nickel and copper increase toughness and hardenability but have different
Original Description:
efecto de aliantes
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document discusses the effects of various alloying elements on the microstructure and properties of ductile iron. It explains that alloying affects the diffusion rate of carbon within the matrix and can result in different matrix microstructures depending on cooling rate. Graphite morphology also influences the ease of forming ferrite. Certain elements like phosphorus and chromium strongly segregate between graphite nodules. The ability to form pearlite, which is important for hardenability, depends on cooling rate, nodule count, and alloy content. Elements like copper, tin, and antimony promote pearlite formation, while molybdenum and manganese stabilize pearlite. Nickel and copper increase toughness and hardenability but have different
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
19 views2 pagesEfectos Aleantes Nodular
Efectos Aleantes Nodular
Uploaded by
jose.figueroa@foseco.comThis document discusses the effects of various alloying elements on the microstructure and properties of ductile iron. It explains that alloying affects the diffusion rate of carbon within the matrix and can result in different matrix microstructures depending on cooling rate. Graphite morphology also influences the ease of forming ferrite. Certain elements like phosphorus and chromium strongly segregate between graphite nodules. The ability to form pearlite, which is important for hardenability, depends on cooling rate, nodule count, and alloy content. Elements like copper, tin, and antimony promote pearlite formation, while molybdenum and manganese stabilize pearlite. Nickel and copper increase toughness and hardenability but have different
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
85
BASIC DUCTILE IRON ALLOYING
by
James D. Mullins, Sorelmetal Technical Services
One of the characteristics of alloying is that it affects
the diffusion rate of the carbon, which is the ability of
the carbon to move within the matrix. Upon cooling
and depending upon the rate of cooling, after
solidification, but before we reach the point where
transformation stops, we find that the carbon is able to
move/diffuse. The amount of this movement will vary
and as a result if it is not fast enough (limited by
alloys) to clear all of the matrix structure before the
end of transformation; this is one of the major reasons
why we get various mixtures of matrix microstructures
in Ductile Irons.
Ductile Irons form ferrite much more easily than gray
irons even with the same alloy content. Graphite
morphology is the reason for this. Graphite nodules
are encased within an austenite shell.
The
composition of the austenite affects the microstructure
and subsequent iron properties, again because if
affects the ease of carbon to move away from pearlite
leaving ferrite, as mentioned earlier. Ferrite contains
practically zero carbon. The average thickness of the
ferrite shell around a nodule of graphite does not
change whether the structure is from an as-cast or
heat treated sample. The other reason is that when
the nodule count is increased the amount of ferrite is
also increased, since the shell thickness remains
essentially the same but the number of places where
the carbon can diffuse to is increased. And so by
increasing the nodule count we see changes in the
structure and properties.
Another factor that we must consider is that during
solidification some elements are pushed into or
segregate to the last areas to freeze. These areas are
located between the graphite nodules and are called
the intercellular regions. Elements that have high
tendencies to segregate are P, Mn, Cr, V, Ti and Mo
in increasing order of tendency to segregate. (For
further information about this see Chapter 4, The
Sorelmetal Book of Ductile Iron). See Figure 1. Even
when the concentration of an element appears to be
low or in a normal range we find that they can
concentrate up to extremely high levels in those
intercellular areas. And when they do, they influence
what happens there. So we must be careful which
elements that we use for alloying to avoid forming
carbides in these intercellular regions. They increase
the hardenability in those areas, and the structures
change becoming harder, less ductile and more brittle.
Increasing the nodule count will reduce this effect and
then it may not make as much difference or it will
make less difference.
SEGREGATION OF VARIOUS ELEMENTS
Element
Mo
Ti
V
Cr
Mn
P
Si
Co
Ni
Cu
Segregation Factor
25.3
25.0
13.2
11.6
1.7 3.5
2.0
0.7
0.4
0.3
0.1
Figure 1.
There are some alloying elements, specifically
antimony, copper and tin, which surround the graphite
spheroids and act as barriers to the diffusion of
carbon. The use of these elements gives us the ability
to form nearly 100% pearlite in the matrix, stabilizing it
to keep it from transforming further.
Most of the time when we are talking about
hardenability we are talking about the ability to form
martensite. The term hardenability by itself means the
response to heat treating. But in cast irons we are
primarily interested in pearlitic hardenability. The
ability to form pearlite is the function of those items
previously mentioned. Matrix structures are functions
of cooling rate, nodule count and alloy content.
Looking at what some of the individual elements do.
Silicon has an effect on carbon equivalent. Its a solid
solution strengthening agent, so increasing silicon
greatly effects the embrittlement of Ductile Iron,
particularly at lower temperatures. For these reasons
the overall amount must be controlled. However,
silicon reduces chill and undercooling and promotes
graphite. It segregates negatively, which means that
the highest concentration is near the graphite nodule.
Nickel is also a graphitizing element and acts similarly
to silicon except that it does not embrittle, but rather
increases the toughness of the matrix. It contributes
to the carbon equivalent in the same way that silicon
does but not as effectively. It promotes pearlite but it
does not stabilize pearlite. With nickel you get more
pearlite but the pearlite breaks down more easily
during heat treatment. It increases hardenability and
through hardenability, it promotes the formation of
austenite and it also makes it easier to heat treat to
form martensite or ADI structures. It segregates
negatively in the same way that silicon does. It
doesnt cause problems.
All the allowing/trace elements that have been
mentioned above are recovered upon remelting, so
when calculating subsequent additions, the addition
must be reduced by the amounts recovered from the
return scrap.
Copper is similar to nickel in many ways, except that
is a much stronger pearlite promoter. At a 1% level,
usually a 100% pearlitic matrix can be obtained. It is
the most widely used alloy for pearlitic Ductile Iron
production, because of its effectiveness and cost. It
only gives a modest increase in hardenability (See
Figure 2).
Manganese is generally a pearlite former/stabilizer,
and forms carbides. It segregates strongly to grain
boundaries and increases hardenability.
Chromium is not a strong pearlite former, but is a
carbide stabilizer and can cause many problems with
respect to segregation as mentioned earlier.
Molybdenum is a pearlite stabilizer and promotes
hardenability. It is most often used in combination
with copper or nickel, but at a much lower addition
rate, because of its serious segregation tendency.
Looking at some of the other elements that we might
use. Antimony is a fantastic pearlite former, but is
used only in heavy sections with balanced cerium
additions, otherwise it can certainly change the nodule
structure/shape towards flake. Arsenic is another very
potent pearlite stabilizer, but for some reason or
another people are not enthusiastic about having
arsenic around in the foundry, but it is very good. It is
another one like antimony in that with a combination
with rare earth, it increases the nodule count and
produces better nodule shape.
The last major alloying element is tin. It is used in
small amounts because it is a strong pearlite former
and stabilizer. It can form flake graphite if the
additions are too large. Tin also strongly segregates if
you have excess tin. If there is not enough room for
the tin atoms to line up around the graphite spheroids,
the excess tin segregates into the last areas to freeze
and it greatly deteriorates the toughness of the
material.
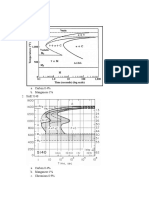
See Figure 3 for the relative pearlite promoting
strength of some elements.
Figure 2. Comparison between the pearlite promoting effects of Mn and Cu
(TC: 3.85%, Si: 2.00%. Unalloyed Mn: 0.25%).
Cerium and magnesium are not used specifically as
alloying elements in Ductile Iron, but they can act to
change the structure and promote carbides. It is best
to add only the necessary amounts. Excessive cerium
causes graphite shape problems especially when you
have high purity charge materials. You must always
balance these things out.
If you want to change the mechanical properties you
must change the microstructure. With no other
process changes this can be done easily with alloys.
The alloying elements can affect both the solidification
process and the cooling from red heat to black heat
where changes to the structure occurs.
Relative
Element
Pearlite Promoting Effectiveness
39.00
Sn
7.90
Mo
5.60
P
4.90
Cu
4.40
Ti
0.44
Mn
0.37
Ni or Cr
Figure 3.
Rev March 2006
You might also like
- 1 TRE300S Chapter 1 To 4 FINALDocument54 pages1 TRE300S Chapter 1 To 4 FINALSamkelo Mduna100% (1)
- Modes of Failure of A Bolted/ Riveted ConnectionDocument4 pagesModes of Failure of A Bolted/ Riveted ConnectionNajib A. CasanNo ratings yet
- NDT of Precipitation Hardened SteelsDocument12 pagesNDT of Precipitation Hardened SteelsMahade Hasan DipuNo ratings yet
- 085 (2006)Document2 pages085 (2006)Uma KoduriNo ratings yet
- Topic 4: Classification, Properties and Applications of S.G. and C.G.Iron S.G.IRONDocument11 pagesTopic 4: Classification, Properties and Applications of S.G. and C.G.Iron S.G.IRONsandeep kumarNo ratings yet
- Additives and Refractories Report in FoundryDocument20 pagesAdditives and Refractories Report in FoundryWalton BangladeshNo ratings yet
- Gray IronDocument4 pagesGray IronLaboratorio CalidadNo ratings yet
- 03.effects of Alloying ElementsDocument8 pages03.effects of Alloying Elementsandrian hermanNo ratings yet
- Toughness Revisited: Effect of Alloying Elements On ToughnessDocument3 pagesToughness Revisited: Effect of Alloying Elements On ToughnessskluxNo ratings yet
- Introduction To Metal - Ferrous AlloyDocument54 pagesIntroduction To Metal - Ferrous AlloydenzNo ratings yet
- Introduction To Metal - Ferrous AlloyDocument54 pagesIntroduction To Metal - Ferrous Alloynailus sa'adahNo ratings yet
- Material EngineeringDocument16 pagesMaterial EngineeringmewmewNo ratings yet
- Effects of Alloying Elements in SteelDocument12 pagesEffects of Alloying Elements in SteelJenna BaileyNo ratings yet
- Metals Used in ProsthodonticsDocument43 pagesMetals Used in ProsthodonticsnavneetkhanNo ratings yet
- Welding Metallurgy and Weldability of Stainless SteelsDocument6 pagesWelding Metallurgy and Weldability of Stainless SteelsoifhiudsnfNo ratings yet
- Materials Engineering ProblemDocument1 pageMaterials Engineering ProblemNieves GuardianNo ratings yet
- Bulacan State University City of Malolos, Bulacan College of EngineeringDocument22 pagesBulacan State University City of Malolos, Bulacan College of EngineeringKenty BoydonNo ratings yet
- L6 Metalic Denture BaseDocument9 pagesL6 Metalic Denture BaseAaaNo ratings yet
- Heat TreatmentDocument33 pagesHeat TreatmentColeNo ratings yet
- Aircraft Maintenance Ferrous MetalsDocument8 pagesAircraft Maintenance Ferrous MetalsRocker HuzzNo ratings yet
- Workshop ReportDocument8 pagesWorkshop ReportAloshNo ratings yet
- Cast Iron Handbook PDFDocument144 pagesCast Iron Handbook PDFsachinguptachdNo ratings yet
- Cast Iron HandbookDocument144 pagesCast Iron Handbooksachinguptachd100% (2)
- Alloys in FPDDocument6 pagesAlloys in FPDharshita parasharNo ratings yet
- Tugas Individu 1 - Radifan Abrar Tahrizi - 1806187732Document6 pagesTugas Individu 1 - Radifan Abrar Tahrizi - 1806187732Radifan AbrarNo ratings yet
- Welding Metallurgy, Part Three: The Inspection ProcessDocument6 pagesWelding Metallurgy, Part Three: The Inspection ProcessGomma AbdallaNo ratings yet
- 21 Chemical Elements and Effects On Steel Mechanical PropertiesDocument12 pages21 Chemical Elements and Effects On Steel Mechanical Propertieshaidv254100% (1)
- Steel and Its AlloyDocument23 pagesSteel and Its AlloyHemang ChopraNo ratings yet
- Effect of Alloying Elements Rev.03 (Ahmed Nabi)Document4 pagesEffect of Alloying Elements Rev.03 (Ahmed Nabi)Harry AnsariNo ratings yet
- Toughness Revisited: The Heat Treat DoctorDocument2 pagesToughness Revisited: The Heat Treat DoctorAgustine SetiawanNo ratings yet
- Alloying Elements ExcelDocument18 pagesAlloying Elements ExcelRavindra ErabattiNo ratings yet
- SG Cast IronDocument3 pagesSG Cast IronSurendra KamalNo ratings yet
- Cast Iron - WikipediaDocument48 pagesCast Iron - WikipediaLAliNo ratings yet
- UNIT 3 Ferrous and Non Ferrous MetalsDocument68 pagesUNIT 3 Ferrous and Non Ferrous MetalsAmutha PSGRKCWNo ratings yet
- CI Casting Failre AnalysisDocument4 pagesCI Casting Failre Analysisdelta lab sangliNo ratings yet
- Assignment 7 MSMDocument6 pagesAssignment 7 MSMJAY KACHANo ratings yet
- Assignment 7 MSMDocument6 pagesAssignment 7 MSMJAY KACHANo ratings yet
- Assignment 7 MSMDocument6 pagesAssignment 7 MSMJAY KACHANo ratings yet
- Microstructure Study of Ferrous and Non Ferrous Alloys Under Various Compositions and Heat Treatment Conditions Lab ReportDocument7 pagesMicrostructure Study of Ferrous and Non Ferrous Alloys Under Various Compositions and Heat Treatment Conditions Lab Reportzrro50% (4)
- LMA Graphs Figs.Document25 pagesLMA Graphs Figs.Jaswanth NalluriNo ratings yet
- Cast Iron: Cast Iron Is A Group of Iron-Carbon Alloys With A Carbon Content Greater ThanDocument8 pagesCast Iron: Cast Iron Is A Group of Iron-Carbon Alloys With A Carbon Content Greater ThanspibluNo ratings yet
- Effect of Alloying Elements On Steel PropertiesDocument5 pagesEffect of Alloying Elements On Steel PropertiesgovimanoNo ratings yet
- Metals Are Combinations of Metallic ElementsDocument4 pagesMetals Are Combinations of Metallic ElementsGuido MorlachettiNo ratings yet
- Lab 2 MaterialDocument22 pagesLab 2 MaterialMon LuffyNo ratings yet
- Summary of AssignmentDocument4 pagesSummary of AssignmentMd Ashraful RahmanNo ratings yet
- Dental Casting and Soldering AlloysDocument40 pagesDental Casting and Soldering AlloysMella Bella WilsonNo ratings yet
- Material Science: Prof. Satish V. KailasDocument12 pagesMaterial Science: Prof. Satish V. KailasAlvin SmithNo ratings yet
- 1996 Bombay Foundry Congress - Inoculation of Grey and Ductile Iron PDFDocument23 pages1996 Bombay Foundry Congress - Inoculation of Grey and Ductile Iron PDFhabibi1328100% (1)
- Ch13 Materials ApplicationsDocument69 pagesCh13 Materials ApplicationsRhanganath ArivudainambiNo ratings yet
- AssignmentDocument11 pagesAssignmentnkar037No ratings yet
- Ch13 Materials ApplicationsDocument63 pagesCh13 Materials ApplicationsThefairman UnkownNo ratings yet
- S.G. Iron : MouldingDocument11 pagesS.G. Iron : MouldingsureshbabuamalaNo ratings yet
- ABSTRACT Lab2 MaterialDocument7 pagesABSTRACT Lab2 MaterialFitri AzraienNo ratings yet
- MetallurgyDocument25 pagesMetallurgyPandu Damay PutraNo ratings yet
- Chemical Composition SteelDocument6 pagesChemical Composition SteelSahil JhambNo ratings yet
- Metallograpy Study of Cast Iron StructureDocument9 pagesMetallograpy Study of Cast Iron StructureMahrukh JavedNo ratings yet
- Cast Iron - WikipediaDocument11 pagesCast Iron - WikipediaBhumikNo ratings yet
- ESDEP Vol0302Document124 pagesESDEP Vol0302aladinmf1No ratings yet
- Metals 2023 2024Document36 pagesMetals 2023 2024Joshua TupasNo ratings yet
- Cast IronDocument12 pagesCast IronCarlos EsparzaNo ratings yet
- To Be EditedDocument6 pagesTo Be EditedSasaleleNo ratings yet
- Selenium cells: The construction, care and use of selenium cells with special reference to the Fritts cellFrom EverandSelenium cells: The construction, care and use of selenium cells with special reference to the Fritts cellNo ratings yet
- Duplex Stainless Steels, A Review After Dss '07 Held in GradoDocument22 pagesDuplex Stainless Steels, A Review After Dss '07 Held in Gradojose.figueroa@foseco.comNo ratings yet
- FundicionDocument397 pagesFundicionjose.figueroa@foseco.comNo ratings yet
- Seven Refractories Aluminio PDFDocument15 pagesSeven Refractories Aluminio PDFjose.figueroa@foseco.comNo ratings yet
- Libro Procedings British Institute 1934Document710 pagesLibro Procedings British Institute 1934jose.figueroa@foseco.comNo ratings yet
- Ni Resist CarbidesDocument11 pagesNi Resist Carbidesjose.figueroa@foseco.comNo ratings yet
- Ambos Ol 2010Document3 pagesAmbos Ol 2010jose.figueroa@foseco.comNo ratings yet
- Dycote 1000 PyrotekDocument1 pageDycote 1000 Pyrotekjose.figueroa@foseco.comNo ratings yet
- Calculo de Dureza Del AceroDocument9 pagesCalculo de Dureza Del Acerojose.figueroa@foseco.comNo ratings yet
- Feeding Systems PDFDocument174 pagesFeeding Systems PDFjose.figueroa@foseco.comNo ratings yet
- Project: Bending BrakeDocument3 pagesProject: Bending Brakejose.figueroa@foseco.comNo ratings yet
- Filtros para HierroDocument4 pagesFiltros para Hierrojose.figueroa@foseco.comNo ratings yet
- Effect of SR, Na, Ca & P On The Castability of Foundry Alloy A356.2Document10 pagesEffect of SR, Na, Ca & P On The Castability of Foundry Alloy A356.2jose.figueroa@foseco.comNo ratings yet
- AllowancesDocument4 pagesAllowancesAlok Dubey100% (1)
- Chunyi ZHAN, Shengshan FENG, Shuzhong XIE, Chunjing LIU, Jiahao LIANG and Yunhua GAODocument6 pagesChunyi ZHAN, Shengshan FENG, Shuzhong XIE, Chunjing LIU, Jiahao LIANG and Yunhua GAOjose.figueroa@foseco.comNo ratings yet
- Gas PurgingDocument2 pagesGas Purgingjose.figueroa@foseco.com100% (1)
- Alimentacion de Hierro y AceroDocument22 pagesAlimentacion de Hierro y Acerojose.figueroa@foseco.comNo ratings yet
- Hard Coating of Tool-Report PDFDocument43 pagesHard Coating of Tool-Report PDFRam TejaNo ratings yet
- 14 330soilstresses PDFDocument24 pages14 330soilstresses PDFClaudio Enrique Nuñez ReyesNo ratings yet
- EIM212 16 EIM212Nov2013Document6 pagesEIM212 16 EIM212Nov2013Priviledge MuzotaNo ratings yet
- Lecture 1 - Ideal Chains PDFDocument8 pagesLecture 1 - Ideal Chains PDFBrandon RawsonNo ratings yet
- MP206 03Document32 pagesMP206 03Weiller MLNo ratings yet
- Manganese SteelDocument4 pagesManganese Steelsaifullah629No ratings yet
- Phase DiagramDocument21 pagesPhase DiagramEdison LimbagaNo ratings yet
- Tutorial Sol 6Document16 pagesTutorial Sol 6Avijit SahaNo ratings yet
- Chapter 4Document24 pagesChapter 4afewerkNo ratings yet
- Book 5Document4 pagesBook 5vasuNo ratings yet
- 2018 05 Ms TranDocument182 pages2018 05 Ms TranSolomon AhimbisibweNo ratings yet
- More or Less Silicone WackerDocument25 pagesMore or Less Silicone WackerAndrea GrajalesNo ratings yet
- Direct and Indirect Band Gap in SemiconductorsDocument6 pagesDirect and Indirect Band Gap in SemiconductorsArvind SharmaNo ratings yet
- Time Monday Registration Opening Ceremony Plenary Lecture, Ogden Coffee BreakDocument19 pagesTime Monday Registration Opening Ceremony Plenary Lecture, Ogden Coffee Breakconmec.crplNo ratings yet
- ME3251 RevisionDocument9 pagesME3251 RevisionJordan NgNo ratings yet
- Numero 35 Art 25 PDFDocument10 pagesNumero 35 Art 25 PDFAhmed GomaaNo ratings yet
- Nano-Fiber Fabric - SNSDocument6 pagesNano-Fiber Fabric - SNSMehdi NaderiNo ratings yet
- M. Tech Surface Coating 2Document18 pagesM. Tech Surface Coating 2Ankit KumarNo ratings yet
- Sound - Ideas - Phononic CrystalsDocument6 pagesSound - Ideas - Phononic Crystalsslysoft.20009951No ratings yet
- Issue No 47 - Yield Strength and Other Near-Elastic PropertiesDocument2 pagesIssue No 47 - Yield Strength and Other Near-Elastic Properties6541646No ratings yet
- Is 883 1994 PDFDocument21 pagesIs 883 1994 PDFkrishnanunniNo ratings yet
- No17c-Bundle-H250x250-R13-BS EP-4InteriorColumn-B2 6, C2 6-Rev3Document50 pagesNo17c-Bundle-H250x250-R13-BS EP-4InteriorColumn-B2 6, C2 6-Rev3Nguyễn Duy QuangNo ratings yet
- Al Busbar 6060 DatasheetDocument1 pageAl Busbar 6060 DatasheetpiirsaluNo ratings yet
- EM-Lab 2-Cold Work and AnnealedDocument7 pagesEM-Lab 2-Cold Work and AnnealedSeneida BiendarraNo ratings yet
- Finite Element Method Analysis of Rectangular Plate With Circular Hole Using Ansys PDFDocument12 pagesFinite Element Method Analysis of Rectangular Plate With Circular Hole Using Ansys PDFVikashNo ratings yet
- 1994 - Stratified Three Phase Flow in Pipes PDFDocument8 pages1994 - Stratified Three Phase Flow in Pipes PDFBonnie JamesNo ratings yet
- A Four-Node Plate Bending Element Based On Mindlin Reissner Plate Theory and A Mixed InterpolationDocument17 pagesA Four-Node Plate Bending Element Based On Mindlin Reissner Plate Theory and A Mixed InterpolationAdnan AhmedNo ratings yet