Professional Documents
Culture Documents
Chem 315 - Lab 7 - Gas Chromatography
Chem 315 - Lab 7 - Gas Chromatography
Uploaded by
kOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem 315 - Lab 7 - Gas Chromatography
Chem 315 - Lab 7 - Gas Chromatography
Uploaded by
kCopyright:
Available Formats
Experiment:
Date:
Gas Chromatography: Distillate
Name
Katheryn Soto
Purpose:
Partners
10/22/25
Drawer No.
Course / Section
CHEM
315/204
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
Katheryn Soto
Course / Section
CHEM
315/204
Approach:
References
Text
Pavia, D.L., Lampman, G.M., Kriz, G.S., Engel, .G.R., 2011, Introduction to Organic Laboratory
Techniques, A Small Scale Approach, GMU Edition, Chem 315/318, Cengage Learning: pp. 817-836
Slayden, S., Stalick, W., Roth, R, 2014, Organic Chemistry Laboratory Manual, 2nd Edition: Pearson
Custom Publishing: pp. 61-62
Web Site URL
Dr Schornicks Website: http:/mason.gmu.edu/~jschorni/gaschromatography
Unknown or Synthesized Compound
CRC Handbook of Chemistry & Physics, 84th Edition, Lide, D.R., Editor-in-chief, 2003-2004, CRC
Press, page no., item no.
Ibid.
Experiment:
Date:
Gas Chromatography: Distillate
Name
10/22/25
Partners
Drawer No.
CHEM
315/204
Katheryn Soto
Proc # 1
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Unk No.
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc # 2
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc # 3
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
10/22/25
Partners
Drawer No.
Course / Section
CHEM
315/204
Katheryn Soto
NOTE: This procedure page is to be numbered and placed in the appropriate
place in the report; normally after the computation of Moles in a Synthesis
experiment. Delete this page if not used.
Proc #
Experimental Reactions / Molar Ratio
Stoichiometric Balanced Equation (Use Structural Formulas)
Example:
Molar Ratio
: 1
Reaction Mechanism
The reaction mechanism may be hand printed in this space or you can use a
chemical drawing program to create the reaction.
http:/www.acdlabs.com
(Chemsketch 10.0 - Free Download)
http:/www.cambridgesoft.com
(ChemDraw 10.0 - Not sure of cost)
DO NOT USE the REACTION Mechanism in the WEB Notes; you must derive
you own mechanism.
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Determine Limiting Reagent
Materials
Desc:
Results
Equipment
Equation Setup:
Proc #
Compute Theoretical Yield
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Results
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Theoretical Yield Summary Table
Course / Section
Results
Desc:
NOTE: If this experiment is not a Synthesis then DELETE this page
For a Synthesis Experiment, use either of the Theoretical Yield Computation Summary Tables
below for most Synthesis experiments. Delete the one not being used.
Balanced
Equation
Molecular
Weight
Mass
(grams)
Moles
Balanced
Equation
Molecular
Weight
Mass
(grams)
Moles
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Partners
10/22/25
Drawer No.
CHEM
315/204
Katheryn Soto
Proc #
Results
Materials
Desc:
Equation Setup:
Course / Section
Equipment
Experiment:
Date:
Gas Chromatography: Distillate
Name
Katheryn Soto
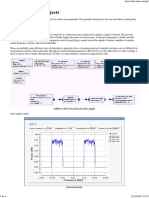
Summary of Results:
Partners
10/22/25
Drawer No.
Course / Section
CHEM
315/204
Experiment:
Date:
Gas Chromatography: Distillate
Name
Katheryn Soto
Analysis & Conclusions:
Partners
10/22/25
Drawer No.
Course / Section
CHEM
315/204
Experiment:
Date:
Gas Chromatography: Distillate
Name
10/22/25
Partners
Drawer No.
Course / Section
CHEM
315/204
Katheryn Soto
Literature Summary (Unknowns, Synthesized Compounds)
Unknown No.
CAS No.
Name (IUPAC)
Synonyms
Melting Point
(oC)
Lit
Exp
Lit
Exp
Lit
Exp
Lit
Exp
Boiling Point
(oC)
Lit
Exp
Lit
Exp
Lit
Exp
Lit
Exp
Refractive Index Lit
(nD20)
Exp
Lit
Exp
Lit
Exp
Lit
Exp
Solubility
(Rel to Water)
Lit
Exp
Lit
Exp
Lit
Exp
Lit
Exp
Density
Rel to Water
Lit
Exp
Lit
Exp
Lit
Exp
Lit
Exp
Molecular
Formula
Structural
Formula
You might also like
- Eln402 Assess 1Document17 pagesEln402 Assess 1api-3546316120% (2)
- A Divine Revelation of The Spirit Realm - Mary Baxter (TheGospel - NG)Document166 pagesA Divine Revelation of The Spirit Realm - Mary Baxter (TheGospel - NG)Mufutau Adegoke100% (5)
- Lab Report Gas Chromatography (GC)Document6 pagesLab Report Gas Chromatography (GC)Nurmazillazainal78% (9)
- m2 Act3 Apply What You Learned Using Dennis and Menaces IepDocument4 pagesm2 Act3 Apply What You Learned Using Dennis and Menaces Iepapi-516574894No ratings yet
- Stevens Institute of Technology Fall 2018: CH117 A, B, C, D, E, F, G, H, I, J, K, L, M: General Chemistry Laboratory IDocument7 pagesStevens Institute of Technology Fall 2018: CH117 A, B, C, D, E, F, G, H, I, J, K, L, M: General Chemistry Laboratory IShawnNo ratings yet
- CDB 3082 Chemical Engineering Lab Iv: - Flame PropagationDocument8 pagesCDB 3082 Chemical Engineering Lab Iv: - Flame PropagationBhinitha ChandrasagaranNo ratings yet
- Gpa 2186-05Document42 pagesGpa 2186-05Guiver Suarez V.No ratings yet
- Melting Point and Boiling Point of Organic CompoundsDocument3 pagesMelting Point and Boiling Point of Organic CompoundsCarlo Aguas Tayag71% (7)
- Guest Speaker Lesson PlanDocument2 pagesGuest Speaker Lesson PlanKelley Thompson NeumannNo ratings yet
- Unit f336 Chemistry Individual Investigation Scheme of Work and Lesson Plan BookletDocument37 pagesUnit f336 Chemistry Individual Investigation Scheme of Work and Lesson Plan BookletArsalan BaigNo ratings yet
- Ebffiledoc - 431download PDF Sat Subject Test Chemistry Crash Course Sat Psat Act College Admission Prep 1St Edition Adrian Dingle Ebook Full ChapterDocument54 pagesEbffiledoc - 431download PDF Sat Subject Test Chemistry Crash Course Sat Psat Act College Admission Prep 1St Edition Adrian Dingle Ebook Full Chapterharry.quinn120100% (1)
- Chem 315 - Lab 10 - Qualitative Organic AnalysisDocument20 pagesChem 315 - Lab 10 - Qualitative Organic Analysisk50% (2)
- 2012 PHD Thesis OrlandoCastellanosDiaz-bitumDocument348 pages2012 PHD Thesis OrlandoCastellanosDiaz-bitumDragos AronNo ratings yet
- Hon Quiz 4 Retake GuideDocument1 pageHon Quiz 4 Retake Guideapi-213645632No ratings yet
- Molecular Modeling of The Thermal Decomposition of Polymers: DOT/FAA/AR-05/32Document17 pagesMolecular Modeling of The Thermal Decomposition of Polymers: DOT/FAA/AR-05/32vytoNo ratings yet
- Wardunit5schedule 1Document1 pageWardunit5schedule 1api-332249032No ratings yet
- Gas Chromatography HomeworkDocument8 pagesGas Chromatography Homeworkegy04nvm100% (1)
- Sma Negeri 3 Semarang: Lesson PlanDocument8 pagesSma Negeri 3 Semarang: Lesson PlanRirin AlchinNo ratings yet
- Chem TestDocument2 pagesChem TestBindu M PillaiNo ratings yet
- Gas Chromatography Research Paper PDFDocument8 pagesGas Chromatography Research Paper PDFngqcodbkf100% (2)
- Access To ChemistryDocument422 pagesAccess To Chemistrythphuongster100% (11)
- Gas Chromatography - Chemistry LibreTextsDocument22 pagesGas Chromatography - Chemistry LibreTextsMohammad NadimNo ratings yet
- Writing An Organic Chemistry Filename: Writing An Organic Chemistry Lab ReportDocument6 pagesWriting An Organic Chemistry Filename: Writing An Organic Chemistry Lab ReportNikon SinghNo ratings yet
- Name: - Gas Laws Webquest: Fahrenheit Celsius KelvinDocument4 pagesName: - Gas Laws Webquest: Fahrenheit Celsius KelvinKamari HillNo ratings yet
- Group 10 Composition Lab ReportDocument7 pagesGroup 10 Composition Lab ReportEthan MuddNo ratings yet
- PDF Basic Gas Chromatography Third Edition Nicholas H Snow Ebook Full ChapterDocument42 pagesPDF Basic Gas Chromatography Third Edition Nicholas H Snow Ebook Full Chapterjoseph.murphy879100% (5)
- Simulation of Reactive Distillation ColumnDocument6 pagesSimulation of Reactive Distillation ColumnthanhndbNo ratings yet
- Chemical Engineering FundamentalsDocument15 pagesChemical Engineering FundamentalsFarwa MalikNo ratings yet
- Impact of Different Li-Ion Cell Test Conditions On Thermal RunawayDocument13 pagesImpact of Different Li-Ion Cell Test Conditions On Thermal RunawayΤσιμπινός ΣπύροςNo ratings yet
- MEC 205 Thermodynamics and Fluid LabDocument75 pagesMEC 205 Thermodynamics and Fluid LabChirithoti Sai CharanNo ratings yet
- Course Syllabus FormatDocument9 pagesCourse Syllabus FormatAleks OpsNo ratings yet
- TCEQ Final Report Oil Gas Storage Tank ProjectDocument73 pagesTCEQ Final Report Oil Gas Storage Tank ProjectC.E. Ishmeet SinghNo ratings yet
- Report Contribution Form: Che Laboratory CompositionDocument18 pagesReport Contribution Form: Che Laboratory CompositionEthan MuddNo ratings yet
- Sample Syllabus 2Document4 pagesSample Syllabus 2JanNo ratings yet
- Chem 315 - Lab 5 - Gas Chromatography - AcetatesDocument13 pagesChem 315 - Lab 5 - Gas Chromatography - AcetateskNo ratings yet
- Properties Data For Pure Materials ComponentsDocument13 pagesProperties Data For Pure Materials ComponentsmoheauNo ratings yet
- ChemistryDocument56 pagesChemistryJaxon Isack90% (10)
- 080312080X 1-100Document100 pages080312080X 1-100phuongthu15267% (3)
- Lab Module: Composition Group: 4 Date: 10/18/2019Document11 pagesLab Module: Composition Group: 4 Date: 10/18/2019Ethan MuddNo ratings yet
- Laboratory Safety and Assessment FormDocument5 pagesLaboratory Safety and Assessment FormKPNo ratings yet
- Gas ChromatographyDocument5 pagesGas ChromatographyassertivailNo ratings yet
- Ruzicka CP Estimation MethodDocument11 pagesRuzicka CP Estimation MethodAndreea Cristina PetcuNo ratings yet
- Investigating Gas Chromatography: Figure 1 Sample Gas ChromatogramDocument9 pagesInvestigating Gas Chromatography: Figure 1 Sample Gas ChromatogramPanduka EkanayakeNo ratings yet
- Syllabus Chem405 Baker 3tDocument9 pagesSyllabus Chem405 Baker 3tTha KantanaNo ratings yet
- Applications and Uncertainties Associated With Measurements Using FTIR SpectrometryDocument37 pagesApplications and Uncertainties Associated With Measurements Using FTIR Spectrometrysalekojic5332No ratings yet
- Chemistry Lab-I: Atomic Structure, Bonding, General Organic Chemistry and Aliphatic HydrocarbonsDocument112 pagesChemistry Lab-I: Atomic Structure, Bonding, General Organic Chemistry and Aliphatic HydrocarbonsyashitaNo ratings yet
- Calor Equipo SDocument604 pagesCalor Equipo SchylergNo ratings yet
- J Ijhydene 2018 04 208Document10 pagesJ Ijhydene 2018 04 208KASHVINWARMA A/L BASKARANNo ratings yet
- 03 VP MitigationDocument52 pages03 VP Mitigationario13No ratings yet
- Thermochemical Properties Estimation For Biodiesel Related MixturesDocument11 pagesThermochemical Properties Estimation For Biodiesel Related Mixturesalberth_carantónNo ratings yet
- Open Notebook Science Melting Point Data BookDocument699 pagesOpen Notebook Science Melting Point Data Bookl7aniNo ratings yet
- Experiments in Analytical ChemistryDocument38 pagesExperiments in Analytical ChemistryBan AliNo ratings yet
- Installation and OverviewDocument9 pagesInstallation and OverviewSharon C. LunaNo ratings yet
- General Chemistry Laboratory Manual ShortDocument20 pagesGeneral Chemistry Laboratory Manual Shortzekarias wondafrashNo ratings yet
- Formal Lab Report FormatDocument10 pagesFormal Lab Report FormatrebbiegNo ratings yet
- Gas Testing LabDocument21 pagesGas Testing LabBankaram ChoudharyNo ratings yet
- CHM 205-Fos 205 Lab ManualDocument140 pagesCHM 205-Fos 205 Lab ManualMostafa AbdelrahmanNo ratings yet
- 6-Ok-Derivatizatio, GC MS, Organic Acids مهم جداDocument366 pages6-Ok-Derivatizatio, GC MS, Organic Acids مهم جداfarkad rawiNo ratings yet
- Department of Chemical Engineering: (An Autonomous Institution Affiliated To JNTUK, AP)Document6 pagesDepartment of Chemical Engineering: (An Autonomous Institution Affiliated To JNTUK, AP)sagarNo ratings yet
- Gas GCDocument15 pagesGas GCEko FirmansyahNo ratings yet
- Methods For Sampling Biogas and Biomethane For Assessment of The Gas Chemical and Physical PropertiesDocument2 pagesMethods For Sampling Biogas and Biomethane For Assessment of The Gas Chemical and Physical PropertiesJuan Enrique Vergel MarinNo ratings yet
- Green Energetic MaterialsFrom EverandGreen Energetic MaterialsTore BrinckNo ratings yet
- Process Intensification Technologies for Green Chemistry: Engineering Solutions for Sustainable Chemical ProcessingFrom EverandProcess Intensification Technologies for Green Chemistry: Engineering Solutions for Sustainable Chemical ProcessingKamelia BoodhooNo ratings yet
- Modern Thermodynamics: From Heat Engines to Dissipative StructuresFrom EverandModern Thermodynamics: From Heat Engines to Dissipative StructuresNo ratings yet
- Chem 315 - Lab 10 - Qualitative Organic AnalysisDocument20 pagesChem 315 - Lab 10 - Qualitative Organic Analysisk50% (2)
- Chem 315 - Lab 6 - Simple and Fractional DistilationDocument27 pagesChem 315 - Lab 6 - Simple and Fractional DistilationkNo ratings yet
- Chem 315 - Lab 8 - Synth of T Pent ChlorideDocument22 pagesChem 315 - Lab 8 - Synth of T Pent Chloridek100% (1)
- Chem 315 - Extraction of CaffeineDocument18 pagesChem 315 - Extraction of CaffeinekNo ratings yet
- Chem 315 - Lab 5 - Gas Chromatography - AcetatesDocument13 pagesChem 315 - Lab 5 - Gas Chromatography - AcetateskNo ratings yet
- Chem 315 - Lab 2 - RecrystallizationDocument14 pagesChem 315 - Lab 2 - RecrystallizationkNo ratings yet
- Test #4 Study GuideDocument1 pageTest #4 Study GuidekNo ratings yet
- Test #3 Study Guide PDFDocument1 pageTest #3 Study Guide PDFkNo ratings yet
- Chem 315 - Lab 1 - Melting Point and Refractive IndexDocument10 pagesChem 315 - Lab 1 - Melting Point and Refractive IndexkNo ratings yet
- Chapter 4 Study Guide PDFDocument57 pagesChapter 4 Study Guide PDFkNo ratings yet
- Chapter 12 Study Guide PDFDocument44 pagesChapter 12 Study Guide PDFkNo ratings yet
- No.1-3 Test CritiquesDocument21 pagesNo.1-3 Test CritiquesYusef John NebridaNo ratings yet
- Thesis (Live in Relationship)Document20 pagesThesis (Live in Relationship)Shaynie Tañajura DuhaylungsodNo ratings yet
- Smart Goals TemplateDocument3 pagesSmart Goals Templateapi-257746864No ratings yet
- Degi DishDocument1 pageDegi DishFuad TalNo ratings yet
- Donjon: Town Name: Town Size: Normalize? Walled? Environment: Coastal? River? Race: Culture: Save As PNGDocument2 pagesDonjon: Town Name: Town Size: Normalize? Walled? Environment: Coastal? River? Race: Culture: Save As PNG2ooneyNo ratings yet
- Yob 2014Document564 pagesYob 2014chuNo ratings yet
- Federico Kauffmann Doig - The Chachapoyas CultureDocument320 pagesFederico Kauffmann Doig - The Chachapoyas CultureChachapoyaNo ratings yet
- Anna GorskaDocument10 pagesAnna GorskaRey-ann Kyle L. ManansalaNo ratings yet
- SHS Science Laboratory Project ProposalDocument5 pagesSHS Science Laboratory Project ProposalJahna Liel Alipio100% (2)
- Noor Ul Bayaan EnglishDocument115 pagesNoor Ul Bayaan EnglishSemiu KareemNo ratings yet
- The Little Red HenDocument3 pagesThe Little Red HenCarylle silveoNo ratings yet
- 4Ms of Production ManagementDocument84 pages4Ms of Production ManagementVincent L. SantiagoNo ratings yet
- Gnuradio ProjectosDocument4 pagesGnuradio ProjectosartovolastiNo ratings yet
- Numbers and PolynomialsDocument52 pagesNumbers and PolynomialsAhmet KayaNo ratings yet
- Smrithi A: ContactDocument3 pagesSmrithi A: ContactAshwathi NarayananNo ratings yet
- CS 108 - Human Computer InteractionDocument17 pagesCS 108 - Human Computer InteractionWILSON LLAVENo ratings yet
- Project Camelot Marcia Schafer LA Awake and Aware Conference TranscriptDocument23 pagesProject Camelot Marcia Schafer LA Awake and Aware Conference TranscriptChristian UFO IlluminatiNo ratings yet
- Inisial A-H Sertifikat Belajar Bersama (28-31 Mei 2024)Document89 pagesInisial A-H Sertifikat Belajar Bersama (28-31 Mei 2024)Dendy RahmatNo ratings yet
- Manila Int Climate PDFDocument3 pagesManila Int Climate PDFLevy SorianoNo ratings yet
- Graduation Invocation PrayerDocument5 pagesGraduation Invocation PrayerDarlene AradanasNo ratings yet
- People Vs SendaydiegoDocument22 pagesPeople Vs SendaydiegoryuseiNo ratings yet
- 2023 Biology f3 p2 Qs t2 Exam Teacher - Co .KeDocument12 pages2023 Biology f3 p2 Qs t2 Exam Teacher - Co .Kealooben2100% (1)
- Charette ProcedureDocument1 pageCharette ProcedureAnnie PrinceNo ratings yet
- Ap ch4 SQ PDFDocument17 pagesAp ch4 SQ PDFAdeel AhmedNo ratings yet
- 2.1 State and Essentials of StateDocument55 pages2.1 State and Essentials of Stateleeni liniNo ratings yet
- Liste Jeux PS Vita Boites PALDocument1 pageListe Jeux PS Vita Boites PALDenis Alibor MajhenNo ratings yet