Professional Documents
Culture Documents
Mazabraud Syndrome: American Journal of Orthopedics (Belle Mead, N.J.) July 2012
Mazabraud Syndrome: American Journal of Orthopedics (Belle Mead, N.J.) July 2012
Uploaded by
irvanieOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mazabraud Syndrome: American Journal of Orthopedics (Belle Mead, N.J.) July 2012
Mazabraud Syndrome: American Journal of Orthopedics (Belle Mead, N.J.) July 2012
Uploaded by
irvanieCopyright:
Available Formats
See
discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/230672249
Mazabraud syndrome
ARTICLE in AMERICAN JOURNAL OF ORTHOPEDICS (BELLE MEAD, N.J.) JULY 2012
Source: PubMed
CITATIONS
READS
43
3 AUTHORS, INCLUDING:
David Dreizin
University of Maryland Medical Center/S
27 PUBLICATIONS 61 CITATIONS
SEE PROFILE
Available from: David Dreizin
Retrieved on: 18 February 2016
Imaging Series
Mazabraud Syndrome
David Dreizin, MD, Charles Glenn, MD, and Jean Jose, DO
Abstract
Mazabraud syndrome is defined by an association
between fibrous dysplasia and intramuscular myxomas,
and is thought to fall within the spectrum of protean
disorders of syndromic fibrous dysplasia that includes
McCune-Albright syndrome. In this article, we briefly
discuss the history and evolution of the term Mazabraud
syndrome, then detail the spectrum of imaging findings
as they relate to the variable pathology and clinical presentations that may be encountered. Differential diagnostic considerations and potential diagnostic pitfalls
are also considered.
he term fibrous dysplasia was coined in 1938 by
Lichtenstein1 to describe a nonheritable, aberrant development of fibro-osseous tissue replacing normal cancellous bone. The word myxoma,
which describes a benign mesenchymal tumor of stellate
and spindle cells within a polysaccharide rich avascular
stroma, is derived from the Greek word myxo, meaning
mucus or slime, first used by Virchow2 to characterize the
tissue comprising Whartons Jelly in the umbilical cord.
Myxomas were recognized as distinct lesions by Stout3
in 1948. While a link was first observed in the German
literature by Henschen4 in 1926, it was not until 1967 that
Mazabraud,5 a French physician, re-examined the association between these 2 entities in their presently defined
forms. In 1971, Wirth and colleagues,6 subsequently
described 11 cases of intramuscular myxoma and fibrous
dysplasia for the first time in the English literature. Since
then, more than 68 cases of what has come to be known
as Mazabraud syndrome have been reported.7
tomic distribution of the disorder. The same mutation
is also responsible for the manifestations of McCuneAlbright syndrome,8 in which fibrous dysplasia is classically accompanied by endocrine abnormalities (eg, precocious puberty, thyroid dysfunction) and caf au lait spots.
Wirth and colleagues6 described 5 cases of McCuneAlbright syndrome among 11 patients with Mazabraud
syndrome in 1971. However, evidence linking fibrous
dysplasia and myxomas remains observational to this
day. There are a number of distinct features that support
the association. Mazabraud syndrome appears to lie
on the severe end of the spectrum of fibrous dysplasia
expression; nonsyndromic fibrous dysplasia is monostotic 75% of the time,9 but myxomas are seen with the
polyostotic form in the great majority of cases10 and are
themselves multiple in approximately 70%.7
While there have been no reported instances of
malignant degeneration of myxoma, 4 cases of sarcomatous transformation of fibrous dysplasia in patients
with Mazabraud syndrome have been reported in the
literature.7 This constitutes a significantly higher incidence of malignant transformation than that reported
in the non-syndromic form of fibrous dysplasia, which
is estimated at 0.5%. Although fibrous dysplasia is rare,
representing only 7% of benign bone lesions,11 Ireland
and colleagues12 identified fibrous dysplasia in 3 out
of a series of 58 patients with myxomas. This may be
a small proportion, but it is clearly much more than
would be expected in the general population.
Curiously, whereas myxomas may occur in any number of locations including the heart and genitourinary
tract, and are intramuscular in only 17% of cases,13 the
majority of those described in Mazabraud syndrome
originated within muscle, typically occurring in close
proximity to the most severely affected bones.14 Finally,
while fibrous dysplasia in isolation more commonly
afflicts males, for unclear reasons, approximately 70%
of Mazabraud patients are women.10
AJO
DO NOT COPY
Pathophysiology
Within the past decade, an activating post-zygotic mutation in the GNAS1 gene encoding a G-protein involved
in cell proliferation has been identified as causative of
fibrous dysplasia, with the resulting mosaic distribution
of the mutation accounting for the widely variable anaDr. Dreizin is Chief Resident, Department of Radiology, Dr.
Glenn is Resident, Department of Pathology, and Dr. Jose
is Assistant Professor of Clinical Radiology, Musculoskeletal
Imaging Section, Department of Radiology, University of Miami
Miller School of Medicine, Miami, Florida.
Address correspondence to: David Dreizin, MD, University of
Miami Miller School of Medicine, Jackson Memorial Hospital,
Department of Radiology, West Wing 279, 1611 N.W. 12th
Avenue, Miami, FL 33136 (tel, 305-585-8178; fax, 305-585-5743;
e-mail, ddreizin@med.miami.edu).
Am J Orthop. 2012;41(7):332-335. Copyright Quadrant HealthCom
Inc. 2012. All rights reserved.
332 The American Journal of Orthopedics
Clinical And Imaging Presentation
The natural history of Mazabraud syndrome is quite
variable. Myxoma may be diagnosed in adolescents
or young adults with symptomatic polyostotic fibrous
dysplasia.15 More commonly though, myxomas present
as enlarging, either painful or painless masses in older
patients (mean age of diagnosis, 46 years), in whom
fibrous dysplasia is incidentally found at imaging.10
Mazabraud syndrome is a rare but probably underreported entity; given the late presentation, it is likely
that many cases go unrecognized. An appreciation of
the varying radiographic appearances of both fibrous
www.amjorthopedics.com
Copyright AJO 2012. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher.
D. Dreizin et al
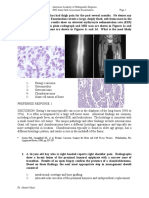
Figure 1. Large field of view coronal T1 (A), STIR (B), and postcontrast fat suppressed T1 weighted (C) MR images of bilateral thighs
demonstrate typical findings of polyostotic fibrous dysplasia. The heterogeneous intramedullary signal intensity in the proximal femurs
reflects a disorganized arrangement of low T1 signal intensity fibrovascular tissue and poorly formed osteoid, interspersed with cystic
components, fat, and hemorrhage, which confer high signal intensity on T2. A typical rim-like enhancement pattern is also demonstrated. There is multifocal osseous scalloping and expansile remodeling, with characteristic Shepherds crook deformity (curved arrows).
Note the circumscribed soft tissues mass along the medial margin of the left femoral neck, which is isointense to skeletal muscle on
T1, uniformly hyperintense to skeletal muscle on STIR, and demonstrates minimal peripheral enhancement, in keeping with myxoma
(straight arrow) in this patient with Mazabraud syndrome.
AJO
DO NOT COPY
A
Figure 2. Axial fat suppressed T2 weighted (A), T1 weighted (B), and post contrast fat suppressed T1 weighted (C). There are 3 solid
circumscribed soft tissues masses within the left hip adductor, short external rotator and gluteal muscles, reflecting myxomas (straight
arrows). The tumors demonstrate uniform fluid-like signal, hyperintense to skeletal muscle on T2, isointense to skeletal muscle on
T1, and with peripheral enhancement. Polyostotic fibrous dysplasia involving the ischium and proximal femur is denoted by asterisks.
dysplasia and myxomas is essential.
Fibrous dysplasia occurs in a wide range of locations
with no predilection for the appendicular or axial skeleton. The femur, humerus, tibia, phalanges, ribs, skull,
and ischium may be involved.11,15 On plain radiographs,
diffusely increased ground glass density is typically seen
and may be associated with scalloping or expansile
remodeling. The findings reflect intramedullary conglomerations of fibrovascular tissue and poorly formed
osteoid. Varying proportions of these histologies correspond to different signal intensities on magnetic
resonance imaging (MRI).16 A high degree of collagen
or bony trabeculae confers hypointensity on both T1
and T2 weighted images, as do internal septations, and
characteristic sclerotic rinds of reactive bone (Figure
1A).15,16 Rests of cartilage occur within fibrous dysplasia with some frequency, with resulting calcifications
that may appear hypointense on all sequences. Lesions
are hyperintense to fat on T2WI in 60% of cases, with
www.amjorthopedics.com
high signal seen in the presence of cystic components,
fat, or hemorrhage, and inversely related to the degree
of cellularity (Figure 1B).15,16 On post-contrast images,
enhancement is usually rim-like,15 but may also be
heterogeneous or solid (Figure 1C). Aneurysmal bone
cysts may be seen in lesions of fibrous dysplasia and
should be suspected when fluid-fluid levels are present.15,16 MRI defines the extent of fibrous dysplasia
more accurately than radiography, however, while cortical disruption and adjacent soft tissue involvement
should raise the possibility of low grade osteosarcoma
or sarcomatous degenration, soft tissue extension was
described in nearly a third of cases of fibrous dysplasia
in one series.15 In rare cases, histopathologic correlation
may be necessary to confidently exclude malignancy.
Bone scan may demonstrate confluent asymmetric
areas of high uptake,10 and increased activity has also
been described on fluorodeoxyglucose position emission
tomography (FDG-PET).11,17
July 2012 333
Copyright AJO 2012. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher.
Mazabraud Syndrome
Figure 3: Short axis gray scale (A) and color doppler (B) ultrasound images show a solid circumscribed soft tissue mass within a left hip
adductor muscle reflecting a myxoma. The lesion is heterogenously hypoechoic, and demonstrates a relative lack of internal vascularity
on color doppler sampling (B).
AJO
DO NOT COPY
Figure 4. A 40x light microscopy image (A) of a hemotoxylin-eosin stained slide from a resected intramuscular mass in the same
patient reveals hypocellular tissue with bland stellate cells (straight arrow) and spindle cells (curved arrow) within a polysaccharide rich
myxoid stroma. The appearance is diagnostic of myxoma, confirming the diagnosis of Mazabraud syndrome. At 4x magnification (B),
the hypovascular myxoma (black asterisk) has a well-defined border with the adjacent skeletal muscle (white asterisk), corresponding
with the pseudocapsules seen on MR.
As fibrous dysplasia commonly affects the femur,
it stands to reason that the thigh is the most common
location of myxomas in Mazabraud syndrome.7 On
MRI, myxomas typically appear as well defined ovoid
intramuscular masses that are low signal intensity on T1,
with homogeneous fluid-like signal on T2WI (Figure 2).
Myxomas may demonstrate avid heterogeneous enhancement in some cases, reflecting focal areas of hypervascularity, or may contain enhancing fibrous septa
within a matrix of nonenhancing solid myxoid tissue.9,18
Additional specific features of myxoma include a rind of
adipose tissue, seen as high signal on T1 weighted images,
and hyperintensity on fluid sensitive sequences involving
the adjacent muscle, corresponding with reactive muscular atrophy and edema.18 The masses have an average size
of 5 cm and typically do not come into contact with adjacent bone.18 The tumors are usually hypoattenuating on
computed tomography and hypo-echoic on ultrasound
(Figure 3). A rind of adipose tissue is often seen and is
attributable to atrophy from a slowly expanding mass.18
Myxomas are hypometabolic and demonstrate lack of
activity on PET.11
334 The American Journal of Orthopedics
Differential Diagnosis and Pitfalls
Differential diagnostic considerations of myxomatous
masses includes sarcoma with myxoid degeneration, particularly liposarcoma.9,18 Metastatic lesions, lymphoma,
peripheral nerve sheath tumors, and degenerating desmoids are other possibilities. Intramuscular lymphoma
is rarely focal and usually spans multiple muscle compartments.19 Metastatic lesions are distinguished by a
greater degree of peri-tumoral high signal.14 The presence of fibrous dysplasia greatly narrows the differential
and while biopsy of myxoma is nearly ubiquitous in the
literature, Mazabraud syndrome should be a principal
consideration in these cases pending histopathologic confirmation (Figure 4).
Due to the rarity of this syndrome, delayed diagnosis and unnecessary biopsy of additionally discovered myxomas is common. Misdiagnosis may lead to
the initiation of inappropriate therapy for sarcoma.9
Management involves continued radiographic and clinical follow-up of fibrous dysplastic lesions, particularly
when involving weight-bearing bones. Pain and swelling
suggests a complication such as pathologic fracture or
www.amjorthopedics.com
Copyright AJO 2012. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher.
D. Dreizin et al
sarcomatous degeneration, the former treated with excision and curettage, as well as fixation in amenable cases.
MRI may be particularly useful in the follow-up of
treated lesions.16 Myxomas may grow to enormous sizes
and have a propensity to recur.20 Painful or bothersome
masses can be removed with wide excision.
Conclusion
In conclusion, knowledge of the association between
fibrous dysplasia and myxomas known as Mazabraud
syndrome and the individual radiographic appearances of
these entities is important in guiding appropriate management and preventing incorrect or delayed diagnosis.
Authors Disclosure
Statement
The authors report no actual or potential conflict of interest in relation to this article.
References
1. Lichtenstein L. Polyostotic fibrous dysplasia. Arch Surg. 1938;36(5):874-898.
2. Virchow R. Die cellularpathologie in ihrer Beegrundung auf physiologische
and pathologische Gewebelehre. Berlin, Germany, Verlag von August
Hirschwald. 1871:563.
3. Stout AP. Myxoma, the tumor of primitive mesenchyme. Ann Surg.
1948;127(4):706-719.
4. Henschen F. Fall von ostitis fibrosa mit multiplen tumoren in der umgebenden muskulatur. Verh Dtsch Ges Pathol. 1926;21:93-97.
5. Mazabraud A, Semat P, Roze R. A propos de lassociation de fibro-
myxomes des tissus mous a la dysplasie fibreuse des os. Presse Med.
1967;75:2223-2228.
6. Wirth W, Leavitt D, Enzinger FM. Multiple intramuscular myxomas. Another
extraskeletal manifestation of fibrous dysplasia. Cancer. 1971;27(5):1167-1173.
7. Zoccali C, Teori, G, Prencipe, U, Erba, F. Mazabrauds syndrome: a new
case and review of the literature. Int Orthop. 2009;33(3):605-610.
8. Cohen MM Jr, Howell RE. Etiology of fibrous dysplasia and McCuneAlbright syndrome. Int J Oral Maxillofac Surg. 1999;28(5):366-371.
9. Iwasko N, Steinbach, L, Disler, D, et al. Imaging findings in Mazabrauds
syndrome: seven new cases. Skeletal Radiol. 2002;31(2):81-87.
10. McLaughlin A, Stalley P, Magee M, Soper J, Van der Wall H. Correlative
imaging in an atypical case of Mazabraud syndrome. AJR Am J Roentgenol.
2007;189(6):W353-356.
11. Case DB, Chapman CN Jr, Freeman JK, Polga JP. Best cases from
AFIP: Atypical presentation of polyostotic fibrous dysplasia with myxoma
(Mazabraud syndrome). Radiographics. 2010;30(3):827-832.
12. Ireland DC, Soule EH, Ivins JC. Myxoma of somatic soft tissues. A report
of 58 patients, 3 with multiple tumors and fibrous dysplasia of bone. Mayo
Clin Proc. 1973;48(6):401-410.
13. Enzinger F. Intramuscular myxoma; a review and follow-up study of 34
cases. Am J Clin Pathol. 1965;43:104-113.
14. Kransdorf MJ, Murphey MD. Diagnosis please. Case 12: Mazabraud syndrome. Radiology. 1999;212(1):129-132.
15. Jee WH, Choi KH, Choe BY, Park JM, Shinn KS. Fibrous dysplasia:
MR imaging characteristics with radiopathologic correlation. AJR Am J
Roentgenol. 1996;167(6):1523-1527.
16. Shah ZK, Peh WC, Koh WL, Shek TW. Magnetic resonance imaging
appearances of fibrous dysplasia. Br J Radiol. 2005;78(936):1104-1115.
17. Fitzpatrick KA, Taljanovic MS, Speer DP, et al. Imaging findings of fibrous
dysplasia with histopathologic and intraoperative correlation. AJR Am J
Roentgenol. 2004;182(6):1389-1398.
18. Bancroft LW, Kransdorf MJ, Menke DM, OConnor MI, Foster WC.
Intramuscular myxoma: characteristic MR imaging features. AJR Am J
Roentgenol. 2002;178(5):1255-1259.
19. Lee VS, Martinez S, Coleman RE. Primary muscle lymphoma: clinical and
imaging findings. Radiology. 1997;203(1):237-244.
20. Szendri M, Rahty P, Antal I, Kiss J. Fibrous dysplasia associated with
intramuscular myxoma (Mazabrauds syndrome): a long-term follow-up of
three cases. J Cancer Res Clin Oncol. 1998;124(7):401-406.
AJO
DO NOT COPY
This paper will be judged for the Resident Writers Award.
www.amjorthopedics.com
July 2012 335
333
Copyright AJO 2012. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher.
You might also like
- The Borax ConspiracyDocument0 pagesThe Borax Conspiracyapi-240541707No ratings yet
- Hand and Wrist AAOS MCQ. 2019 PDFDocument158 pagesHand and Wrist AAOS MCQ. 2019 PDFantonio ferrer100% (2)
- Drug StudyDocument13 pagesDrug StudyGi Ey ElNo ratings yet
- Fruit Extract (Capsicum Frutescens) As Coackroaches KillerDocument17 pagesFruit Extract (Capsicum Frutescens) As Coackroaches KillerKristine Jill Fuentes LlegueNo ratings yet
- Calcific Tendinitis of The Rotator Cuff State of The Art in Diagnosis PDFDocument8 pagesCalcific Tendinitis of The Rotator Cuff State of The Art in Diagnosis PDFItai IzhakNo ratings yet
- IB Psychology Revision Guide - FINALDocument108 pagesIB Psychology Revision Guide - FINALDhyan Valle93% (14)
- Blood InvestigationDocument70 pagesBlood InvestigationRohit RaiNo ratings yet
- Collagenous Fibroma (Desmoplastic Fibroblastoma) : Clinical, Pathological and Imaging FindingsDocument41 pagesCollagenous Fibroma (Desmoplastic Fibroblastoma) : Clinical, Pathological and Imaging FindingsAnonymous bE4VegCcNo ratings yet
- Secondary Aneurysmal Bone Cyst in Fibrous Dysplasia of The Proximal FemurDocument5 pagesSecondary Aneurysmal Bone Cyst in Fibrous Dysplasia of The Proximal FemurdamdamienaNo ratings yet
- Becker Muscular DystrophyDocument9 pagesBecker Muscular DystrophyManusama HasanNo ratings yet
- Lymphatic Malformation (Cystic Hygroma)Document12 pagesLymphatic Malformation (Cystic Hygroma)afrisiammyNo ratings yet
- Fribous Displasia 234Document9 pagesFribous Displasia 234ManuelGonzalezGaitanoNo ratings yet
- Osteo Sarko Ma 1Document10 pagesOsteo Sarko Ma 1merizNo ratings yet
- Jop 12797 PDFDocument7 pagesJop 12797 PDFNada lathifahNo ratings yet
- Osteocondroma & OsteochondromatosisDocument24 pagesOsteocondroma & OsteochondromatosisrazalihamidNo ratings yet
- 08 - MSK Acr 2010Document16 pages08 - MSK Acr 2010ANAS ALINo ratings yet
- Transverse Myelitis: Differential DiagnosisDocument14 pagesTransverse Myelitis: Differential DiagnosissekiannNo ratings yet
- Boroumand 2016Document3 pagesBoroumand 2016Winston FontesNo ratings yet
- Osteoid Osteoma: BackgroundDocument11 pagesOsteoid Osteoma: BackgroundputriNo ratings yet
- Congenital Rib AnomaliesDocument7 pagesCongenital Rib AnomaliesAlberto MedinNo ratings yet
- Neurofibromatosis Etiology, Commonly Encountered Spinal Deformities and Surgical TreatmentDocument10 pagesNeurofibromatosis Etiology, Commonly Encountered Spinal Deformities and Surgical TreatmentChristoforos KampouridisNo ratings yet
- Surg Recon F&ADocument13 pagesSurg Recon F&AJeffNo ratings yet
- Limb Salvage Procedure For Liposarcoma of The Left Thigh: A CaseDocument3 pagesLimb Salvage Procedure For Liposarcoma of The Left Thigh: A CaseL Yudhantoro YudhaNo ratings yet
- AAOS2002 TumorDocument59 pagesAAOS2002 TumorHizkyas KassayeNo ratings yet
- Ctoij MS Id 555925Document8 pagesCtoij MS Id 555925lucasnegromonte0001No ratings yet
- Sindrome de LarsenDocument8 pagesSindrome de LarsenReyes Aguilar SalinasNo ratings yet
- Orphanet Journal of Rare Diseases: Mccune-Albright SyndromeDocument12 pagesOrphanet Journal of Rare Diseases: Mccune-Albright Syndromesagarjangam123No ratings yet
- Ijms 19 00311Document26 pagesIjms 19 00311Hidayat ArifinNo ratings yet
- Mi OsteoartritisDocument23 pagesMi OsteoartritisJULISSA MARICIELO GONZALEZ DE LA CRUZNo ratings yet
- Osteonecrosis of The Femoral Head: Evaluation and TreatmentDocument10 pagesOsteonecrosis of The Femoral Head: Evaluation and TreatmentHector Ulises Quintanilla SotoNo ratings yet
- 07 BMDocument9 pages07 BMMajid KhanNo ratings yet
- Fibrous Dysplasia - Radiology Reference Article - RadiopaediaDocument18 pagesFibrous Dysplasia - Radiology Reference Article - RadiopaediaManuelGonzalezGaitanoNo ratings yet
- Case Report 1Document11 pagesCase Report 1Ussy FmsNo ratings yet
- Review Article Enchondromatosis: Insights On The Different SubtypesDocument13 pagesReview Article Enchondromatosis: Insights On The Different SubtypesCassNo ratings yet
- Low-Grade Central Osteosarcoma Versus Fibrous Dysplasia: Carrie Y. Inwards, MDDocument6 pagesLow-Grade Central Osteosarcoma Versus Fibrous Dysplasia: Carrie Y. Inwards, MDManuelGonzalezGaitanoNo ratings yet
- Journal of AMCDocument6 pagesJournal of AMCMarko SimonceliNo ratings yet
- Review Article: Diagnosing Arthrogryposis Multiplex Congenita: A ReviewDocument7 pagesReview Article: Diagnosing Arthrogryposis Multiplex Congenita: A ReviewnuriajunitNo ratings yet
- Descripon Viejita Pero BuenaDocument16 pagesDescripon Viejita Pero BuenaJorge Octavio Ibarra ChairezNo ratings yet
- 5 MSDocument37 pages5 MSKim GonzalesNo ratings yet
- Giant Cell Tumor of The Tendon Sheath: A Rare Case in The Left Knee of A 15-Year-Old BoyDocument3 pagesGiant Cell Tumor of The Tendon Sheath: A Rare Case in The Left Knee of A 15-Year-Old BoyFriadi NataNo ratings yet
- Modpathol 201792 ADocument11 pagesModpathol 201792 AMariana UsatiiNo ratings yet
- Cervical 1Document5 pagesCervical 1hendrayatiranyNo ratings yet
- Magnetic Resonance Imaging of Diabetic Foot Complications: RticleDocument12 pagesMagnetic Resonance Imaging of Diabetic Foot Complications: RticlenessimNo ratings yet
- Jurnal 0steosarcoma 2Document27 pagesJurnal 0steosarcoma 2Rika Irena DwiputriNo ratings yet
- Pearson Syndrome - A Multisystem Mitochondrial Disease With Bone Marrow Failure - PMCDocument23 pagesPearson Syndrome - A Multisystem Mitochondrial Disease With Bone Marrow Failure - PMCLeslie CordovaNo ratings yet
- Osteomielitis PDFDocument14 pagesOsteomielitis PDFAngela TorresNo ratings yet
- Ovarian Myeloid Sarcoma, Gynecol Oncol Rep 2023Document7 pagesOvarian Myeloid Sarcoma, Gynecol Oncol Rep 2023Semir VranicNo ratings yet
- Spinal Cord EnhancementDocument3 pagesSpinal Cord EnhancementGabriel PinillaNo ratings yet
- TB Tulang 3 PDFDocument6 pagesTB Tulang 3 PDFkeysmeraudjeNo ratings yet
- Activation of T-Cells-Major Key To Multiple SclerosisDocument14 pagesActivation of T-Cells-Major Key To Multiple Sclerosisbabansub9542No ratings yet
- Aneurysmal Bone CystDocument5 pagesAneurysmal Bone CystAustine OsaweNo ratings yet
- Vascular Malformations: A Review: Joshua A. Cox, MD Erica Bartlett, MD Edward I. Lee, MDDocument6 pagesVascular Malformations: A Review: Joshua A. Cox, MD Erica Bartlett, MD Edward I. Lee, MDYusmiatiNo ratings yet
- Ew WikiDocument8 pagesEw WikidesyonkNo ratings yet
- Ni Hms 278458Document8 pagesNi Hms 278458VidinikusumaNo ratings yet
- Fibrous DysplasiaDocument29 pagesFibrous Dysplasiadr_adjie100% (2)
- Hereditary Influence in Thoracic Aortic Aneurysm and DissectionDocument14 pagesHereditary Influence in Thoracic Aortic Aneurysm and DissectionFede WeckesserNo ratings yet
- Cervical Spondylotic Myelopathy: A Guide To Diagnosis and ManagementDocument11 pagesCervical Spondylotic Myelopathy: A Guide To Diagnosis and ManagementSurya PangeranNo ratings yet
- Diagnosis, Classification, and Management of Soft Tissue SarcomasDocument17 pagesDiagnosis, Classification, and Management of Soft Tissue SarcomasSafrilia GandhiNo ratings yet
- 1998 - Hawkey (Ankilosis y Discapacidad)Document15 pages1998 - Hawkey (Ankilosis y Discapacidad)Alejandro SernaNo ratings yet
- A Rare Case of Large Size Undifferentiated Pleomorphic Sarcoma of AnkleDocument5 pagesA Rare Case of Large Size Undifferentiated Pleomorphic Sarcoma of AnkleIJAR JOURNALNo ratings yet
- Distrofia Muscular - DUCHENNEDocument4 pagesDistrofia Muscular - DUCHENNEMariano RamisNo ratings yet
- Neuroendocrine Tumors: Surgical Evaluation and ManagementFrom EverandNeuroendocrine Tumors: Surgical Evaluation and ManagementJordan M. CloydNo ratings yet
- Fast Facts: Myelodysplastic Syndromes: Determining risk, tailoring therapy, supporting patientsFrom EverandFast Facts: Myelodysplastic Syndromes: Determining risk, tailoring therapy, supporting patientsNo ratings yet
- Multiple Myeloma and Other Plasma Cell NeoplasmsFrom EverandMultiple Myeloma and Other Plasma Cell NeoplasmsMeletios A. DimopoulosNo ratings yet
- Myoclonus-Dystonia Syndrome: Case ReportDocument6 pagesMyoclonus-Dystonia Syndrome: Case ReportIzhar DedekNo ratings yet
- Dgab 475Document17 pagesDgab 475stone riverNo ratings yet
- Muscle Tissue: Matthew Velkey, PH.DDocument40 pagesMuscle Tissue: Matthew Velkey, PH.DREMAN ALINGASANo ratings yet
- 2 CarbohydratesDocument54 pages2 CarbohydratesoecologieNo ratings yet
- Case Report 3 MukokelDocument3 pagesCase Report 3 MukokelWidychii GadiestchhetyaNo ratings yet
- Disorders of Water and Sodium BalanceDocument21 pagesDisorders of Water and Sodium BalanceStanNo ratings yet
- Chordal Preservation TechniquesDocument8 pagesChordal Preservation TechniquesKunwar Sidharth SaurabhNo ratings yet
- January VIPDocument52 pagesJanuary VIPbeaumontenterpriseNo ratings yet
- Levofloxacin 500mg Film-Coated Tablets - Summary of Product Characteristics (SMPC)Document10 pagesLevofloxacin 500mg Film-Coated Tablets - Summary of Product Characteristics (SMPC)OdunlamiNo ratings yet
- Region Iv-A Calabarzon Francisco P. Felix Memorial National High SchoolDocument4 pagesRegion Iv-A Calabarzon Francisco P. Felix Memorial National High SchoolLumahan, Jazmine Julia F.No ratings yet
- Vein of Galen MalformationDocument69 pagesVein of Galen MalformationBhupendra GuptaNo ratings yet
- Differential Diagnosis: Giant Cystic Abdominal Masses in Children and Adolescents: UltrasonicDocument5 pagesDifferential Diagnosis: Giant Cystic Abdominal Masses in Children and Adolescents: UltrasonicGoran MaliNo ratings yet
- My LogDocument229 pagesMy Logsowpij290laslNo ratings yet
- Mun Position Paper - USADocument1 pageMun Position Paper - USAAksharaNo ratings yet
- Managment of Trauma Patient in ERDocument46 pagesManagment of Trauma Patient in ERtofanNo ratings yet
- Zoo Dentists - Quiz NATALY YULIET GOMEZ CHAGUA PDFDocument2 pagesZoo Dentists - Quiz NATALY YULIET GOMEZ CHAGUA PDFNATALYYULIETNo ratings yet
- Group 15 DM ModuleDocument38 pagesGroup 15 DM ModuleJerremy LuqueNo ratings yet
- Complications of 3rd Molar SurgeryDocument12 pagesComplications of 3rd Molar Surgeryhamad_kayani_774954No ratings yet
- MINI International Neuropsychiatric Schedule: Clinical Utility and Patient AcceptanceDocument4 pagesMINI International Neuropsychiatric Schedule: Clinical Utility and Patient AcceptanceRafael MartinsNo ratings yet
- Immunology Micro LabDocument56 pagesImmunology Micro LabMarielle Anne TuazonNo ratings yet
- SEMINAR 3 Neoplastic, Myeloproliferative and Myelodysplastic DisordersDocument6 pagesSEMINAR 3 Neoplastic, Myeloproliferative and Myelodysplastic DisordersMICHELLE RAPELONo ratings yet
- Chapter 20 EnzymesDocument5 pagesChapter 20 Enzymesmariel_rockNo ratings yet
- ACG Guideline GERD March 2013Document21 pagesACG Guideline GERD March 2013Arri KurniawanNo ratings yet
- Infectious Diseases in Dogs and CatsDocument7 pagesInfectious Diseases in Dogs and Catslmanero2690No ratings yet
- 2013 (4 6-59)Document18 pages2013 (4 6-59)Dani GarnidaNo ratings yet