Professional Documents
Culture Documents
Journal Article For Pet
Journal Article For Pet
Uploaded by
MEOW41Copyright:
Available Formats
You might also like
- Solutions Manual For: Processes and SystemsDocument10 pagesSolutions Manual For: Processes and SystemsBanbona AlkurdshNo ratings yet
- Youkoso Jitsuryoku Shijou Shugi No Kyoushitsu e Vol.1Document423 pagesYoukoso Jitsuryoku Shijou Shugi No Kyoushitsu e Vol.1MEOW4194% (33)
- Original Texts For Demo EditsDocument7 pagesOriginal Texts For Demo EditsIvana JeremicNo ratings yet
- National Program in Weather Mod 1966.pdf"Document97 pagesNational Program in Weather Mod 1966.pdf"Thom Lee ChapmanNo ratings yet
- CT-PET Shielding DesingDocument24 pagesCT-PET Shielding DesingGezim Hodolli100% (1)
- Journal of Microscopy SubmissionDocument16 pagesJournal of Microscopy SubmissionNorhan MadinaNo ratings yet
- Simulation of The Expected Performance of A Seamless Scanner For Brain Pet Based On Highly Pixelated Cdte DetectorsDocument8 pagesSimulation of The Expected Performance of A Seamless Scanner For Brain Pet Based On Highly Pixelated Cdte DetectorsjuanNo ratings yet
- SPECT-MRI InstrumentationDocument13 pagesSPECT-MRI Instrumentationjannine arrietaNo ratings yet
- Al-Sharify 2020 IOP Conf. Ser. Mater. Sci. Eng. 870 0120431Document11 pagesAl-Sharify 2020 IOP Conf. Ser. Mater. Sci. Eng. 870 0120431Hua Hidari YangNo ratings yet
- Physica MedicaDocument10 pagesPhysica MedicaZulvan AviviNo ratings yet
- Site Planning and Radiation Safety in The PET FacilitDocument13 pagesSite Planning and Radiation Safety in The PET FacilitMustafa KamelNo ratings yet
- Absorbed Dose in Mgy From CT ScannersDocument9 pagesAbsorbed Dose in Mgy From CT Scannerscebuano88No ratings yet
- A NIM PETCT Phantom For Evaluating The PET Image Quality of Micro Lesions and The Performance Parameters of CTDocument13 pagesA NIM PETCT Phantom For Evaluating The PET Image Quality of Micro Lesions and The Performance Parameters of CTp110054No ratings yet
- Https:opg Optica Org:directpdfaccess: - 417296:boe-10-9-4896 Pdf?da 1&id 417296&seq 0&mobile NoDocument11 pagesHttps:opg Optica Org:directpdfaccess: - 417296:boe-10-9-4896 Pdf?da 1&id 417296&seq 0&mobile NoHarsh PanchalNo ratings yet
- TP5 João MachadoDocument3 pagesTP5 João MachadoJoao MachadoNo ratings yet
- Notes To Students - List of ProjectsDocument46 pagesNotes To Students - List of ProjectsDileep KumarNo ratings yet
- WorldJNuclMed143189-8541241 234332Document8 pagesWorldJNuclMed143189-8541241 234332arief oktavaNo ratings yet
- Simultaneous Measurement of Ionizing Radiation and Heart Rate Using A Smartphone CameraDocument8 pagesSimultaneous Measurement of Ionizing Radiation and Heart Rate Using A Smartphone CameraOcnos78No ratings yet
- Design and Validation of An Osteochondral Bioreactor For The Screening of Treatments For OsteoarthritisDocument8 pagesDesign and Validation of An Osteochondral Bioreactor For The Screening of Treatments For OsteoarthritisBrian VasquezNo ratings yet
- Estimating The Shielding Requirements For A Newly Planned PET-CT FacilityDocument6 pagesEstimating The Shielding Requirements For A Newly Planned PET-CT FacilitySelim HossainNo ratings yet
- Duma 15 Handheld Scanning Probes For OCTDocument13 pagesDuma 15 Handheld Scanning Probes For OCTMainakNo ratings yet
- FTP IvanenkioDocument5 pagesFTP Ivanenkiorwong1231No ratings yet
- Estimating The Size of Onion Epidermal Cells From Diffraction PatternsDocument5 pagesEstimating The Size of Onion Epidermal Cells From Diffraction PatternsAlexis Nathán RuedaNo ratings yet
- History and Future Technical Innovation in Positron Emission TomographyDocument18 pagesHistory and Future Technical Innovation in Positron Emission TomographyTuấn Long Diệp VũNo ratings yet
- Corr - Chapter1'Document4 pagesCorr - Chapter1'Costin GabrielaNo ratings yet
- 1 s2.0 S2212440312000442 MainDocument6 pages1 s2.0 S2212440312000442 MainMarinaNo ratings yet
- A Digital Preclinical PET/MRI Insert and Initial Results: Abstract - Combining Positron Emission Tomography (PET)Document13 pagesA Digital Preclinical PET/MRI Insert and Initial Results: Abstract - Combining Positron Emission Tomography (PET)dwid jansNo ratings yet
- Implications of CBCT in Pediatric Dentistry-A ReviewDocument8 pagesImplications of CBCT in Pediatric Dentistry-A ReviewIJAR JOURNALNo ratings yet
- Omicron Detection With X-Ray and CT-Scan Using Machine LearningDocument6 pagesOmicron Detection With X-Ray and CT-Scan Using Machine LearningIJRASETPublicationsNo ratings yet
- Micro PillsDocument33 pagesMicro PillsAmshala SrikanthNo ratings yet
- Bio-Medical X-Ray Imaging With Spectroscopic Pixel DetectorsDocument6 pagesBio-Medical X-Ray Imaging With Spectroscopic Pixel DetectorsEvelynNo ratings yet
- Ciocca Et Al. - 2019 - Design and Commissioning of The Nondedicated Scanning Proton Beamline For Ocular Treatment at The SynchrotronDocument12 pagesCiocca Et Al. - 2019 - Design and Commissioning of The Nondedicated Scanning Proton Beamline For Ocular Treatment at The SynchrotronKanit TanthanawigraiNo ratings yet
- Deep Learning-Based Cell Identification and Disease Diagnosis Using Spatio-Temporal Cellular Dynamics in Compact Digita PDFDocument18 pagesDeep Learning-Based Cell Identification and Disease Diagnosis Using Spatio-Temporal Cellular Dynamics in Compact Digita PDFJeffreyNo ratings yet
- The Future of Hybrid Imaging-Part 2: PET/CT: Insights Into Imaging June 2011Document11 pagesThe Future of Hybrid Imaging-Part 2: PET/CT: Insights Into Imaging June 2011maaddy001No ratings yet
- Post PrintDocument28 pagesPost PrintSusi SusantiNo ratings yet
- 08 - Alkek NM-PET Suite Radiation Shielding Recommendation3Document13 pages08 - Alkek NM-PET Suite Radiation Shielding Recommendation3Vinil CariappaNo ratings yet
- Computed TomographyDocument31 pagesComputed TomographyAvik Mukherjee100% (3)
- Pet SpectDocument32 pagesPet SpectgpillajoNo ratings yet
- 10 1155@2017@3848207Document10 pages10 1155@2017@3848207hafidh akbarNo ratings yet
- Routine IMRT Verification by Means of An Automated Monte Carlo Simulation SystemDocument11 pagesRoutine IMRT Verification by Means of An Automated Monte Carlo Simulation SystemMuhammad IrsyadNo ratings yet
- Diagnostics 11 00161 v2Document14 pagesDiagnostics 11 00161 v2sara.dj.7cuNo ratings yet
- Role of Gamma Camera Components in Radiological DiagnosisDocument7 pagesRole of Gamma Camera Components in Radiological DiagnosisCentral Asian StudiesNo ratings yet
- Small Field Dosimetry For The Small Animal Radiotherapy Research Platform (SARRP)Document11 pagesSmall Field Dosimetry For The Small Animal Radiotherapy Research Platform (SARRP)ammar.herratiNo ratings yet
- BulganDocument10 pagesBulganIkh nahia Ikh Nakhia PharmNo ratings yet
- Priyanka and Om Prakash ChoudharyDocument6 pagesPriyanka and Om Prakash ChoudharyAditya RifNo ratings yet
- Distribution Statement A Approved For Public Release Distribution UnlimitedDocument15 pagesDistribution Statement A Approved For Public Release Distribution Unlimitedapi-255706616No ratings yet
- 2010 Artigo - X - Ray microCT LandisDocument12 pages2010 Artigo - X - Ray microCT LandisAna Kely SouzaNo ratings yet
- Pancreas Disease Detection and Segmentation Using Abdominal CT ScanDocument9 pagesPancreas Disease Detection and Segmentation Using Abdominal CT ScanIJAR JOURNALNo ratings yet
- Essay Writing in Biophotonics: December 24, 2011Document14 pagesEssay Writing in Biophotonics: December 24, 2011tanbir-hasan-6730No ratings yet
- Radiation Shielding and Bunker Design: Review ArticleDocument13 pagesRadiation Shielding and Bunker Design: Review Articlefadli puteraNo ratings yet
- Evaluation of Image Quality and Recovery Coefficient of Corn Starchbonded Rhizophora Spp. Particleboards As Phantom For SPECT/CT ImagingDocument8 pagesEvaluation of Image Quality and Recovery Coefficient of Corn Starchbonded Rhizophora Spp. Particleboards As Phantom For SPECT/CT ImagingPuteriNo ratings yet
- Calculo Ontecarlo MetodologiaDocument5 pagesCalculo Ontecarlo MetodologiaCarlos GutierrezNo ratings yet
- Radiopharmaceuticals For PET ImagingDocument7 pagesRadiopharmaceuticals For PET ImagingmemeiniNo ratings yet
- Fully 3-D Integrated Pixel Detectors For X-RaysDocument10 pagesFully 3-D Integrated Pixel Detectors For X-RaysHuy Anh BùiNo ratings yet
- Editor Response ExampleDocument5 pagesEditor Response ExampleCamilo PerezNo ratings yet
- First Clinical Trial of The Miro'' Capsule Endoscope by Using A Novel Transmission Technology: Electric-Field PropagationDocument7 pagesFirst Clinical Trial of The Miro'' Capsule Endoscope by Using A Novel Transmission Technology: Electric-Field PropagationfadzisomashavaNo ratings yet
- Notes On PETDocument13 pagesNotes On PETAbhi SachdevNo ratings yet
- DL 3Document20 pagesDL 3Gabbar IsBackNo ratings yet
- Complementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionDocument10 pagesComplementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionhesoyamyecgaaaNo ratings yet
- Simulation and Lithographic Fabrication of A Triple Band Terahertz Metamaterial Absorber Coated On Flexible Polyethylene Terephthalate SubstrateDocument22 pagesSimulation and Lithographic Fabrication of A Triple Band Terahertz Metamaterial Absorber Coated On Flexible Polyethylene Terephthalate SubstratekoyaunivNo ratings yet
- Artificial Neural Network For Bubbles Pattern Recognition On The ImagesDocument15 pagesArtificial Neural Network For Bubbles Pattern Recognition On The ImagesGustavo ArellanoNo ratings yet
- PetDocument346 pagesPetbdalcin5512No ratings yet
- Micro-computed Tomography (micro-CT) in Medicine and EngineeringFrom EverandMicro-computed Tomography (micro-CT) in Medicine and EngineeringKaan OrhanNo ratings yet
- MATH115 REYES Sample Quiz2Document2 pagesMATH115 REYES Sample Quiz2MEOW41No ratings yet
- Slide - Infinite Series of Constant Terms PDFDocument36 pagesSlide - Infinite Series of Constant Terms PDFMEOW41No ratings yet
- Math115 Lo#2Document1 pageMath115 Lo#2MEOW41No ratings yet
- Math115 Lo#1Document1 pageMath115 Lo#1MEOW41No ratings yet
- MATH115 Sample Long Quiz 4 Answers 08 July 2015Document2 pagesMATH115 Sample Long Quiz 4 Answers 08 July 2015MEOW41No ratings yet
- Training Program TemplateDocument2 pagesTraining Program TemplateMEOW41No ratings yet
- Importance of Biochemistry in Medicine - Human Nutrition - Diagnosis of Diseases - Treament of Disease States - Creation of "Designer" DrugsDocument1 pageImportance of Biochemistry in Medicine - Human Nutrition - Diagnosis of Diseases - Treament of Disease States - Creation of "Designer" DrugsMEOW41No ratings yet
- AndroidNotesForProfessionals PDFDocument1,329 pagesAndroidNotesForProfessionals PDFIlter KocamanNo ratings yet
- Universal Principles of Biomedical EthicsDocument16 pagesUniversal Principles of Biomedical EthicsMEOW41100% (3)
- Activities For ICpEP TBDocument5 pagesActivities For ICpEP TBMEOW41No ratings yet
- OS 216: Hematology: Topic Conference 1: AnemiasDocument7 pagesOS 216: Hematology: Topic Conference 1: AnemiasMEOW41No ratings yet
- Slide 6Document8 pagesSlide 6MEOW41No ratings yet
- Annual Occupational Dose Limit: Skin - 0.5 SVDocument7 pagesAnnual Occupational Dose Limit: Skin - 0.5 SVMEOW41No ratings yet
- Permanent Magnet Materials - A N PatelDocument64 pagesPermanent Magnet Materials - A N PatelRishabhNo ratings yet
- Continuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeDocument5 pagesContinuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeKasra GolbanNo ratings yet
- DXR Raman Ficha TecnicaDocument4 pagesDXR Raman Ficha TecnicaFernando Torres PérezNo ratings yet
- Lubrication Solutions For Industrial Applications: Energizing Performance. Every DayDocument68 pagesLubrication Solutions For Industrial Applications: Energizing Performance. Every DayEid EeidNo ratings yet
- Statistical Mechanics: Course Code - PPHY 302Document29 pagesStatistical Mechanics: Course Code - PPHY 302Siva ArunNo ratings yet
- Melting Point and Boiling PointDocument14 pagesMelting Point and Boiling PointMALOU ELEVERANo ratings yet
- BVD 2065 GBDocument4 pagesBVD 2065 GBФизули АбиловNo ratings yet
- ISO 717-1 Rate of Sound InsulationDocument20 pagesISO 717-1 Rate of Sound InsulationFYNo ratings yet
- Scramjet Combustion Fundamentals and Advances Gautam Choubey Full Download ChapterDocument51 pagesScramjet Combustion Fundamentals and Advances Gautam Choubey Full Download Chaptermargaret.hoffman258100% (21)
- Structure and Di Ffusion Behavior of Trioctyl Trimellitate (TOTM) in PVC Film Studied by ATR-IR SpectrosDocument11 pagesStructure and Di Ffusion Behavior of Trioctyl Trimellitate (TOTM) in PVC Film Studied by ATR-IR SpectrosGanciarov MihaelaNo ratings yet
- Particle Morphology and Powder Properties During - Wageningen University and Research 513788Document6 pagesParticle Morphology and Powder Properties During - Wageningen University and Research 513788Gabriel Henrique De Melo SilvaNo ratings yet
- Extended Elastic Impedance EEI Inversion PDFDocument37 pagesExtended Elastic Impedance EEI Inversion PDFsonu420No ratings yet
- MCQ Chapter 2 ElectrochemistryDocument4 pagesMCQ Chapter 2 ElectrochemistryFact GamerNo ratings yet
- Table of Personnel ProtectionDocument1 pageTable of Personnel ProtectionnincitoNo ratings yet
- 8 Legged Walking RobotDocument15 pages8 Legged Walking RobotAnuj TripathiNo ratings yet
- Experimental Investigation of Mustard Oil Based Nano Cutting Fluid On CNC Turning OperationDocument13 pagesExperimental Investigation of Mustard Oil Based Nano Cutting Fluid On CNC Turning OperationIJRASETPublicationsNo ratings yet
- Flexural Properties of Stringer Sections (Lipped and Unlipped Angles, Channels and Z-Sections)Document6 pagesFlexural Properties of Stringer Sections (Lipped and Unlipped Angles, Channels and Z-Sections)Lênon Guimarães Silva AlípioNo ratings yet
- Aer Conditionat Multisplit LG Unitati Externe Pliant Date Tehnice PDFDocument9 pagesAer Conditionat Multisplit LG Unitati Externe Pliant Date Tehnice PDFClau HerleaNo ratings yet
- 8.2 Aerodynamics 8.2.1: Airflow Around A BodyDocument6 pages8.2 Aerodynamics 8.2.1: Airflow Around A BodyNayem Hashan NayemNo ratings yet
- MAE 241-Lec4-Summer 2011Document14 pagesMAE 241-Lec4-Summer 2011kostas.sierros9374No ratings yet
- Module 02 Dan 03 Plate Tectonic, Gerak Dan Sifat InteraksiDocument48 pagesModule 02 Dan 03 Plate Tectonic, Gerak Dan Sifat InteraksiNurAjiNo ratings yet
- Chemical Kinetics Part 5 PDFDocument10 pagesChemical Kinetics Part 5 PDFShaikh FarooqNo ratings yet
- Solved Problem of First Three Chapters by Asif RasheedDocument10 pagesSolved Problem of First Three Chapters by Asif RasheedAsif Rasheed RajputNo ratings yet
- Progressive GR 9 2nd Q CDocument21 pagesProgressive GR 9 2nd Q CRAMIL BAUTISTANo ratings yet
- Deslandres Table N2DataDocument5 pagesDeslandres Table N2DataPiyali GhoshNo ratings yet
- Interaction CurveDocument126 pagesInteraction CurveNikhilNo ratings yet
- Peskin & Schroeder - The Quantum SpinDocument6 pagesPeskin & Schroeder - The Quantum SpinGiggs SandyNo ratings yet
- Topic 2 Pastpapers AllDocument44 pagesTopic 2 Pastpapers AllYoutube Foroau100% (1)
Journal Article For Pet
Journal Article For Pet
Uploaded by
MEOW41Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Journal Article For Pet
Journal Article For Pet
Uploaded by
MEOW41Copyright:
Available Formats
www.sciedu.
ca/jbgc
Journal of Biomedical Graphics and Computing, June 2012, Vol. 2, No. 1
ORIGINAL RESEARCH
Potential shielding for a positron emission
tomography (PET) suite
Michelle E. Bresnahan1, Bijaya Shrestha2
1. Department of Environmental Health and Safety, Missouri University of Science and Technology, Missouri, United
States. 2. Department of Electrical and Computer Engineering, Missouri University of Science and Technology, Missouri,
United States.
Correspondence: Bijaya Shrestha. Address: 215 EE Hall, Missouri S&T, Rolla, MO. 65401. Telephone: 573-341-6068.
Email: Shrestha@mst.edu.
Received: February 12, 2012
DOI: 10.5430/jbgc.v2n1p89
Accepted: May 6, 2012
Published: June 1, 2012
URL: http://dx.doi.org/10.5430/jbgc.v2n1p89
Abstract
Due to the amount of radioisotopes needed for use in positron emission tomography scans, it is important to have a careful
planning of procedures and design of such facilities. Using the Monte Carlo N-Particle Transport Code (MCNP), a
potential suite for a positron emission tomography (PET) facility was designed. The design covered three different areas as
required in a PET facility: the scan room, the waiting area, and the hot lab. The design used two different shielding options,
lead and concrete. Both materials were found to be enough to lower dose rates below the public dose limits set by the U.S.
Nuclear Regulatory Commission.
Key words
Tomography, Monte Carlo, Radiation dose, Simulation, Monte Carlo N-Particle Transport Code, Positron emission,
Neutral particle transport, Medical imaging
1 Introduction
Positron emission tomography is a nuclear medicine imaging technology [1]. It employs the use of radioisotopes that decay
by positron emission followed by annihilation phenomena resulting in coincident pair of photons with 0.511 MeV. These
photons emerge in opposite directions. The photon detectors of a PET scanner are arranged around the patient in such a
way as to be able to detect the pair of emitted photons simultaneously. After enough coincident photon events are
collected, reconstruction by computer results an image with the information of the positron-emitting isotope
distribution [2]. This image is generally paired with another type of computer tomography image, such as a computed axial
tomography scan, and then used to create detailed views of metabolic functions within the human body. These images are
used for detection of cancer cells and evaluation of coronary artery disease. Due to the amount needed of radioisotopes for
PET, it is very important to have a careful planning and design of facilities. Particular shielding design is required due to
exposure of workloads and potential occupancies of the facility. More often than not, the shield materials for a PET facility
can be combined with common building materials (i.e. gypsum wallboard combined with lead). Additionally, shielding
material can be incorporated into the building structure (i.e. concrete brick) to cut down on the cost of material needed [3].
Published by Sciedu Press
89
www.sciedu.ca/jbgc
Journal of Biomedical Graphics and Computing, June 2012, Vol. 2, No. 1
For this work the MCNP code was used to design and calculate the amount of shielding needed for the facility. Since there
is no PET facility available at Missouri S&T for study, a contact was made with a health physicist at Washington
University in St. Louis, MO [4] as a result of which the floor plan and structural information were provided about the PET
located at the Mallinckrodt Institute of Radiology (MIR) [5] which is the basis of the PET suite modeled in MCNP for this
work (see Figure 1).
Figure 1. Floor plan of the Center for Clinical Imaging Research (CCIR) at MIR
(http://ccir.wustl.edu/facilities/floorplan.shtml#)
There were several assumptions made in the design of this facility which are discussed in more detail in the following
sections. The main focus for design in this project was based on a facility able to utilize only one PET scanner.
1.1 Facility
The PET facility in this work was designed using MCNP and consists of a three room area: the hot lab, the waiting room,
and the scanner room (see Figure 2).
Figure 2. MCNP design of PET facility
The area that has the greatest potential for exposure is the hot lab. This is the area where the radioisotopes are stored,
prepared, and injected into the patient. In this work, the facility was designed on the assumption that whole body scans
would occur more than scans focused just on a particular area of the body, therefore only one injection room is modeled.
The hot lab area design focused on conservative estimates in order to ensure that adequate shielding would be used. In this
90
ISSN 1925-4008 E-ISSN 1925-4016
www.sciedu.ca/jbgc
Journal of Biomedical Graphics and Computing, June 2012, Vol. 2, No. 1
case, the hot lab area is a five foot by ten foot room with walls made of gypsum and a small cylindrical source located
inside.
The patient waits in the waiting area after being injected with the radioisotope before they begin the PET scan. The wait is
needed before beginning the scan to allow the radioisotope to collect in the area of the body it needs to. It is also important
that the patient remain in relative seclusion during this time because the patient will be a source of radiation immediately
after injection. For this design, the waiting area was designed as a five foot by ten foot room with walls made of gypsum
and a person, temporarily considered a source, located inside.
The scanner room physically houses the PET scanner (see Figure 3). By the time patient comes to the scan room, the
patient is no longer a valid source of radiation due to the relatively short half-life of the radioisotope injected; as total
activity would have been reduced in the patient body. The physical scan area, where the exposures are expected to be the
least for both the medical personnel and the people who occupy the surrounded areas, is a ten foot by ten foot room with
walls made of gypsum containing the scanner and a radiating person, the source.
Figure 3. MCNP design of PET scanner
1.2 Shielding
The shielding for each area in the PET suite was determined placing a layer of material, either lead or concrete, outside a
single wall of each room. Each unshielded wall showed an equal dose rate; therefore it was only necessary to experiment
with one wall to determine appropriate shielding for all four. For the hot lab, the wall with the highest dose rate measured
was selected to achieve the most conservative estimate.
1.3 Source
There were three sources used in this work, each one in a different room in the suite. In the scanner room the source was
modeled as a 58 person, simulated by a cylinder of water, with a spherical head, also filled with water. The hot lab area
contained a source modeled as an eight inch plastic vial to simulate the radioisotope before it was injected into the patient.
The waiting area contained a source modeled as a 58 person, also simulated by a cylinder of water with a spherical head.
All sources were designed to emit photon energy at 0.511 MeV.
1.4 Workload & workflow
As previously stated, the PET suite was designed principally for whole body scans. The most widely used PET
radiopharmaceutical is 18FDG (18F-fluorodeoxyglucose), an analog of the bodys metabolic energy source, glucose, used
by brain tissue, malignant cancer cell, and other tissues [6]. For a whole body scan, the amount of injection ranges from 3
mCi to 15 mCi [2]. In this work, the dose was assumed to be 12 mCi of 18F, to be injected into the person before the
Published by Sciedu Press
91
www.sciedu.ca/jbgc
Journal of Biomedical Graphics and Computing, June 2012, Vol. 2, No. 1
beginning of the scan. The facility would, however, receive a larger amount of the isotope in use if more than one scan is to
be performed that day, and store it. Using syringes and dose calibrators, medical technologists would divide the larger
amount of the isotope into the correct amounts to be injected into each patient receiving a PET scan. A total amount of 300
mCi of 18F was assumed to be delivered to the facility at the start of each day. The patient will be held for approximately
one hour after injection to allow for isotope uptake into the body. The patient will undergo emission scans once the waiting
period expires.
In this case, since only one scanner is in use at this facility, it will only be able to deal with one patient at a time. Assuming
a nine hour day, the facility would only be able to handle eight patients per day at a maximum. This particular PET was
designed as a conventional bismuth germanium scanner, taking approximately fifty minutes per whole body scan [2]. Since
the annual number of nuclear medicine procedures has increased three-fold (from seven million to twenty million)
between 1985 and 2005, a reasonable approach was to assume that the facility would remain busy throughout the year [7].
The facility would need to be taken out of service seven weeks each year for maintenance and calibration.
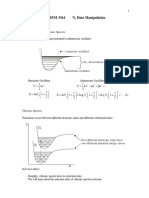
2 Methodology: simulation parameters
We developed an MCNP model of the PET facility, which consisted of the hotlab, waiting room, and scanning room with
the patient modeled as a water-filled cylindrical phantom with a spherical head. All of the walls in the model were
considered to be made of gypsum with half an inch thickness. Using combinatory, the geometry of the rooms and the
patient were modeled and input to the program using cell and surface cards as required. The dimensions of the hotlab and
the waiting room were modeled to be 5 ft by 10 ft where as that of the scanning room was 10 ft by 10 ft. The patient was
modeled as lying on the platform. These geometrical modeling are shown in Figures 2 and 3.
All of the sources including the plastic vial containing 18FDG in the hotlab and the patient were all considered to emit
0.511 MeV photons. Using importance card, a calculation optimization technique within MCNP, the simulation was
optimized and dose deposited by the photons were tallied for million histories, so that the uncertainty in the dose
calculations are below 1%. The MCNP modeling was done to create a realistic situation. Therefore certain approximations
were made for the sake of simplicity such as modeling the patient by a cylinder filed with water. In this regard, some of the
limitations in our modeling are the following:
2.1 Physics limitation
18
FDG is considered solely as 18F as far as the radiation us considered. All sources are considered to emit 0.511 MeV
photons
2.2 Design limitation
Patient was modeled as a cylinder of water with a spherical head
2.3 Radiation Protection
Regulations
The U.S. Nuclear Regulatory Commission (NRC) protects the health and well-being of persons who could potentially be
exposed to ionizing radiation occupationally. These people are often willing to be exposed to minimal amounts of
radiation for their occupation. The NRC also protects members of the public from radiation found in licensed activities [8].
NRC regulations limit exposure to ensure that no individual will receive more than 100 millirems per year from licensed
operations [9, 10]. Additionally, the NRC requires that the dose rate in areas accessible to members of the public not exceed
2 mrem in any given hour. Radiation workers are limited to an exposure of five rem per year. Along with the regulations,
the ALARA (as low as reasonably achievable) guideline is a radiation exposure concept intended to encourage protection
practices.
92
ISSN 1925-4008 E-ISSN 1925-4016
www.sciedu.ca/jbgc
Journal of Biomedical Graphics and Computing, June 2012, Vol. 2, No. 1
3 Results and discussion
3.1 PET scan room
Figure 4. Dose rates on the outside of the PET scan room walls using lead and concrete has shielding material in varying
thickness
Figure 5. Dose rates on the outside of the Hot lab room walls using lead and concrete has shielding material in varying
thickness
By the time the patient reaches the PET scan room, the 12 mCi dose of 18F they were originally injected has decayed to
about 8.22 mCi. This activity was used to calculate the exposure on the outsides of the PET room walls. With no shielding
in place, the dose rate is below the 2 mrem per hour limit set in the NRC regulations. It is important to note, however, that
while the dose rate is within NRC regulations, when the workload of the facility is taken into account, the exposure rates
Published by Sciedu Press
93
www.sciedu.ca/jbgc
Journal of Biomedical Graphics and Computing, June 2012, Vol. 2, No. 1
quickly reach greater than one hundred mrem per year. The dose with no shielding is approximately 0.3 mrem per hour. If
eight patients are seen per day, five days per week, forty five weeks each year, the total dose rate is approximately 540
mrem per year; over five times the limit. An unshielded facility could process no more than seven people per day, once a
week. If one centimeter of lead shielding is used, the dose rate falls below regulatory limits both hourly and annually.
As seen from Figures 4 and 5, for 5 cm of concrete, dose rate of ~5.10-2 mRem/hr was found when 8.22 mCi was used.
When 300 mCi is used, dose rate of ~5.100 mRem/hr was found for the same 5 cm thickness of concrete. The results are
proportional to fluency/activity of the source (ratio of 102), which is quite reasonable.
3.2 Hot lab room
The hot lab room, where the radioisotopes are delivered and stored, presents the biggest shielding challenge and greatest
amount of radiation exposure. For this project, it was assumed that approximately 300 mCi of 18F would be stored in the
hot lab. The amount can then be separated out into individual syringes for injection into patients. With no shielding in
place, the dose rate on the outside of the walls was calculated to be approximately 47.32 mrem/hr, far exceeding the limits
set by the NRC.
Shielding in this area is a must. It was determined that using two inch lead brick to line the walls of this area would lower
the dose rate to within regulatory limits. Eight inch concrete bricks would also be sufficient.
Figure 6. Dose rates on the outside of the Holding room walls using lead and concrete has shielding material in varying
thickness
3.3 Holding room
The shielding for the holding room was calculated using the same amount injected into the patient, 12 mCi of 18F. Dose
rates in this area are very similar to those in the PET scan room. With no shielding, the dose rate on the outside of the
holding room wall about 0.31 mrem/hr, again is below the regulated hourly limit. However, after the workload of the
facility is taken into consideration, the dose rate is found to be above the annual exposure limit. The MCNP design
confirmed that one centimeter of lead sheeting was sufficient to shield the area to within acceptable limits. Also, only two
inches of concrete block is necessary to shield within acceptable limits.
94
ISSN 1925-4008 E-ISSN 1925-4016
www.sciedu.ca/jbgc
Journal of Biomedical Graphics and Computing, June 2012, Vol. 2, No. 1
4 Conclusion
Both lead and concrete are sufficient to shield a PET scan facility. The most economical decision when deciding how to
shield a PET facility would be to either design or pick a location that already has shielding material in place. For example,
most buildings are built with concrete block exterior walls. If the PET suite could be located in an area where it uses an
outer wall, the concrete from the building should be sufficient to shield within acceptable dose limits. Ideally, the hot lab
would be placed in a corner room where it can utilize two concrete walls, considerably cutting down the cost of materials
like lead needed to shield the inner walls. The PET scan room exhibited the smallest dose rate and therefore, could be
placed in a central location. Since the holding room and the hot lab need to be in close proximity, the holding room would
also utilize a concrete outer wall.
References
[1] "Positron Emission Tomography - Computed Tomography (PET/CT)." Radiology Info. 11 Sept. 2008. Radiological Society of
North America [Internet]. 2008. Available from: http://www.radiologyinfo.org/en/info.cfm?pg=pet&bhcp=1.
[2] Anderson, Jon A., and Dana Mathews. Site Planning and Radiation Safety in the PET Facility. Ts. The University of Texas
Southwestern Medical Center at Dallas, Dallas, TX, 2002.
[3] Methe, Brian M. "Shielding Design for a PET Imaging Suite: A Case Study." Operation Radiation Safety. 2004; S83-88.
[4] Szatkowski, Dan. "Information on PET." E-mail to the author. 16 Oct. 2008.
[5] "Floor plan for the CCIR." Center for Clinical Imaging Research. 8 May 2007. Washington University in St. Louis. 11 Dec. 2008
Available from: http://ccir.wustl.edu/facilities/floorplan.shtml.
[6] Erdman, Mike, Steve King, and Ken Miller. "Recent Experiences with Shielding a PET/CT Facility." Operational Radiation Safety
87 (2004); S37-39.
[7] Zanzonico, Pat, Lawrence Dauer, and Jean St. Germain. "Operational Radiation Safety." Health Physics. 2008: 554-70.
PMid:18849690 http://dx.doi.org/10.1097/01.HP.0000327651.15794.f7
[8] Coker, Audra L. "PET/CT Shielding Design Comparisons." Thesis. College Station, Texas
[9] Nuclear Regulation Commission, Code of Federal Regulations 10CFR20.1201, Occupational dose limits for adults, (U.S
Government Printing Office, 1999).
[10] Nuclear Regulatory Commission, Code of Federal Regulations 10CFR20.1301, Dose limits for individual members of the
public, (U.S Government Printing Office, 1999).
Published by Sciedu Press
95
You might also like
- Solutions Manual For: Processes and SystemsDocument10 pagesSolutions Manual For: Processes and SystemsBanbona AlkurdshNo ratings yet
- Youkoso Jitsuryoku Shijou Shugi No Kyoushitsu e Vol.1Document423 pagesYoukoso Jitsuryoku Shijou Shugi No Kyoushitsu e Vol.1MEOW4194% (33)
- Original Texts For Demo EditsDocument7 pagesOriginal Texts For Demo EditsIvana JeremicNo ratings yet
- National Program in Weather Mod 1966.pdf"Document97 pagesNational Program in Weather Mod 1966.pdf"Thom Lee ChapmanNo ratings yet
- CT-PET Shielding DesingDocument24 pagesCT-PET Shielding DesingGezim Hodolli100% (1)
- Journal of Microscopy SubmissionDocument16 pagesJournal of Microscopy SubmissionNorhan MadinaNo ratings yet
- Simulation of The Expected Performance of A Seamless Scanner For Brain Pet Based On Highly Pixelated Cdte DetectorsDocument8 pagesSimulation of The Expected Performance of A Seamless Scanner For Brain Pet Based On Highly Pixelated Cdte DetectorsjuanNo ratings yet
- SPECT-MRI InstrumentationDocument13 pagesSPECT-MRI Instrumentationjannine arrietaNo ratings yet
- Al-Sharify 2020 IOP Conf. Ser. Mater. Sci. Eng. 870 0120431Document11 pagesAl-Sharify 2020 IOP Conf. Ser. Mater. Sci. Eng. 870 0120431Hua Hidari YangNo ratings yet
- Physica MedicaDocument10 pagesPhysica MedicaZulvan AviviNo ratings yet
- Site Planning and Radiation Safety in The PET FacilitDocument13 pagesSite Planning and Radiation Safety in The PET FacilitMustafa KamelNo ratings yet
- Absorbed Dose in Mgy From CT ScannersDocument9 pagesAbsorbed Dose in Mgy From CT Scannerscebuano88No ratings yet
- A NIM PETCT Phantom For Evaluating The PET Image Quality of Micro Lesions and The Performance Parameters of CTDocument13 pagesA NIM PETCT Phantom For Evaluating The PET Image Quality of Micro Lesions and The Performance Parameters of CTp110054No ratings yet
- Https:opg Optica Org:directpdfaccess: - 417296:boe-10-9-4896 Pdf?da 1&id 417296&seq 0&mobile NoDocument11 pagesHttps:opg Optica Org:directpdfaccess: - 417296:boe-10-9-4896 Pdf?da 1&id 417296&seq 0&mobile NoHarsh PanchalNo ratings yet
- TP5 João MachadoDocument3 pagesTP5 João MachadoJoao MachadoNo ratings yet
- Notes To Students - List of ProjectsDocument46 pagesNotes To Students - List of ProjectsDileep KumarNo ratings yet
- WorldJNuclMed143189-8541241 234332Document8 pagesWorldJNuclMed143189-8541241 234332arief oktavaNo ratings yet
- Simultaneous Measurement of Ionizing Radiation and Heart Rate Using A Smartphone CameraDocument8 pagesSimultaneous Measurement of Ionizing Radiation and Heart Rate Using A Smartphone CameraOcnos78No ratings yet
- Design and Validation of An Osteochondral Bioreactor For The Screening of Treatments For OsteoarthritisDocument8 pagesDesign and Validation of An Osteochondral Bioreactor For The Screening of Treatments For OsteoarthritisBrian VasquezNo ratings yet
- Estimating The Shielding Requirements For A Newly Planned PET-CT FacilityDocument6 pagesEstimating The Shielding Requirements For A Newly Planned PET-CT FacilitySelim HossainNo ratings yet
- Duma 15 Handheld Scanning Probes For OCTDocument13 pagesDuma 15 Handheld Scanning Probes For OCTMainakNo ratings yet
- FTP IvanenkioDocument5 pagesFTP Ivanenkiorwong1231No ratings yet
- Estimating The Size of Onion Epidermal Cells From Diffraction PatternsDocument5 pagesEstimating The Size of Onion Epidermal Cells From Diffraction PatternsAlexis Nathán RuedaNo ratings yet
- History and Future Technical Innovation in Positron Emission TomographyDocument18 pagesHistory and Future Technical Innovation in Positron Emission TomographyTuấn Long Diệp VũNo ratings yet
- Corr - Chapter1'Document4 pagesCorr - Chapter1'Costin GabrielaNo ratings yet
- 1 s2.0 S2212440312000442 MainDocument6 pages1 s2.0 S2212440312000442 MainMarinaNo ratings yet
- A Digital Preclinical PET/MRI Insert and Initial Results: Abstract - Combining Positron Emission Tomography (PET)Document13 pagesA Digital Preclinical PET/MRI Insert and Initial Results: Abstract - Combining Positron Emission Tomography (PET)dwid jansNo ratings yet
- Implications of CBCT in Pediatric Dentistry-A ReviewDocument8 pagesImplications of CBCT in Pediatric Dentistry-A ReviewIJAR JOURNALNo ratings yet
- Omicron Detection With X-Ray and CT-Scan Using Machine LearningDocument6 pagesOmicron Detection With X-Ray and CT-Scan Using Machine LearningIJRASETPublicationsNo ratings yet
- Micro PillsDocument33 pagesMicro PillsAmshala SrikanthNo ratings yet
- Bio-Medical X-Ray Imaging With Spectroscopic Pixel DetectorsDocument6 pagesBio-Medical X-Ray Imaging With Spectroscopic Pixel DetectorsEvelynNo ratings yet
- Ciocca Et Al. - 2019 - Design and Commissioning of The Nondedicated Scanning Proton Beamline For Ocular Treatment at The SynchrotronDocument12 pagesCiocca Et Al. - 2019 - Design and Commissioning of The Nondedicated Scanning Proton Beamline For Ocular Treatment at The SynchrotronKanit TanthanawigraiNo ratings yet
- Deep Learning-Based Cell Identification and Disease Diagnosis Using Spatio-Temporal Cellular Dynamics in Compact Digita PDFDocument18 pagesDeep Learning-Based Cell Identification and Disease Diagnosis Using Spatio-Temporal Cellular Dynamics in Compact Digita PDFJeffreyNo ratings yet
- The Future of Hybrid Imaging-Part 2: PET/CT: Insights Into Imaging June 2011Document11 pagesThe Future of Hybrid Imaging-Part 2: PET/CT: Insights Into Imaging June 2011maaddy001No ratings yet
- Post PrintDocument28 pagesPost PrintSusi SusantiNo ratings yet
- 08 - Alkek NM-PET Suite Radiation Shielding Recommendation3Document13 pages08 - Alkek NM-PET Suite Radiation Shielding Recommendation3Vinil CariappaNo ratings yet
- Computed TomographyDocument31 pagesComputed TomographyAvik Mukherjee100% (3)
- Pet SpectDocument32 pagesPet SpectgpillajoNo ratings yet
- 10 1155@2017@3848207Document10 pages10 1155@2017@3848207hafidh akbarNo ratings yet
- Routine IMRT Verification by Means of An Automated Monte Carlo Simulation SystemDocument11 pagesRoutine IMRT Verification by Means of An Automated Monte Carlo Simulation SystemMuhammad IrsyadNo ratings yet
- Diagnostics 11 00161 v2Document14 pagesDiagnostics 11 00161 v2sara.dj.7cuNo ratings yet
- Role of Gamma Camera Components in Radiological DiagnosisDocument7 pagesRole of Gamma Camera Components in Radiological DiagnosisCentral Asian StudiesNo ratings yet
- Small Field Dosimetry For The Small Animal Radiotherapy Research Platform (SARRP)Document11 pagesSmall Field Dosimetry For The Small Animal Radiotherapy Research Platform (SARRP)ammar.herratiNo ratings yet
- BulganDocument10 pagesBulganIkh nahia Ikh Nakhia PharmNo ratings yet
- Priyanka and Om Prakash ChoudharyDocument6 pagesPriyanka and Om Prakash ChoudharyAditya RifNo ratings yet
- Distribution Statement A Approved For Public Release Distribution UnlimitedDocument15 pagesDistribution Statement A Approved For Public Release Distribution Unlimitedapi-255706616No ratings yet
- 2010 Artigo - X - Ray microCT LandisDocument12 pages2010 Artigo - X - Ray microCT LandisAna Kely SouzaNo ratings yet
- Pancreas Disease Detection and Segmentation Using Abdominal CT ScanDocument9 pagesPancreas Disease Detection and Segmentation Using Abdominal CT ScanIJAR JOURNALNo ratings yet
- Essay Writing in Biophotonics: December 24, 2011Document14 pagesEssay Writing in Biophotonics: December 24, 2011tanbir-hasan-6730No ratings yet
- Radiation Shielding and Bunker Design: Review ArticleDocument13 pagesRadiation Shielding and Bunker Design: Review Articlefadli puteraNo ratings yet
- Evaluation of Image Quality and Recovery Coefficient of Corn Starchbonded Rhizophora Spp. Particleboards As Phantom For SPECT/CT ImagingDocument8 pagesEvaluation of Image Quality and Recovery Coefficient of Corn Starchbonded Rhizophora Spp. Particleboards As Phantom For SPECT/CT ImagingPuteriNo ratings yet
- Calculo Ontecarlo MetodologiaDocument5 pagesCalculo Ontecarlo MetodologiaCarlos GutierrezNo ratings yet
- Radiopharmaceuticals For PET ImagingDocument7 pagesRadiopharmaceuticals For PET ImagingmemeiniNo ratings yet
- Fully 3-D Integrated Pixel Detectors For X-RaysDocument10 pagesFully 3-D Integrated Pixel Detectors For X-RaysHuy Anh BùiNo ratings yet
- Editor Response ExampleDocument5 pagesEditor Response ExampleCamilo PerezNo ratings yet
- First Clinical Trial of The Miro'' Capsule Endoscope by Using A Novel Transmission Technology: Electric-Field PropagationDocument7 pagesFirst Clinical Trial of The Miro'' Capsule Endoscope by Using A Novel Transmission Technology: Electric-Field PropagationfadzisomashavaNo ratings yet
- Notes On PETDocument13 pagesNotes On PETAbhi SachdevNo ratings yet
- DL 3Document20 pagesDL 3Gabbar IsBackNo ratings yet
- Complementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionDocument10 pagesComplementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionhesoyamyecgaaaNo ratings yet
- Simulation and Lithographic Fabrication of A Triple Band Terahertz Metamaterial Absorber Coated On Flexible Polyethylene Terephthalate SubstrateDocument22 pagesSimulation and Lithographic Fabrication of A Triple Band Terahertz Metamaterial Absorber Coated On Flexible Polyethylene Terephthalate SubstratekoyaunivNo ratings yet
- Artificial Neural Network For Bubbles Pattern Recognition On The ImagesDocument15 pagesArtificial Neural Network For Bubbles Pattern Recognition On The ImagesGustavo ArellanoNo ratings yet
- PetDocument346 pagesPetbdalcin5512No ratings yet
- Micro-computed Tomography (micro-CT) in Medicine and EngineeringFrom EverandMicro-computed Tomography (micro-CT) in Medicine and EngineeringKaan OrhanNo ratings yet
- MATH115 REYES Sample Quiz2Document2 pagesMATH115 REYES Sample Quiz2MEOW41No ratings yet
- Slide - Infinite Series of Constant Terms PDFDocument36 pagesSlide - Infinite Series of Constant Terms PDFMEOW41No ratings yet
- Math115 Lo#2Document1 pageMath115 Lo#2MEOW41No ratings yet
- Math115 Lo#1Document1 pageMath115 Lo#1MEOW41No ratings yet
- MATH115 Sample Long Quiz 4 Answers 08 July 2015Document2 pagesMATH115 Sample Long Quiz 4 Answers 08 July 2015MEOW41No ratings yet
- Training Program TemplateDocument2 pagesTraining Program TemplateMEOW41No ratings yet
- Importance of Biochemistry in Medicine - Human Nutrition - Diagnosis of Diseases - Treament of Disease States - Creation of "Designer" DrugsDocument1 pageImportance of Biochemistry in Medicine - Human Nutrition - Diagnosis of Diseases - Treament of Disease States - Creation of "Designer" DrugsMEOW41No ratings yet
- AndroidNotesForProfessionals PDFDocument1,329 pagesAndroidNotesForProfessionals PDFIlter KocamanNo ratings yet
- Universal Principles of Biomedical EthicsDocument16 pagesUniversal Principles of Biomedical EthicsMEOW41100% (3)
- Activities For ICpEP TBDocument5 pagesActivities For ICpEP TBMEOW41No ratings yet
- OS 216: Hematology: Topic Conference 1: AnemiasDocument7 pagesOS 216: Hematology: Topic Conference 1: AnemiasMEOW41No ratings yet
- Slide 6Document8 pagesSlide 6MEOW41No ratings yet
- Annual Occupational Dose Limit: Skin - 0.5 SVDocument7 pagesAnnual Occupational Dose Limit: Skin - 0.5 SVMEOW41No ratings yet
- Permanent Magnet Materials - A N PatelDocument64 pagesPermanent Magnet Materials - A N PatelRishabhNo ratings yet
- Continuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeDocument5 pagesContinuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeKasra GolbanNo ratings yet
- DXR Raman Ficha TecnicaDocument4 pagesDXR Raman Ficha TecnicaFernando Torres PérezNo ratings yet
- Lubrication Solutions For Industrial Applications: Energizing Performance. Every DayDocument68 pagesLubrication Solutions For Industrial Applications: Energizing Performance. Every DayEid EeidNo ratings yet
- Statistical Mechanics: Course Code - PPHY 302Document29 pagesStatistical Mechanics: Course Code - PPHY 302Siva ArunNo ratings yet
- Melting Point and Boiling PointDocument14 pagesMelting Point and Boiling PointMALOU ELEVERANo ratings yet
- BVD 2065 GBDocument4 pagesBVD 2065 GBФизули АбиловNo ratings yet
- ISO 717-1 Rate of Sound InsulationDocument20 pagesISO 717-1 Rate of Sound InsulationFYNo ratings yet
- Scramjet Combustion Fundamentals and Advances Gautam Choubey Full Download ChapterDocument51 pagesScramjet Combustion Fundamentals and Advances Gautam Choubey Full Download Chaptermargaret.hoffman258100% (21)
- Structure and Di Ffusion Behavior of Trioctyl Trimellitate (TOTM) in PVC Film Studied by ATR-IR SpectrosDocument11 pagesStructure and Di Ffusion Behavior of Trioctyl Trimellitate (TOTM) in PVC Film Studied by ATR-IR SpectrosGanciarov MihaelaNo ratings yet
- Particle Morphology and Powder Properties During - Wageningen University and Research 513788Document6 pagesParticle Morphology and Powder Properties During - Wageningen University and Research 513788Gabriel Henrique De Melo SilvaNo ratings yet
- Extended Elastic Impedance EEI Inversion PDFDocument37 pagesExtended Elastic Impedance EEI Inversion PDFsonu420No ratings yet
- MCQ Chapter 2 ElectrochemistryDocument4 pagesMCQ Chapter 2 ElectrochemistryFact GamerNo ratings yet
- Table of Personnel ProtectionDocument1 pageTable of Personnel ProtectionnincitoNo ratings yet
- 8 Legged Walking RobotDocument15 pages8 Legged Walking RobotAnuj TripathiNo ratings yet
- Experimental Investigation of Mustard Oil Based Nano Cutting Fluid On CNC Turning OperationDocument13 pagesExperimental Investigation of Mustard Oil Based Nano Cutting Fluid On CNC Turning OperationIJRASETPublicationsNo ratings yet
- Flexural Properties of Stringer Sections (Lipped and Unlipped Angles, Channels and Z-Sections)Document6 pagesFlexural Properties of Stringer Sections (Lipped and Unlipped Angles, Channels and Z-Sections)Lênon Guimarães Silva AlípioNo ratings yet
- Aer Conditionat Multisplit LG Unitati Externe Pliant Date Tehnice PDFDocument9 pagesAer Conditionat Multisplit LG Unitati Externe Pliant Date Tehnice PDFClau HerleaNo ratings yet
- 8.2 Aerodynamics 8.2.1: Airflow Around A BodyDocument6 pages8.2 Aerodynamics 8.2.1: Airflow Around A BodyNayem Hashan NayemNo ratings yet
- MAE 241-Lec4-Summer 2011Document14 pagesMAE 241-Lec4-Summer 2011kostas.sierros9374No ratings yet
- Module 02 Dan 03 Plate Tectonic, Gerak Dan Sifat InteraksiDocument48 pagesModule 02 Dan 03 Plate Tectonic, Gerak Dan Sifat InteraksiNurAjiNo ratings yet
- Chemical Kinetics Part 5 PDFDocument10 pagesChemical Kinetics Part 5 PDFShaikh FarooqNo ratings yet
- Solved Problem of First Three Chapters by Asif RasheedDocument10 pagesSolved Problem of First Three Chapters by Asif RasheedAsif Rasheed RajputNo ratings yet
- Progressive GR 9 2nd Q CDocument21 pagesProgressive GR 9 2nd Q CRAMIL BAUTISTANo ratings yet
- Deslandres Table N2DataDocument5 pagesDeslandres Table N2DataPiyali GhoshNo ratings yet
- Interaction CurveDocument126 pagesInteraction CurveNikhilNo ratings yet
- Peskin & Schroeder - The Quantum SpinDocument6 pagesPeskin & Schroeder - The Quantum SpinGiggs SandyNo ratings yet
- Topic 2 Pastpapers AllDocument44 pagesTopic 2 Pastpapers AllYoutube Foroau100% (1)