Professional Documents
Culture Documents
HW 2
HW 2
Uploaded by
DubistWhite0 ratings0% found this document useful (0 votes)
20 views2 pagesThis document contains plots of bonding energy (Ea, Er, En) versus interatomic separation for an unknown diatomic molecule.
The plots show that at an interatomic separation of 0.24nm, the bonding energy is at a minimum of -4.58eV.
It also provides the electronegativity values for elements Ga, P, Cs, F, Fe and O and uses those values to calculate the percentage ionic character of bonds in GaP (6.06%), CsF (93.4%) and FeO (51.4%).
Original Description:
material science
Original Title
hw2
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains plots of bonding energy (Ea, Er, En) versus interatomic separation for an unknown diatomic molecule.
The plots show that at an interatomic separation of 0.24nm, the bonding energy is at a minimum of -4.58eV.
It also provides the electronegativity values for elements Ga, P, Cs, F, Fe and O and uses those values to calculate the percentage ionic character of bonds in GaP (6.06%), CsF (93.4%) and FeO (51.4%).
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
20 views2 pagesHW 2
HW 2
Uploaded by
DubistWhiteThis document contains plots of bonding energy (Ea, Er, En) versus interatomic separation for an unknown diatomic molecule.
The plots show that at an interatomic separation of 0.24nm, the bonding energy is at a minimum of -4.58eV.
It also provides the electronegativity values for elements Ga, P, Cs, F, Fe and O and uses those values to calculate the percentage ionic character of bonds in GaP (6.06%), CsF (93.4%) and FeO (51.4%).
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
R
0
0.1
0.2
0.3
0.4
Ea
Er
-14.36
-7.18
-4.79
-3.59
732
2.86
0.113
0.0113
0.5
-2.87
0.6
-2.39
0.7
-2.05
0.8
-1.8
0.9
1
-1.6
-1.436
En
717.64
-4.32
-4.677
-3.5787
0.00187 2.86813
0.000436 2.38956
0.000127 2.04987
0.0000436 1.79996
0.000017 1.59998
7.32E-14
-1.436
(A.)
4
B
o
n
d
i
n
g
e
n
e
r
g
y
2
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.1
-2
Ea
Er
En
-4
-6
-8
interatomic seperation, nm
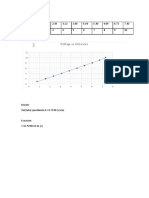
(b) From this plot Ro =
0.24nm
E0 = -4.58eV,
Question 2: Compute the percentage ionic character of the interatomic bond for each of the following
compounds: GaP, CsF, and FeO.
The percent ionic character is a function of the electron negativities of the ions XA and XB according to
Equation 2.10.The electro negativities of the elements are found in Figure 2.7.
For GaP XGa = 1.6XP = 2.1 %IC = [1 e ((-0.25) (2.1-1.6)2)]*100] = 6.06%
For CsF Xcs = 0.7 XF = 4.0
%IC = [1 e ((-0.25) (4.0-0.7)2)]*100] = 93.4%
For FeO XFe = 1.8 XO = 3.5
%IC = [1 e ((-0.25) (3.5-1.8)2)]*100] = 51.4%
You might also like
- The Rotary Cement KilnDocument388 pagesThe Rotary Cement KilnDubistWhite100% (6)
- Chapter 7 Solutions Modern Physics 4th EditionDocument32 pagesChapter 7 Solutions Modern Physics 4th EditionhabanerochildNo ratings yet
- Set 7 AnsDocument4 pagesSet 7 AnsArturo Hernández MoralesNo ratings yet
- Small Scale Dosimetry: Beta Emitters (SS-) : Manuel Bardiès, INSERM UMR 892, Nantes, France Manuel - Bardies@Document39 pagesSmall Scale Dosimetry: Beta Emitters (SS-) : Manuel Bardiès, INSERM UMR 892, Nantes, France Manuel - Bardies@Madalina-Elena CostacheNo ratings yet
- 2016 Jiang Zhang Anotherdummys SupportinginformationDocument21 pages2016 Jiang Zhang Anotherdummys SupportinginformationalejandraNo ratings yet
- Hrs. Studies Grade On Exam Xi Yi Xi - Xbar Yi - Ybar (Xi - Xbar) (Yi - Ybar)Document6 pagesHrs. Studies Grade On Exam Xi Yi Xi - Xbar Yi - Ybar (Xi - Xbar) (Yi - Ybar)ElizabethNo ratings yet
- 11) Tanimo1986Document5 pages11) Tanimo1986Mohammad JawadNo ratings yet
- Analisis Korelasi - ZainabDocument7 pagesAnalisis Korelasi - ZainabAhmadi NavalNo ratings yet
- Cover PageDocument5 pagesCover PageA DNo ratings yet
- CoV - Ex 1Document1 pageCoV - Ex 1Venkata Aditya SomisettyNo ratings yet
- EXAMPLE 8.32: N-HeptaneDocument7 pagesEXAMPLE 8.32: N-HeptaneAria DarmawanNo ratings yet
- Ao9b00622 Si 001Document9 pagesAo9b00622 Si 001AnushanNo ratings yet
- Electron Stopping Powers: Cedric J. PowellDocument2 pagesElectron Stopping Powers: Cedric J. PowellantonioNo ratings yet
- Critical Behaviour of Anisotropic Spiral Self Avoiding WalksDocument5 pagesCritical Behaviour of Anisotropic Spiral Self Avoiding WalksFrontiersNo ratings yet
- Instructional Module On Correlation and Regression ProceduresDocument23 pagesInstructional Module On Correlation and Regression ProceduresAlbyra Bianca Sy TamcoNo ratings yet
- The Study of Modulation Schemes 変調方式に関する研究Document20 pagesThe Study of Modulation Schemes 変調方式に関する研究संजय यादवNo ratings yet
- Answer 79236Document2 pagesAnswer 79236tiesNo ratings yet
- Labo1 GraficosDocument5 pagesLabo1 GraficosVieri Sergio Ocrospoma CallupeNo ratings yet
- Tugas Praktek Kecerdasan Buatan: Universitas Methodist Indonesia Fakultas Ilmu Komputer TA. 2013/2014Document16 pagesTugas Praktek Kecerdasan Buatan: Universitas Methodist Indonesia Fakultas Ilmu Komputer TA. 2013/2014AnggaNo ratings yet
- Solutions To HW Exercises - Ch. 14Document3 pagesSolutions To HW Exercises - Ch. 14ShardulNo ratings yet
- CBSE 10th Most Important Questions (NCERT)Document1 pageCBSE 10th Most Important Questions (NCERT)RoyaleNo ratings yet
- Variabel X2 Motivasi Guru: NotesDocument13 pagesVariabel X2 Motivasi Guru: NotesAndi Jumardi Al-BugizyNo ratings yet
- (Maths Assign2)Document16 pages(Maths Assign2)anmol agrawalNo ratings yet
- 3 Dan 4Document10 pages3 Dan 4NorhalisahNo ratings yet
- Improving Pattern Recognition Using Several Feature Vectors: 1 IntroductionDocument7 pagesImproving Pattern Recognition Using Several Feature Vectors: 1 IntroductionIvan lopezNo ratings yet
- Manual SAS GEOESTADISTICADocument21 pagesManual SAS GEOESTADISTICAcamilofon77No ratings yet
- CHIFAMBA MELBAH R213866M .Test InclassDocument3 pagesCHIFAMBA MELBAH R213866M .Test InclasswilsonNo ratings yet
- CoDocument4 pagesCoMissiles dodgedNo ratings yet
- 2010 APhO Experimental Question 2 - SolutionDocument12 pages2010 APhO Experimental Question 2 - SolutionSiddharth AcharyaNo ratings yet
- Chemrj 2017 02 04 84 90Document7 pagesChemrj 2017 02 04 84 90editor chemrjNo ratings yet
- Ambrose 1975Document6 pagesAmbrose 1975Ankur PatelNo ratings yet
- A AssmtDocument85 pagesA AssmtNiroj MaharjanNo ratings yet
- Molecular Hyperpolarizabilities of Coumarin Dyes: MoylanDocument4 pagesMolecular Hyperpolarizabilities of Coumarin Dyes: MoylanIsmael Vargas RodriguezNo ratings yet
- ParallelDocument9 pagesParallelSagar RawalNo ratings yet
- Data Kematian Angola: Menghitung Mean Square ErrorDocument6 pagesData Kematian Angola: Menghitung Mean Square ErrorDevi AfsanahNo ratings yet
- Statistics and Probability ExampleDocument3 pagesStatistics and Probability ExampleAndrea AldeaNo ratings yet
- BIO&Epi Final22Document3 pagesBIO&Epi Final22ERICA ROSE ENRIQUEZNo ratings yet
- Solutions Manual Ch13 - 2012Document37 pagesSolutions Manual Ch13 - 2012thegreatllNo ratings yet
- BEC Finals - 2Document2 pagesBEC Finals - 2Lorenz BerroyaNo ratings yet
- IE101 Problem SetDocument6 pagesIE101 Problem SetGray Fiore FullbusterNo ratings yet
- BP-C4-o+ (3-4°-J6+ (3-Y) : Euidisant YaisDocument7 pagesBP-C4-o+ (3-4°-J6+ (3-Y) : Euidisant YaisadithyanNo ratings yet
- 27 AnswersDocument25 pages27 AnswersYadana1No ratings yet
- Curtin University of Technology Department of Mechanical EngineeringDocument10 pagesCurtin University of Technology Department of Mechanical Engineeringalan_kit_2No ratings yet
- Diskusi Sesi 5 AriefWijaya 041324513Document2 pagesDiskusi Sesi 5 AriefWijaya 041324513Jupi TerzNo ratings yet
- Homework 4: Emmanuel Nkansah Econometrics IVDocument5 pagesHomework 4: Emmanuel Nkansah Econometrics IVemmanuel nkansahNo ratings yet
- Empirical Formula For Beta-Particle-Induced Bremsstrahlung YieldsDocument6 pagesEmpirical Formula For Beta-Particle-Induced Bremsstrahlung YieldsTanmoy BanerjeeNo ratings yet
- 58 B 8 Eef 6 BC 97 Fa 1 CDB 7 FDocument2 pages58 B 8 Eef 6 BC 97 Fa 1 CDB 7 Fapi-441441400No ratings yet
- Dacs Class Work 10augDocument4 pagesDacs Class Work 10augBharath SNo ratings yet
- AML 170423siDocument14 pagesAML 170423siAleksa SesicNo ratings yet
- This Study Resource Was: Informe #4: Medida de Reactancia Inductiva, Capacitiva E ImpedanciaDocument9 pagesThis Study Resource Was: Informe #4: Medida de Reactancia Inductiva, Capacitiva E ImpedanciaDavid Sencia TorresNo ratings yet
- KYM332 11. HaftaDocument8 pagesKYM332 11. HaftaHande KorkmazNo ratings yet
- O E O E (O E) (O E) /E: Chi-Square AnalysisDocument6 pagesO E O E (O E) (O E) /E: Chi-Square Analysismraj24No ratings yet
- Ibf RiskDocument3 pagesIbf RiskAnas4253No ratings yet
- Doubly Excited 1,3P e Resonances in He Between The N 2 and 3 He + ThresholdsDocument8 pagesDoubly Excited 1,3P e Resonances in He Between The N 2 and 3 He + Thresholdsaa022551006No ratings yet
- ME-4 CertificateDocument4 pagesME-4 CertificateFRoblesNo ratings yet
- Nama: Salwa Fasha Wijaya NPM: A1A020089 Kelas: 4C Prodi: Pendidikan Bahasa IndonesiaDocument12 pagesNama: Salwa Fasha Wijaya NPM: A1A020089 Kelas: 4C Prodi: Pendidikan Bahasa IndonesiaSalwa fasha WijayaNo ratings yet
- Informe2 1Document22 pagesInforme2 1Wilfredo Sirhua VargasNo ratings yet
- Influence of Focal Mechanism On Shape of Isoseismals: Irpinia Earthquake of November 23Document6 pagesInfluence of Focal Mechanism On Shape of Isoseismals: Irpinia Earthquake of November 23Najeb PendiamanNo ratings yet
- Latih Tubi Persediaan Ujian 1 (Question) KIM3301 Sem II 20-21 K1 K2Document2 pagesLatih Tubi Persediaan Ujian 1 (Question) KIM3301 Sem II 20-21 K1 K2Business MatterNo ratings yet
- NMR Spectroscopy Explained: Simplified Theory, Applications and Examples for Organic Chemistry and Structural BiologyFrom EverandNMR Spectroscopy Explained: Simplified Theory, Applications and Examples for Organic Chemistry and Structural BiologyNo ratings yet
- Method of Lines PDE Analysis in Biomedical Science and EngineeringFrom EverandMethod of Lines PDE Analysis in Biomedical Science and EngineeringNo ratings yet
- 5 BlastingDocument75 pages5 BlastingDubistWhiteNo ratings yet
- 9 - Quarry RehabilitationDocument36 pages9 - Quarry RehabilitationDubistWhiteNo ratings yet
- Crushing Principles and Equipment: by Evgueni Porokhovoi. 2019Document53 pagesCrushing Principles and Equipment: by Evgueni Porokhovoi. 2019DubistWhite100% (1)
- 14 - Raw MixDocument31 pages14 - Raw MixDubistWhite100% (1)
- 2 - Cement Raw MaterialsDocument32 pages2 - Cement Raw MaterialsDubistWhiteNo ratings yet
- Rossin RammbllerDocument15 pagesRossin RammbllerDubistWhiteNo ratings yet
- 1 - General GeologyDocument60 pages1 - General GeologyDubistWhiteNo ratings yet
- Frigate Flyer Air LiftDocument1 pageFrigate Flyer Air LiftDubistWhite100% (1)
- M Aking An Impact at Kohat CementDocument4 pagesM Aking An Impact at Kohat CementDubistWhiteNo ratings yet
- Engineering PaperDocument15 pagesEngineering PaperDubistWhiteNo ratings yet
- Experiment Molarity (Mol DM) Rates (Mol DM / Min) : Chy2018 Tutorial 3 (Kinetics)Document2 pagesExperiment Molarity (Mol DM) Rates (Mol DM / Min) : Chy2018 Tutorial 3 (Kinetics)DubistWhiteNo ratings yet
- Electrochemistry Tut 2008Document3 pagesElectrochemistry Tut 2008DubistWhiteNo ratings yet
- Transport Phenomena Transport CoefficientsDocument3 pagesTransport Phenomena Transport CoefficientsDubistWhiteNo ratings yet
- MAT3004 Work Sheet 2 201314Document3 pagesMAT3004 Work Sheet 2 201314DubistWhiteNo ratings yet
- Hach K - C D: ITS Arbon IoxideDocument2 pagesHach K - C D: ITS Arbon IoxideDubistWhiteNo ratings yet