Professional Documents
Culture Documents
NIH Public Access: Multivariate Pattern Analysis of fMRI: The Early Beginnings
NIH Public Access: Multivariate Pattern Analysis of fMRI: The Early Beginnings
Uploaded by
fiestamixOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
NIH Public Access: Multivariate Pattern Analysis of fMRI: The Early Beginnings
NIH Public Access: Multivariate Pattern Analysis of fMRI: The Early Beginnings
Uploaded by
fiestamixCopyright:
Available Formats
NIH Public Access
Author Manuscript
Neuroimage. Author manuscript; available in PMC 2013 August 15.
NIH-PA Author Manuscript
Published in final edited form as:
Neuroimage. 2012 August 15; 62(2): 852855. doi:10.1016/j.neuroimage.2012.03.016.
Multivariate pattern analysis of fMRI: The early beginnings
James. V. Haxby
Center for Cognitive Neuroscience, Dartmouth College, Hanover, NH, Center for Mind/Brain
Sciences, University of Trento, Italy
Abstract
NIH-PA Author Manuscript
In 2001, we published a paper on the representation of faces and objects in ventral temporal cortex
that introduced a new method for fMRI analysis, which subsequently came to be called
multivariate pattern analysis (MVPA). MVPA now refers to a diverse set of methods that analyze
neural responses as patterns of activity that reflect the varying brain states that a cortical field or
system can produce. This paper recounts the circumstances and events that led to the original
study and later developments and innovations that have greatly expanded this approach to fMRI
data analysis, leading to its widespread application.
Keywords
fMRI; multivariate pattern analysis (MVPA); vision; decoding; machine learning; pattern
classification
NIH-PA Author Manuscript
Multivariate pattern analysis (MVPA) of fMRI data has proven to be more sensitive and
more informative about the functional organization of cortex than is univariate analysis with
the general linear model (GLM). MVPA refers to a set of methods that analyze neural
responses as patterns of activity, thus affording investigation of the varying brain states that
a cortical field or system can produce, thus increasing the amount of information that can be
decoded from brain activity, in contrast to simpler univariate measures that indicate the
extent to which a cortical field or system is globally engaged. We first devised a prototype
MVPA method in the course of investigation of the functional architecture for face and
object recognition in ventral temporal cortex (Haxby et al., 2001). The results of this study
demonstrated the basic concept and power of MVPA. Initially, however, the paper attracted
attention primarily for its neuroscientific content, namely as a strong argument for
distributed representation of high-level visual percepts that stood in contrast to modular
accounts (Kanwisher et al. 1997). Recognition of the significance of the methodological
innovation was slower. The initial disinclination of others to use MVPA in fMRI research
was due, in part, to unfamiliarity and the perceived complexity of these methods. MVPA
does not provide simple answers to the kinds of questions people were asking Where is the
motion area? Where is the face (or place or body parts) area? Where is the numbers area?
and so forth; and partly because they addressed questions that people hadnt thought of
investigating What are the varying brain states in an area and how do they encode different
types of information? These methods were not simply another method to answer the same
2012 Elsevier Inc. All rights reserved.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our
customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of
the resulting proof before it is published in its final citable form. Please note that during the production process errors may be
discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Haxby
Page 2
questions but, rather, challenged cognitive neuroscientists to consider a different model of
cortical organization.
NIH-PA Author Manuscript
In 1991 and 1994, working with Cheryl Grady, Barry Horwitz, Leslie Ungerleider, Mort
Mishkin, and Stanley Rapoport, we published two positron emission tomography (PET)
studies on the division of visual processing in the human brain into the ventral object vision
pathway and the dorsal spatial vision pathway (Haxby et al. 1991, 1994). We had chosen a
face-matching task as our proxy for object vision in the ventral pathway. Without fail,
wherever we presented these data, we were asked why we had made this decision, given the
neuropsychological, developmental, and behavioral evidence that face processing has a
special status that is distinct from processing of other objects.
NIH-PA Author Manuscript
In the meantime, I had moved from the National Institute on Aging to the National Institute
of Mental Health (down two floors) where I developed an independent cognitive
neuroscience research program working with Leslie Ungerleider and Alex Martin. With our
entry into fMRI research, one of my first goals was to address whether face and object
perception activate the same or different cortical regions. As we were still puzzling over our
initial results, others published reports on a specialized area in the fusiform gyrus that
responded more during face perception tasks than during perception of other objects (Puce et
al. 1996; McCarthy et al. 1997; Kanwisher et al. 1997). We decided to conduct two further
experiments one looking at the effect of face inversion (Haxby et al. 1999) and the other
looking at whether two different categories of objects evoked equivalent patterns of
response in the non-face-selective cortex (Ishai et al. 1999). The results of both studies
confirmed the existence of a patch of fusiform cortex that responded more to faces than to
other stimuli but led us to doubt the interpretation that this region is specialized for face
processing and nothing else. As I analyzed the results of the face inversion paper, I also
devised a new method of correlating patterns of response to different conditions, which
became the basis for our paper in 2001 that introduced MVPA (Haxby et al. 2001).
NIH-PA Author Manuscript
The principal finding from these two studies that made us doubt that the face area was
specialized for face perception and nothing else, and that face processing was restricted to
the face area, came from our analysis of responses to faces, houses, and chairs, relative to a
no stimulus baseline, in ventral temporal cortex (Haxby et al. 1999, Figure 2; Ishai et al.
1999, Figure 3). The voxels in the face area clearly showed significant responses to the nonpreferred categories. Similarly, chair-selective voxels and house-selective voxels also
showed positive, and usually significant, responses to non-preferred categories (with the
exception of a negligible response to faces in medial fusiform house-selective voxels). The
strong modularity hypothesis, and the logic of univariate analysis of contrasts in
neuroimaging, implied that these weaker, albeit significant, brain responses played no role in
representation. In essence, they are discarded because they indicate that the stimulus is a
suboptimal fit for the function in those cortical fields. This seemed improbable to us on two
counts. First, discarding information in submaximal responses seemed suboptimal and
conflicted with methods for analyzing population response representations. Second, the
proposal that every possible face, animal, and object category has a specialized region or set
of neurons dedicated to its representation didnt seem possible. There are too many ways
that faces and objects can be categorized. Moreover, category-dedicated systems do not
capture the relationships, or similarities, among categories. Instead, it seemed more probable
that the weak responses play an important role in representation, implying that face and
object categories are encoded as patterns of neural activity, rather than as peak responses in
category-specific modules, and that these patterns involve neural populations that play a role
in the representation of multiple categories.
Neuroimage. Author manuscript; available in PMC 2013 August 15.
Haxby
Page 3
NIH-PA Author Manuscript
We set out to test this hypothesis and began collecting data in 1998 on neural responses to
eight categories of objects, animals, and faces human faces, cats, chairs, shoes, scissors,
bottles, houses, and phase-scrambled images. The broad outlines of the project were clear
from the beginning but working out the details of the analysis took a lot of time. We
hypothesized that each category would evoke a distinct pattern of response in ventral
temporal cortex. We hypothesized further that these distinctive patterns would not be
restricted to category-selective regions, such as the FFA and PPA, leading to the prediction
that distinctive patterns could be detected if such regions were excluded from the analysis.
We also hypothesized that neural activity patterns within category-selective regions would
carry information that discriminates between categories other than those regions preferred
stimuli. The analytic approach would be based on the pattern correlation method that I had
devised for our paper on face inversion (Haxby et al. 1999). In hindsight, the project was
straightforward from the beginning and was completed mostly as planned. At the time, I felt
as if I were groping around in the dark working out how to actually execute these analyses.
My next-door neighbor at NIH, Alex Martin, thought that I would never emerge from these
labors.
NIH-PA Author Manuscript
The idea was straightforward and based on a concept from conventional statistics, namely
split-sample cross-validation. If a given stimulus category evoked a distinct pattern of
activity, then independent observations of the response to that category should be more
similar to each other than to responses to different categories. Correlation of patterns was the
chosen measure of similarity, and I made independent observations by dividing the data for
each subject into two halves even-numbered and odd-numbered runs. Thus, I predicted
that within-category correlations would be higher than between-category correlations. The
subsidiary hypotheses were to be tested in two further analyses. In the first, I identified the
voxels that responded maximally to each category and eliminated those voxels for each
possible pair of categories when comparing within- and between-category correlations for
that pair. In the second, I identified the FFA and PPA and tested for category-specific
patterns for all categories within those areas.
In retrospect, it is hard to remember why devising and executing the analysis took so long.
Working with fMRI analysis systems that were designed for univariate analyses, primarily
AFNI (Cox) and, to a lesser extent, our own home-grown FIDAP (Functional Imaging Data
Analysis Program, written by Jos Maisog in my group, and decommissioned years ago) and
UNIX programming, each step was constructed using tools not designed for this application.
For example, the correlations were all calculated using a little-known function in AFNI,
3ddot, that calculated one correlation at a time.
NIH-PA Author Manuscript
At the time, I was unaware of other pattern classification methods from machine learning.
The relevance and utility of these methods was demonstrated through collaborations with
colleagues who reanalyzed my data using neural net classifiers (Hanson et al. 2004) and
linear discriminant analysis (LDA) (OToole et al. 2005) and through subsequent papers
from groups that analyzed similar datasets collected independently, most prominently Cox
& Savoys (2003) application of support vector machines (SVM). Tom Mitchell later
dignified the split-sample, correlation-based method, which I had devised based on
conventional statistics, by giving it a respectable machine learning pattern classifier name,
calling it a one-nearest neighbor classifier using a correlation-based distance measure
(correlation-based 1NN). Curiously, this method is still used in many reports (e.g. Peelen et
al. 2010), presumably because of its conceptual simplicity and straightforward
interpretation. It also proved to be surprisingly sensitive, although we have found that other
classifiers, in particular SVM (e.g. Connolly et al. in press; Haxby et al. 2011), consistently
outperform correlation-based 1NN. Later, my colleague at Princeton, Ken Norman, took to
calling this approach multivoxel pattern analysis (MVPA), which we subsequently
Neuroimage. Author manuscript; available in PMC 2013 August 15.
Haxby
Page 4
changed to multivariate pattern analysis, to acknowledge, with no need for a new acronym,
its application to feature sets other than voxels.
NIH-PA Author Manuscript
The general adoption of MVPA gained momentum very slowly. I believe that three factors
underlay this inertia. First and foremost, it was unclear what the results of an MVPA
classification analysis meant in terms of neural representation. Second, it seemed
complicated and software for implementation was not available. Third, MVPA analyses
typically were done separately for each individual because the pattern structure that carries
subtle distinctions appeared to be based on fine-grained topographies that did not align well
across brains based on anatomy. Consequently, the cortical topographic features that carry
these subtle distinctions were unspecified and somewhat mysterious.
NIH-PA Author Manuscript
Enthusiasm for MVPA ticked up significantly with two reports that appeared back-to-back
in Nature Neuroscience in 2005 (Kamitani & Tong, 2005; Haynes & Rees, 2005), both of
which showed that MVPA could be applied to a visual feature with a well-understood neural
basis, namely edge orientation. Kamitani & Tong (2005) and later papers from Haynes
group showed further that MVPA could decode cognitive states, such as the target of
selective attention (Kamitani & Tong, 2005), and the intention to perform one task rather
than another (Haynes et al. 2007). Shortly thereafter, a series of review papers began to
appear that presented the principles of MVPA in a clear and accessible manner, further
demystifying the basis of this new analytic approach (Haynes & Rees, 2006; Norman et al.
2006; OToole et al. 2007; Mur et al. 2009; Pereira et al. 2009; Tong and Pratt, 2012).
NIH-PA Author Manuscript
A parallel development was the realization that multivariate response patterns also could be
analyzed in terms of strength of similarities among response patterns, rather than simply as
binary distinctions, affording analysis of the structure of representational spaces. This work
actually began with an underappreciated paper by Edelman et al. (1998). Reanalyses of our
data by Steve Hanson and his colleagues (2004) and Alice OToole and her colleagues
(2005) revealed similarity structures that conformed to intuitions about semantic
relationships namely, a major distinction between animate and inanimate objects and a
secondary distinction between houses and small inanimate objects. This approach was
amplified greatly in a landmark study by Kiani et al. (2007) of similarity structure in the
population responses of large numbers of single cells in monkey IT cortex, followed by a
second landmark study (Kriegeskorte et al. 2008a), which showed that the similarity
structure in monkey IT cortex (from Kiani et al.s single unit data) and human VT cortex
(using fMRI) are remarkably similar, characterized by major distinctions between animate
and inanimate stimuli and, within the animate domain, between faces and bodies. In a later
study, Connolly et al. (in press) found even more structure in the representation of animate
entities in human VT cortex, with a similarity structure that embodies semantic relationships
among classes of animal species, similar to what Kiani et al. (2007) had found in monkey IT
population responses. Kriegeskorte (2008b) has proposed that analysis of similarity structure
could provide a common basis for comparing representational spaces across multiple
neuroscience approaches, such as fMRI, single unit physiology, computational models, and
stimulus models, and gave this approach a new name, representational similarity analysis
or RSA, which has stuck.
Meanwhile, another new approach showed that multivoxel responses to new stimuli could
be predicted based on high-dimensional feature models of stimuli (Kay et al. 2008; Mitchell
et al. 2008; Naselaris et al. 2009; Nishimoto et al. 2011). These methods make the
relationships between stimulus attributes and response patterns explicit, bringing us closer to
understanding how patterns of activity in fMRI data and stimulus representation are related.
Neuroimage. Author manuscript; available in PMC 2013 August 15.
Haxby
Page 5
NIH-PA Author Manuscript
MVPA is more complicated than conventional univariate analysis, and this complexity has
been a major barrier for many. Good software is now readily available, however, that makes
MVPA more accessible to investigators. When I was at Princeton, a graduate student in Ken
Normans laboratory, Greg Detre, took on the project of developing an MVPA toolbox using
Matlab (the Princeton MVPA toolbox (http://code.google.com/p/princeton-mvpa-toolbox/).
Around the same time, Michael Hanke, who at the time was a graduate student studying
with Stefan Pollman at the University of Magdeburg, came to Princeton to learn MVPA in
my laboratory. Michael Hanke turned out to be a brilliant software developer and a fervent
advocate of free and open-source software (FOSS). He decided to develop PyMVPA, a
Python-based MVPA toolbox, in collaboration with Yaroslav Halchenko
(www.pymvpa.org) (Hanke et al. 2009), who was working with Steve Hanson at Rutgers
University. By using Python, this toolbox incorporates software from a vast library of
machine learning algorithms.
NIH-PA Author Manuscript
Another source of wariness about MVPA came from its reliance on fine-scale topographic
features to detect subtle distinctions between patterns. These fine-scale features cannot be
aligned well across brains based on anatomy. Consequently, MVPA generally builds
classifier models for an individual subject based on that subjects own data. As a result, the
population response topographies that carry fine distinctions are difficult to characterize and
appear idiosyncratic. Ten years after our 2001 paper, we published a paper that, we believe,
solves this problem (Haxby et al. 2011). In this report, we presented a new method,
hyperalignment, that enabled us to derive common models of cortical representational
spaces that capture all of the information for MVP classification in a set of a few dozen basis
functions. These basis functions have stimulus tuning functions that are common across
brains and model individual voxel tuning functions as weighted sums. These basis functions
also are associated with individual-specific pattern basis functions that model individual
cortical response patterns as weighted sums. These models thus account for the fine-scale
topographies that underlie the sensitivity of MVPA in a straightforward linear model and
show that these topographies have a basis that is common across individuals.
NIH-PA Author Manuscript
Ten years after our paper that introduced MVPA in 2001, the methods have become vastly
more sophisticated, software has been developed that make implementation with
standardized algorithms widely accessible, and the conceptual foundations have been
clarified and amplified. The methods are more complicated than those of conventional
univariate analysis, requiring more sophistication and computational knowledge on the part
of its users. MVPA, however, allows one to investigate questions about the how information
is encoded in patterns of neural activity, rather than simpler questions about where functions
simply are performed. Many investigators are now finding that the scientific payoff justifies
the effort necessary to use these methods effectively. Now that this approach has engaged a
large community of sophisticated investigators, the rate of development and innovation is, if
anything, accelerating.
References
Connolly AC, Guntupalli JS, Gors J, Hanke M, Halchenko YO, Wu Y-C, Abdi H, Haxby JV. The
representation of biological classes in the human brain. J Neurosci. 2012; 32:26082618. [PubMed:
22357845]
Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) brain reading: detecting and
classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage. 2003; 19:261
270. [PubMed: 12814577]
Edelman S, Grill-Spector K, Kushnir T, Malach R. Toward direct visualization of the internal shape
space by fMRI. Psychobiol. 1998; 26:309321.
Neuroimage. Author manuscript; available in PMC 2013 August 15.
Haxby
Page 6
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Hanke M, Halchenko YO, Sederberg PB, Hanson SJ, Haxby JV, Pollman S. PyMVPA: A Python
toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics. 2009; 7:3753. [PubMed:
19184561]
Hanson SJ, Toshihiko M, Haxby JV. Combinatorial codes in ventral temporal lobe for object
recognition: Haxby (2001) revisited: Is there a face area. Neuroimage. 2004; 23:156167.
[PubMed: 15325362]
Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro
MB, Rapoport SI. Dissociation of object and spatial visual processing pathways in human
extrastriate cortex. Proc Natl Acad Sci, USA. 1991; 88:16211625. [PubMed: 2000370]
Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization
of human extrastriate cortex: A PET-rCBF study of selective attention to faces and locations. J
Neurosci. 1994; 14:63366353. [PubMed: 7965040]
Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A. The effect of face
inversion on activity in human neural systems for face and object perception. Neuron. 1999;
22:189199. [PubMed: 10027301]
Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping
representations of faces and objects in ventral temporal cortex. Science. 2001; 293:24252430.
[PubMed: 11577229]
Haxby JV, Guntupalli JS, Connolly AC, Halchenko YO, Conroy BR, Gobbini MI, Hanke M, Ramadge
PJ. A common, high-dimensional model of the representational space in human ventral temporal
cortex. Neuron. 2011; 72:404416. [PubMed: 22017997]
Haynes JD, Rees G. Predicting the orientation of invisible stimuli from activity in primary visual
cortex. Nature Neurosci. 2005; 8:686691. [PubMed: 15852013]
Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nature Rev Neurosci.
2006; 7:523534. [PubMed: 16791142]
Haynes JD, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE. Reading hidden intentions in the
human brain. Curr Biol. 2007; 17:323328. [PubMed: 17291759]
Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in
the human ventral visual pathway. Proc Natl Acad Sci, USA. 1999; 96:93799384. [PubMed:
10430951]
Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nature
Neurosci. 2005; 8:679685. [PubMed: 15852014]
Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex
specialized for face perception. J Neurosci. 1997; 17:43024311. [PubMed: 9151747]
Kay KN, Naselaris T, Prenger RJ, Gallant JL. Identifying natural images from human brain activity.
Nature. 2008; 452:352355. [PubMed: 18322462]
Kiani R, Esteky H, Mirpour K, Tanaka K. Object category structure in response patterns of neuronal
population in monkey inferior temporal cortex. J Neurophysiol. 2007; 97:42964309. [PubMed:
17428910]
Kriegeskorte N, Mur M, Ruff DA, Kiani R, Bodurka J, Esteky H, Tanaka K, Bandettini PA. Matching
categorical object representations in inferior temporal cortex of man and monkey. Neuron. 2008a;
60:11261141. [PubMed: 19109916]
Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis - connecting the branches of
systems neuroscience. Front Syst Neurosci. 2008b; 2:4. [PubMed: 19104670]
McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. J
Cogn Neurosci. 1997; 9:605610.
Mitchell TM, Shinkareva SV, Carlson A, Chang KM, Malave VL, Mason RA, Just MA. Predicting
human brain activity associated with the meanings of nouns. Science. 2008; 320:11911195.
[PubMed: 18511683]
Mur M, Bandettini PA, Kriegeskorte N. Revealing representational content with pattern-information
fMR: an introductory guide, Soc. Cog Neurosci. 2009; 4:101109.
Nishimoto S, Vu AT, Naselaris T, Bejamini Y, Yu B, Gallant JL. Reconstructing visual experience
from brain activity evoked by natural movies. Curr Biol. 2011; 21:16. [PubMed: 21129968]
Neuroimage. Author manuscript; available in PMC 2013 August 15.
Haxby
Page 7
NIH-PA Author Manuscript
Naselaris T, Prenger RJ, Kay KN, Oliver M, Gallant JL. Bayesian reconstruction of natural images
from human brain activity. Neuron. 2009; 63:902915. [PubMed: 19778517]
Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind- reading: multi-voxel pattern analysis of
fMRI data. Trends Cogn Sci. 2006; 10:424430. [PubMed: 16899397]
OToole AJ, Jiang F, Abdi H, Haxby JV. Partially distributed representations of objects and faces in
ventral temporal cortex. J Cogn Neurosci. 2005; 17:580590. [PubMed: 15829079]
OToole AJ, Jiang F, Abdi H, Pnard N, Dunlop JP, Parent MA. Theoretical, statistical, and practical
perspectives on pattern-based classification approaches to the analysis of functional neuroimaging
data. J Cogn Neurosci. 2007; 19:17351752. [PubMed: 17958478]
Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the
human brain. J Neurosci. 2010; 30:1012710134. [PubMed: 20668196]
Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview.
Neuroimage. 2009; 45(Suppl 1):S199209. [PubMed: 19070668]
Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to
faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci. 2006;
16:52055215. [PubMed: 8756449]
Tong F, Pratte MS. Decoding patterns of human brain activity. Ann Rev Psychol. 2012; 63:483509.
[PubMed: 21943172]
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Neuroimage. Author manuscript; available in PMC 2013 August 15.
You might also like
- S4C01E - SAP S - 4HANA Cloud On-Boarding Fundamentals - SAP TrainingDocument4 pagesS4C01E - SAP S - 4HANA Cloud On-Boarding Fundamentals - SAP Trainingfiestamix13% (8)
- SCM600 - Sales Order Management - SAP TrainingDocument7 pagesSCM600 - Sales Order Management - SAP TrainingfiestamixNo ratings yet
- MDG100 - SAP Master Data Governance - SAP TrainingDocument7 pagesMDG100 - SAP Master Data Governance - SAP Trainingfiestamix0% (3)
- The New Mind Readers: What Neuroimaging Can and Cannot Reveal about Our ThoughtsFrom EverandThe New Mind Readers: What Neuroimaging Can and Cannot Reveal about Our ThoughtsNo ratings yet
- SCM605 - Sales Processing in SAP ERP - SAP TrainingDocument7 pagesSCM605 - Sales Processing in SAP ERP - SAP Trainingfiestamix33% (3)
- Raven Prabhakaran1997 PDFDocument21 pagesRaven Prabhakaran1997 PDFargiaescuNo ratings yet
- Aphasia RehabDocument72 pagesAphasia RehabkicsirekaNo ratings yet
- Neural Substrates of Fluid Reasoning: An fMRI Study of Neocortical Activation During Performance of The Raven's Progressive Matrices TestDocument21 pagesNeural Substrates of Fluid Reasoning: An fMRI Study of Neocortical Activation During Performance of The Raven's Progressive Matrices TestAviraj MahadikNo ratings yet
- Berman Et Al., 2006 Studying Mind and Brain With fMRIDocument4 pagesBerman Et Al., 2006 Studying Mind and Brain With fMRIMarkNo ratings yet
- NIH Public Access: Future Trends in Neuroimaging: Neural Processes As Expressed Within Real-Life ContextsDocument14 pagesNIH Public Access: Future Trends in Neuroimaging: Neural Processes As Expressed Within Real-Life ContextslitrevnmNo ratings yet
- Do We Need To Study The Brain To Understand The MindDocument3 pagesDo We Need To Study The Brain To Understand The MindMARCO ANTONIO OLIVARES HURTADONo ratings yet
- Theoretical, Statistical, and Practical Perspectives On Pattern-Based Classification Approaches To The Analysis of Functional Neuroimaging DataDocument19 pagesTheoretical, Statistical, and Practical Perspectives On Pattern-Based Classification Approaches To The Analysis of Functional Neuroimaging DataNoScriptNo ratings yet
- 1 Functional Neuroimaging of Executive Functions Todd S. Braver and Hannes Ruge Department ... (PDFDrive)Document93 pages1 Functional Neuroimaging of Executive Functions Todd S. Braver and Hannes Ruge Department ... (PDFDrive)Amornchai WongworrakunNo ratings yet
- Contributions of Episodic Retrieval and Mentalizing To Autobiographical ThoughtDocument12 pagesContributions of Episodic Retrieval and Mentalizing To Autobiographical ThoughtFrancisco Ahumada MéndezNo ratings yet
- ConsciousnessDocument4 pagesConsciousnessarhodes777No ratings yet
- The Multiple Functions of Sensory-Motor (2005)Document3 pagesThe Multiple Functions of Sensory-Motor (2005)ANTONIO REYES MEDINANo ratings yet
- Soc Cogn Affect Neurosci 2016 Greven 641 51Document11 pagesSoc Cogn Affect Neurosci 2016 Greven 641 51tefsNo ratings yet
- MVPA Personality - PreprintDocument14 pagesMVPA Personality - PreprintGeo MeNo ratings yet
- The Brain's Default Network: Anatomy, Function, and Relevance To DiseaseDocument38 pagesThe Brain's Default Network: Anatomy, Function, and Relevance To DiseaseMarkoff ChaneyNo ratings yet
- Facial PerceptionDocument7 pagesFacial PerceptionGECJNo ratings yet
- Vinod Goel (2007) - Anatomy of Deductive ReasoningDocument7 pagesVinod Goel (2007) - Anatomy of Deductive ReasoningRodrigo CórdovaNo ratings yet
- Plasticité Nrpsy CogDocument14 pagesPlasticité Nrpsy CogMalak GabsiNo ratings yet
- Depisapia AISC2010Document5 pagesDepisapia AISC2010Nicola De PisapiaNo ratings yet
- Image Search AlgorithmDocument28 pagesImage Search AlgorithmbinzbinzNo ratings yet
- Fnhum 07 00692Document6 pagesFnhum 07 00692bronson.harryNo ratings yet
- Reitan 1994Document38 pagesReitan 1994Sergio Machado NeurocientistaNo ratings yet
- Nancy - Face AreaDocument20 pagesNancy - Face AreaOchopinochoNo ratings yet
- Nihms 474787Document7 pagesNihms 474787ClaraCalvoTorresNo ratings yet
- J Neuroimage 2009 12 098Document8 pagesJ Neuroimage 2009 12 098hanbing yinNo ratings yet
- Executive Functions As Virtual Machines: A New Metaphor For A Neuropsychological Classic ConceptDocument11 pagesExecutive Functions As Virtual Machines: A New Metaphor For A Neuropsychological Classic ConceptsiscupNo ratings yet
- En Lo AmbientalDocument11 pagesEn Lo AmbientalYocelin Espiritu ReynosoNo ratings yet
- Spunt (2011) JOCNDocument12 pagesSpunt (2011) JOCNBUBUSNo ratings yet
- What Can Be Learned About Normal Cognitive Function Using Haemodynamic Imaging Techniques?Document6 pagesWhat Can Be Learned About Normal Cognitive Function Using Haemodynamic Imaging Techniques?Stephen LissendenNo ratings yet
- Superfluous Neuroscience Information Makes Explanations of Psychological Phenomena More AppealingDocument19 pagesSuperfluous Neuroscience Information Makes Explanations of Psychological Phenomena More AppealingBrianNo ratings yet
- Self-Reported Empathy and Neural Activity During Action Imitation andDocument9 pagesSelf-Reported Empathy and Neural Activity During Action Imitation andPheeraphol ThapprasithiNo ratings yet
- Mind The Brain Mind The Brain Mind The Brain Mind The BrainDocument4 pagesMind The Brain Mind The Brain Mind The Brain Mind The BrainMariø YŋgveøhliŋNo ratings yet
- Vivian Wu 260891725 Paper 6Document3 pagesVivian Wu 260891725 Paper 6Vivian WuNo ratings yet
- Affective Mapping An Activation Likelihood Estimation (ALE) Meta-AnalysisDocument12 pagesAffective Mapping An Activation Likelihood Estimation (ALE) Meta-Analysisselina hahnNo ratings yet
- Neural Correlates of The Object-Recall Process in Semantic MemoryDocument12 pagesNeural Correlates of The Object-Recall Process in Semantic MemoryMilenaNo ratings yet
- A Default Mode of Brain Function - A Brief History of An Evolving IdeaDocument8 pagesA Default Mode of Brain Function - A Brief History of An Evolving IdeaEnzimoshNo ratings yet
- An Introduction To Anatomical ROI-based fMRI Classification AnalysisDocument12 pagesAn Introduction To Anatomical ROI-based fMRI Classification AnalysisFridRachmanNo ratings yet
- What Is Self SpecificDocument31 pagesWhat Is Self SpecificchandusgNo ratings yet
- NIH Public Access: Author ManuscriptDocument18 pagesNIH Public Access: Author ManuscriptYeremias EdwinNo ratings yet
- 114-2005 General Specific NeuroImageDocument6 pages114-2005 General Specific NeuroImageEdward Ventura BarrientosNo ratings yet
- Jurnal 2Document10 pagesJurnal 2akreditasimedicalpegadaianNo ratings yet
- Tunik Et Al. 2007Document10 pagesTunik Et Al. 2007Miguel Antonio Barretto GarcíaNo ratings yet
- Abernathy Dylan Context Memo Cover Letter 1 KF 3Document3 pagesAbernathy Dylan Context Memo Cover Letter 1 KF 3api-710551120No ratings yet
- Understanding SelfDocument9 pagesUnderstanding SelfNaja ReyesNo ratings yet
- Burgess 08 SpatialDocument21 pagesBurgess 08 Spatialsarabiba1971No ratings yet
- Cognitive NeuroscienceDocument4 pagesCognitive NeuroscienceAkhwand SaulatNo ratings yet
- Hemispheric Reasoning StrategiesDocument10 pagesHemispheric Reasoning Strategiesrodrigo_rrNo ratings yet
- Editorial: J.Neurol Sci (Turk)Document4 pagesEditorial: J.Neurol Sci (Turk)Nezih OktarNo ratings yet
- Current Topics in Cognitive Psychology, 2010Document15 pagesCurrent Topics in Cognitive Psychology, 2010Hanne Melgård WatkinsNo ratings yet
- Pre Mid Assignment 1 Sania Saddique (Book)Document7 pagesPre Mid Assignment 1 Sania Saddique (Book)Sania SaddiqueNo ratings yet
- VanOverwalle2020 Article ConsensusPaperCerebellumAndSocDocument36 pagesVanOverwalle2020 Article ConsensusPaperCerebellumAndSocTSGNo ratings yet
- Decision Making Brain Rorie 2005Document3 pagesDecision Making Brain Rorie 2005neurocastedNo ratings yet
- Cognitive Synthesis TestDocument33 pagesCognitive Synthesis TestvedroconmioNo ratings yet
- Self-Esteem Modulates Dorsal Medial Prefrontal Cortical Response To Self-Positivity Bias in Implicit Self-Relevant ProcessingDocument5 pagesSelf-Esteem Modulates Dorsal Medial Prefrontal Cortical Response To Self-Positivity Bias in Implicit Self-Relevant ProcessingJoeNo ratings yet
- HHS Public Access: Neural Architecture Underlying Classification of Face Perception ParadigmsDocument26 pagesHHS Public Access: Neural Architecture Underlying Classification of Face Perception Paradigmscecilia martinezNo ratings yet
- fMRI Analysis of Situational and Behavior Description Interview QuestionsDocument12 pagesfMRI Analysis of Situational and Behavior Description Interview QuestionsEszter SzerencsiNo ratings yet
- 2008 Investigating Paranormal Phenomena Functional Brain Imaging of TelepathyDocument6 pages2008 Investigating Paranormal Phenomena Functional Brain Imaging of TelepathymarciascognamilloNo ratings yet
- 2004 Towards Studies of The Social BrainDocument5 pages2004 Towards Studies of The Social BrainAl WilsonNo ratings yet
- Neural Reuse Anderson 2010Document69 pagesNeural Reuse Anderson 2010Katie BanksNo ratings yet
- The Brain has a Mind of its Own: Attachment, Neurobiology, and the New Science of PsychotherapyFrom EverandThe Brain has a Mind of its Own: Attachment, Neurobiology, and the New Science of PsychotherapyNo ratings yet
- Stock Markets Still in The Doldrums - BBC NewsDocument17 pagesStock Markets Still in The Doldrums - BBC NewsfiestamixNo ratings yet
- What'Ll Happen To US Commercial Real Estate As Chinese Money Dries Up - Zero HedgeDocument6 pagesWhat'Ll Happen To US Commercial Real Estate As Chinese Money Dries Up - Zero HedgefiestamixNo ratings yet
- No-Deal Brexit 'To Leave Shelves Empty' Warn Retailers - BBC NewsDocument14 pagesNo-Deal Brexit 'To Leave Shelves Empty' Warn Retailers - BBC NewsfiestamixNo ratings yet
- ? - 40 @abc BB ? - 40 @ibc BBDocument4 pages? - 40 @abc BB ? - 40 @ibc BBfiestamixNo ratings yet
- SapDocument4 pagesSapfiestamixNo ratings yet
- Unit 1: Introduction To Webdynpro ABAPDocument43 pagesUnit 1: Introduction To Webdynpro ABAPfiestamixNo ratings yet
- Which Is A Good Book For SAP SD - QuoraDocument2 pagesWhich Is A Good Book For SAP SD - QuorafiestamixNo ratings yet
- The Mistake That Led To A 1.2bn Business - BBC NewsDocument14 pagesThe Mistake That Led To A 1.2bn Business - BBC NewsfiestamixNo ratings yet
- SAP SD Certification Exam Syllabus - ERPPrepDocument4 pagesSAP SD Certification Exam Syllabus - ERPPrepfiestamix0% (1)
- Curriculum - Application (Solution) Consultant SAP SCM - SAP ERP - Order Fulfillment (Sales Order Management)Document3 pagesCurriculum - Application (Solution) Consultant SAP SCM - SAP ERP - Order Fulfillment (Sales Order Management)fiestamixNo ratings yet
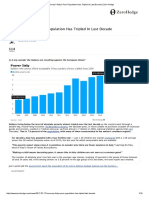
- Recovery - Italy's Poor Population Has Tripled in Last Decade - Zero HedgeDocument19 pagesRecovery - Italy's Poor Population Has Tripled in Last Decade - Zero HedgefiestamixNo ratings yet
- SCM610 - Delivery Processing in SAP ERP - SAP TrainingDocument7 pagesSCM610 - Delivery Processing in SAP ERP - SAP TrainingfiestamixNo ratings yet
- American Students Love Socialism (Just Don't Ask Them What It Is) - Zero HedgeDocument22 pagesAmerican Students Love Socialism (Just Don't Ask Them What It Is) - Zero HedgefiestamixNo ratings yet
- SCM210 - Core Interface (CIF) - SAP TrainingDocument5 pagesSCM210 - Core Interface (CIF) - SAP TrainingfiestamixNo ratings yet
- SCM610 - Delivery Processing in SAP ERP - SAP TrainingDocument7 pagesSCM610 - Delivery Processing in SAP ERP - SAP TrainingfiestamixNo ratings yet
- TEWM10 - Extended Warehouse Management I - SAP TrainingDocument4 pagesTEWM10 - Extended Warehouse Management I - SAP TrainingfiestamixNo ratings yet
- S4F00E - Overview of Financials in SAP S - 4HANA - SAP Training PDFDocument3 pagesS4F00E - Overview of Financials in SAP S - 4HANA - SAP Training PDFfiestamix0% (1)
- S4F00E - Overview of Financials in SAP S - 4HANA - SAP TrainingDocument3 pagesS4F00E - Overview of Financials in SAP S - 4HANA - SAP TrainingfiestamixNo ratings yet
- Only in San Francisco - Couples Making $138k A Year Now Qualify For Subsidized - Affordable Housing - Zero HedgeDocument13 pagesOnly in San Francisco - Couples Making $138k A Year Now Qualify For Subsidized - Affordable Housing - Zero HedgefiestamixNo ratings yet
- SAP Easy Access - SAP Training and CertificationDocument16 pagesSAP Easy Access - SAP Training and Certificationfiestamix100% (1)
- SAP FI Certification (Financial Accounting) - SAP CertificationDocument5 pagesSAP FI Certification (Financial Accounting) - SAP CertificationfiestamixNo ratings yet
- SAP Super User - SAP Training and CertificationDocument5 pagesSAP Super User - SAP Training and CertificationfiestamixNo ratings yet
- Connectivism and Connective KnowledgeDocument616 pagesConnectivism and Connective KnowledgeleeyikshengNo ratings yet
- Appraisal PointsDocument2 pagesAppraisal PointsPiyasha NathNo ratings yet
- CEL2102 Project 1 - DescriptionDocument6 pagesCEL2102 Project 1 - DescriptionAbdul Qhouyyum AiedilNo ratings yet
- The Rise of Digital Intelligence Challenges For Public Relations Education and PracticesDocument18 pagesThe Rise of Digital Intelligence Challenges For Public Relations Education and PracticesTiana AlexeNo ratings yet
- Coaching in Human ResourceDocument21 pagesCoaching in Human Resourcesya_ezNo ratings yet
- QuestionsDocument20 pagesQuestionsapi-295210059No ratings yet
- The Importance of Discomfort On NoFapDocument4 pagesThe Importance of Discomfort On NoFapVAXAEVNo ratings yet
- Senior High School: Organization & Management Quarter IIDocument18 pagesSenior High School: Organization & Management Quarter IICipher Amoz100% (1)
- Final OUTLINE Art&CreativityDocument11 pagesFinal OUTLINE Art&CreativityJohanna GutierrezNo ratings yet
- Mult Intelligence QuizDocument2 pagesMult Intelligence Quizapi-299481084No ratings yet
- Free Sample of An Action Research ProposalDocument18 pagesFree Sample of An Action Research Proposalreginald_adia_1No ratings yet
- Theory Part 4Document4 pagesTheory Part 4Abad ChristineNo ratings yet
- ETHICSDocument7 pagesETHICSErlan Grace HeceraNo ratings yet
- Creative As A Prerequisite To InnovationDocument2 pagesCreative As A Prerequisite To InnovationSaad Rehman100% (2)
- WORKING THESIS (Teavher's Effectiveness in Teaching The English Language As Perceived by The Grade 9 Students of Quezonian Educational College Inc., Atimonan, Quezon S.Y. 2017-2018Document54 pagesWORKING THESIS (Teavher's Effectiveness in Teaching The English Language As Perceived by The Grade 9 Students of Quezonian Educational College Inc., Atimonan, Quezon S.Y. 2017-2018Christofer De Los ReyesNo ratings yet
- Cls 6Document5 pagesCls 6popalina24No ratings yet
- Relationship Between Reading and Writing Performance (Rosita Aminullah) PP 179-182Document4 pagesRelationship Between Reading and Writing Performance (Rosita Aminullah) PP 179-182upenapahangNo ratings yet
- Hall - S The Work of RepresentationDocument30 pagesHall - S The Work of RepresentationmarleneprestesNo ratings yet
- Marketing Keller's Brand Equity ModelDocument3 pagesMarketing Keller's Brand Equity ModelSana Vancy100% (1)
- Mind HackDocument6 pagesMind Hackapi-374080275% (4)
- Module 5 - The Emotional SelfDocument5 pagesModule 5 - The Emotional SelfMonasque PamelaNo ratings yet
- Running Head: Interviewing Types and TechniquesDocument6 pagesRunning Head: Interviewing Types and Techniquesklm klmNo ratings yet
- Week 1 Undefined TermsDocument8 pagesWeek 1 Undefined TermsJo-Amver Valera ManzanoNo ratings yet
- Tweed 2020 Faith in HumanityDocument13 pagesTweed 2020 Faith in Humanitymuiescr1bdNo ratings yet
- By Liam Lindsay: Cover Photo by Andrea RothDocument32 pagesBy Liam Lindsay: Cover Photo by Andrea RothSandraNo ratings yet
- Critical ThikingDocument17 pagesCritical ThikingLen AungNo ratings yet
- Ethic in Public SectorDocument46 pagesEthic in Public SectorCOT Management Training Insitute100% (2)
- Writing Cases: Melodena Stephens BalakrishnanDocument6 pagesWriting Cases: Melodena Stephens BalakrishnanErika L.No ratings yet
- What Is Divided Attention?Document50 pagesWhat Is Divided Attention?LoraNo ratings yet
- Problem Solving StrategiesDocument3 pagesProblem Solving StrategiesJA M ESNo ratings yet