Professional Documents
Culture Documents
Dilutions Protocols Draft
Dilutions Protocols Draft
Uploaded by
Guillermo UriarteOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dilutions Protocols Draft
Dilutions Protocols Draft
Uploaded by
Guillermo UriarteCopyright:
Available Formats
Dilutions and Protocols (Draft)
Calculations
1.1
Area of gold plate
Diameter of gold plates: 500um
Hemisphere (V) of gold plate (in cubic meters) =
=
2
3
r3
2
(2503 106 )3 = 3.272 1011 m3
3
(1)
Volume of solution to fill hemisphere =
3.272 1011 (1000L/1m3 ) (106 uL/L) = 0.032725uL = 32.7259nL
(2)
8 gold plates to transistor 261.799 nL to fill all plates
1.2
Mass of aptamer
MW NS1 Aptamer with biotin and 6T = 12,116 g/mol S15 was 34 BP and NS1 aptamer is 32 BP,
both form g-quadruplex tertiary structures.
S15 sequence: 5 GCACCGGGCAGGACGTCCGGGGTCCTCGGGGGGC 3
Mass in grams for S15: 10646.79 Da = 1.7679409179e-20 grams
Molar Weight S15: grams/mole = (1.7679409179e-20 grams) * (6.022140857(74)1023 mol1) =
10,646.8969 g/mol
1.3
General concentration formula
concentration = mass / volume * 1/molecular weight
moles/liter = grams/[1liter = (.001meters3 )] 1/(grams/mole)
1.4
(3)
Density of aptamer in concentration
Assume 2 uM, 10 uM, 20 uM final concentration of NS1 aptamer in solution (from Goda papers
aptamer concentrations)
2 uM = 2 106 M
10 uM = 10 106 M
20 uM = 20 106 M
30 uM = 30 106 M
20*106
20*106
20*106
30*106
1.5
M = x g / 1 L * 1 / 10,646.8969 g/mol = .02129 grams/L = .02129 ng/nL
M = x g / 1 L * 1 / 10,646.8969 g/mol = .1064 grams/L = .1064 ng/nL
M = x g / 1 L * 1 / 10,646.8969 g/mol = .2129 grams/L = .2129 ng/nL
M= x g / 1 L * 1 / 10,646.8969 g/mol = .3194 grams/L = .3194 ng/nL
Aptamer Density vs. Protein
NS1 protein length:
Width: 120-125 Angstroms = 120 1010 m 125 1010 m
Diagnol Width: 140 Angstroms 140 1010 m
Theoretical surface density of the immobilized DNA aptamers (DN A ) =
1
1.6
Grams to fill one hemisphere
32.7259 nL to fill hemisphere of 1 plate
x nanograms / 32.759 nL = .02129 ng/nL
= .6974 ng at 2 uM concentration to fill one hemisphere
x nanograms / 32.759 nL = .1064 ng/nL
= 3.4856 ng at 10 uM concentration to fill one hemisphere
x nanograms / 32.759 nL = .2129 ng/nL
= 6.9743 ng at 20 uM concentration to fill one hemisphere
x nanograms / 32.759 nL = .3194 ng/nL
= 10.4634 ng at 30 uM concentration to fill one hemisphere
1.7
Grams to fill entire plate
261.799 nL to fill hemispheres of all plates
x nanograms /261.799 nL = .02129 ng/nL
= 5.5737 ng at 2 uM concentration to fill all hemispheres
x nanograms /261.799 nL = .1064 ng/nL
= 27.855 ng at 10 uM concentration to fill all hemispheres
x nanograms /261.799 nL = .2129 ng/nL
= 55.737 ng at 20 uM concentration to fill all hemispheres
x nanograms /261.799 nL = .3194 ng/nL
= 83.6189 ng at 30 uM concentration to fill all hemispheres
1.8
Number of tests
Amount of aptamer ordered = 25 ug
2 uM: .02129 ng
How many single gold plate tests can be run: 25000 ng / .69743 ng = 35845.89 tests
How many full transistor tests can be run: 25000 ng / 5.5737 ng = 4485.35 tests
10 uM: .1064 ng
How many single gold plate tests can be run: 25000 ng / 3.4856 ng = 7172.366 tests
How many full transistor tests can be run: 25000 ng / 27.855 ng = 897.504 tests
20 uM: .2129 ng
How many single gold plate tests can be run: 25000 ng / 6.9743 ng = 3584.589 tests
How many full transistor tests can be run: 25000 ng / 55.737 ng = 448.535 tests
30 uM: .3194 ng
How many single gold plate tests can be run: 25000 ng / 10.4634 ng = 2389.28 tests
How many full transistor tests can be run: 25000 ng / 83.6189 ng = 298.975 tests
1.9
Aptamer Reduction Buffer
TE buffer: 10 mM Tris, 0.1 mM EDTA, pH 7.5
10 mM Tris 121.14 100G : 121.14 g/mol
.01 M = x g / L * 1/121.14 g/mol = 1.2114 g/L
0.1 mM EDTA, pH 7.5 100G : 292.24 g/mol
.0001 M = x g / L * 1/292.24 g/mol = .029224 g/L
TCEP 1g : 286.65 MW
.01M = xg / 1L * 1/286.65 g/mole = 2.8665 g/L
1.10
Folding buffer concentrations and dilutions
1 mM MgCl2, 1X PBS, pH 7.5 (137 mM NaCl, 2.7 mM Potassium Chloride, 8 mM Sodium Phosphate dibasic, 2 mM Potassium Phosphate monobasic)
1 mM MgCl2 - 100 ml (1 M) : MW - 95.211 g/mol
1mL of solution - 100 buffers
137 mM NaCl : MW - 58.44 g/mol
.137 M = x g / L * 1/58.44 g/mol = 8 g/L
2.7 mM KCl : MW 74.5513 g/mol
.0027 M = x g / L * 1/74.5513 g/mol = .2013 g/L
8 mM Sodium Phosphate dibasic 250G : 141.96 g/mol
.0008 M = x g / L * 1/141.96 g/mol = .113568 g/L
2 mM Potassium Phosphate monobasic 500 g : 136.09 g/mol
.0002 M = x g / L * 1/136.09 g/mol = .027218 g/L
1.11
Reaction Buffer to bind aptamer to gold: concentrations/dilutions
10 mM TrisHCl, 100 mM KCl, 100 mM NaCl, 10 mM CaCl2, 10 mM MgCl2, and 1 mM TCEP
10mM tris-HCL Ph 8.0- 1L (1M) : MW 157.594 g/mol
10 mL of Tris HCL-100 buffer solutions from tris-HCL
100 mM KCl - in lab : MW 74.5513 g/mol
.1 M = x g / 1L * 1/74.5513 g/mol = 7.45513 g/L
10 mM NaCl - in lab : MW - 58.44 g/mol
.01 M = x g / 1L * 1/58.44 g/mol = .5844 g/L
10 mM CaCl2 - 500g : MW - 110.98 g/mol
.01 M = x g / 1L * 1/110.98 g/mol = 1.1098 g/L
10mM MgCl2- 100 ml (1 M) : MW - 95.211 g/mol
1mL of solution - 100 buffers
1 mM TCEP - 1g : MW 286.65 g/mol
.001 M = x g / 1L * 1/286.65 g/mol = .28665 g/L = .28665 mg/mL
1.12
SB Solution: concentrations/dilutions
1mM Sulfobentaine-3-undecanethiol - 10 mg : 353.59 MW
.001M = xg / 1L * 1/353.59 g/mole = .35355 g/L = .35355 ng/nL
1mM TCEPHCl, Tris(2-carboxyethyl)phosphine hydrochloride, 1g : 286.65 MW
.001M = xg / 1L * 1/286.65 g/mole = .28665 g/L = .28665 ug/uL
1.13
Antibody/NS1 binding buffer
1.14
NS1 and measurement buffer
Mass of NS1 with His-tag: 2.5837986e-20 g
MW: 15562.61 g/mole
Concentration: 100 ug at 0.53 mg/ml
10nM:
10*109 M = x g / 1L * 1/15562.6 g/mol = .00015562 g/L
50nM:
50*109 M = x g / 1L * 1/15562.6 g/mol = .0007781 g/L
100nM:
100*109 M = x g / 1L * 1/15562.6 g/mol = .0015562 g/L
500nM:
500*109 M = x g / 1L * 1/15562.6 g/mol = .007781 g/L
1uM:
1*106 M = x g / 1L * 1/15562.6 g/mol = .015562 g/L
Electrolyte composition of measurement buffer (pH 8.2): 10mM TrisHCl, 5mM KCl, 3mM NaCl,
1mM CaCl2 and 1mM MgCl2.
10mM tris-HCL pH 8.0- 1L (1M) : MW 157.594 g/mol
10 mL of Tris HCL-100 buffer solutions from tris-HCL
5 mM KCl - in lab : MW 74.5513 g/mol
.005 M = x g / 1L * 1/74.5513 g/mol = .37276 g/L
3 mM NaCl - in lab : MW - 58.44 g/mol
.003 M = x g / 1L * 1/58.44 g/mol = .17532 g/L
1 mM CaCl2 - 500g : MW - 110.98 g/mol
.001 M = x g / 1L * 1/110.98 g/mol = .11098 g/L
1mM MgCl2- 100 ml (1 M) : MW - 95.211 g/mol
100uL of solution - 1000 buffers
1.15
2
2.1
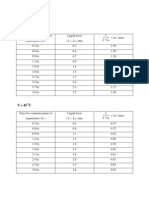
Fluorospectrometry Cuvette Buffers
Protocols
Aptamer Reduction Protocol
1. xBriefly centrifuge the aptamer storage tube to ensure the dried aptamer pellet is at the bottom
of the tube.
2. Resuspend the aptamers pellet in TE buffer (10 mM Tris HCl, 0.1 mM EDTA, pH 7.5) or
non-DPEC treat-ed, nuclease free water to achieve 100uM concentration (using volumes indicated
in the Product Information Sheet that accompanies each order).
3. Incubate at room temperature for 30 minutes.
4. Briefly vortex and quickly spin the sample down ( 10,000g for 1 second).
4
5.Create aliquots and store 20o C.
1.Resuspend and aliquot aptamer in TE buffer to 100 uM according to the instruction on the
Product Information Form from BasePairBio.
2.Prepare a 1:1 v/v dilution of aptamer solution with 20 mM TCEP solution, for a final concentration of 50 uM aptamer and 10 mM TCEP. Incubate the reaction at room temperature for 1
hour.
2.2
Aptamer Folding Protocol
1. Prior to use, dilute the reduced thiol-aptamer to its working concentration in Folding Buffer.
2. Heat the aptamer solution to 85 95 o C for 5 minutes.
3. Allow the solution to cool to room temperature ( 15 minutes).
2.3
Aptamer and SB gold plate binding protocol
Immobilize thiol-modified DNA aptamers in a Reaction Buffer overnight, followed by rinse with
water. Backfill the remaining gold surface with SB solution, followed by rinse with water.
2.4
Measurement Buffer and NS1
Use varying concentration of NS1 in Measurement Buffer ie. go from 0-1400 nm over time and see
the signal difference.
2.5
6x His Tag Antibody
To determine the binding efficacy of the DENV2 NS1 to its related aptamer, well be performing
something similar to an ELISA assay. This document will provide details on how this will be
performed.
Process (components, paragraph form): The process will work much the same as a standard
ELISA assay. The base of our assay will be a layer of gold plating. This is necessary because the
next component, the NS1 aptamer, is thiolated. Thiols bind to gold, thereby anchoring the aptamer
to the gold surface. The next component will be for the NS1, since it binding to the aptamer is
what we want to test. This NS1 well be using has another feature that will be important for this
assay: these particles have 6xHis tags attached to them. Once an adequate amount of time has
passed, the NS1 will have theoretically bound to the aptamer, we will add the antibody detector.
This antibody binds to His tags and will have a dye bound to the functional end. After washing,
what we expect to see is fluorescence (green light) from the bound dyes, which will show that the
NS1 is bound to the aptamer.
Process (components, layered from top to bottom w/ associated quantities needed*):
6x-His tag antibody (DyLight 488) NS1 protein, His-tagged NS1 aptamer, thiolated Gold surface
Process (general ELISA steps):
Details taken from ThermoFisher: https://www.thermofisher.com/us/en/home/life-science/proteinbiology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overviewelisa.html
The direct detection method uses a labeled primary antibody that reacts directly with the
antigen. Direct detection can be performed with antigen that is directly immobilized on the assay
plate or with the capture assay format. Direct detection is not widely used in ELISA but is quite
common for immunohistochemical staining of tissues and cells.
You might also like
- Reviewer For Chemical Engineering Licensure Examination 3 Edition Solutions ManualDocument56 pagesReviewer For Chemical Engineering Licensure Examination 3 Edition Solutions ManualSherry Anne Ynciong Panganiban100% (4)
- Plug Flow Reactor Design Presentation...Document18 pagesPlug Flow Reactor Design Presentation...Nana Kwame BoatengNo ratings yet
- Determination of Mean Astivity Coefficient and Solubility of Potassium Hydrogen Tartrate (KHT) in Aqueous Solution at 30ºCDocument9 pagesDetermination of Mean Astivity Coefficient and Solubility of Potassium Hydrogen Tartrate (KHT) in Aqueous Solution at 30ºCAmeerul Hazeeq100% (10)
- Word Soru 407Document6 pagesWord Soru 407Sahar TehraniNo ratings yet
- Aeration and Agitation 2Document18 pagesAeration and Agitation 2rhia100% (1)
- Format of Lab Report Example 8609Document14 pagesFormat of Lab Report Example 8609herrk167% (3)
- DLL Mathematics 6 q2 w7Document4 pagesDLL Mathematics 6 q2 w7Kymberly Jean Radores Quimpan100% (4)
- Counselling Skills For NursesDocument129 pagesCounselling Skills For Nursesadyna26100% (1)
- MNT Design 2520of 2520equipmentsDocument32 pagesMNT Design 2520of 2520equipmentsshamsabbasNo ratings yet
- 10 Enzymes For Modifying and Labeling DNA and RN - 1987 - Methods in EnzymologDocument17 pages10 Enzymes For Modifying and Labeling DNA and RN - 1987 - Methods in EnzymologMontsZs G-oNo ratings yet
- 18 - 70 Heppy Yessya PutriDocument6 pages18 - 70 Heppy Yessya PutriHeppy YessyaNo ratings yet
- Computer ApplicationsDocument8 pagesComputer Applicationsapi-3728602100% (1)
- Tugas Volume Molar Dan Termo CmpuranDocument6 pagesTugas Volume Molar Dan Termo CmpuranHeppy Yessya100% (1)
- Diffusion and Conduction Problem SolvingDocument8 pagesDiffusion and Conduction Problem Solvingmendoza21203831mNo ratings yet
- Basic Design of A Heat ExchangerDocument10 pagesBasic Design of A Heat ExchangerKvspavan Kumar100% (1)
- sm8 117Document3 pagessm8 117Sadie HnatowNo ratings yet
- Adsorption of Acetic Acid On Charcoal SurfaceDocument3 pagesAdsorption of Acetic Acid On Charcoal SurfaceFrankyFan90% (10)
- TANK Module: Sample PrintoutDocument17 pagesTANK Module: Sample PrintoutAnonymous J1vjrU2No ratings yet
- Enzyme KinecticsDocument25 pagesEnzyme KinecticsRhia80% (5)
- Lab10 CompleteDocument22 pagesLab10 CompleteMastura Ahmad Termizi100% (1)
- Transfderencia de CalorDocument1 pageTransfderencia de CalorDavid Cj AcNo ratings yet
- Ifc Q & A 17 - GeasDocument5 pagesIfc Q & A 17 - GeasBelat Cruz100% (3)
- PCR CalculationsDocument7 pagesPCR Calculationsmarwatsum100% (1)
- cs2c03048 Si 001Document15 pagescs2c03048 Si 001hassnain iqbalNo ratings yet
- Ja5061388 Si 001Document38 pagesJa5061388 Si 001vasut.nakNo ratings yet
- Gas DiffusionDocument6 pagesGas DiffusionAkashoujo AkariNo ratings yet
- Allyl Chloride 1000: CH CHCH CL MW: 76.53 CAS: 107-05-1 RTECS: UC7350000Document3 pagesAllyl Chloride 1000: CH CHCH CL MW: 76.53 CAS: 107-05-1 RTECS: UC7350000Luis TrejoNo ratings yet
- Results PFRDocument3 pagesResults PFRMuhammad ArshadNo ratings yet
- Science - Abm8868 SMDocument154 pagesScience - Abm8868 SMchrissteedNo ratings yet
- Experiment 3 Che 314Document11 pagesExperiment 3 Che 314Seele TlhagaNo ratings yet
- BS 3 MembraneDocument33 pagesBS 3 MembranePratyush GoelNo ratings yet
- Lab Report Bio560Document39 pagesLab Report Bio560Syar QamNo ratings yet
- Tutorial 3 Tutoriaal 3 - MemoDocument2 pagesTutorial 3 Tutoriaal 3 - Memosnalo mdludluNo ratings yet
- Enzym ESDocument21 pagesEnzym ESpoopnoodlemanNo ratings yet
- Solutions Asgn-1,2 2Document4 pagesSolutions Asgn-1,2 2razakhanNo ratings yet
- Determine The Enthalpy Change For Reaction of ZN and Cuso Research Question: What Is The Value of Enthalpy Change For The Reaction of 0.500 MolDocument5 pagesDetermine The Enthalpy Change For Reaction of ZN and Cuso Research Question: What Is The Value of Enthalpy Change For The Reaction of 0.500 Molfrancescosa1No ratings yet
- The Calorimetric Determination of Manganese in Paper ClipsDocument4 pagesThe Calorimetric Determination of Manganese in Paper ClipsOnkarabile MatomeNo ratings yet
- Chapter 3 SBRDocument45 pagesChapter 3 SBRAtlantida Dërmaku100% (1)
- EE Tarek Cal. Eq. Ex. 2019Document7 pagesEE Tarek Cal. Eq. Ex. 2019Ibrahim Sayed AhmedNo ratings yet
- Kappa Number AnalysisDocument3 pagesKappa Number AnalysiskudaNo ratings yet
- Condensation and BoilingDocument14 pagesCondensation and BoilingCrislyn Akilit Bayawa100% (1)
- Handout For Workshop (DAY 01) (KSBT)Document7 pagesHandout For Workshop (DAY 01) (KSBT)Michael KahnwaldNo ratings yet
- Perhitungan PiknometerDocument11 pagesPerhitungan PiknometerEbsNo ratings yet
- Extraction For UPLC of NAM-NMN-NAD-ADPR (NADH) TissueDocument5 pagesExtraction For UPLC of NAM-NMN-NAD-ADPR (NADH) TissueItzel JuárezNo ratings yet
- Adsorption Equilibria ExamplesDocument31 pagesAdsorption Equilibria ExamplesNagwa MansyNo ratings yet
- Protocol For Comet AssayDocument10 pagesProtocol For Comet AssaySari NmNo ratings yet
- Exp6 Result report - 최진호 (2016310092)Document10 pagesExp6 Result report - 최진호 (2016310092)임성민No ratings yet
- The Hill RXN in Isolated Chloroplasts PostDocument12 pagesThe Hill RXN in Isolated Chloroplasts PostKarina Khan100% (12)
- 1 KbladderDocument4 pages1 KbladderTan E-ChuanNo ratings yet
- HW#1Document7 pagesHW#1MahsaNo ratings yet
- Laporan FIlter PressDocument10 pagesLaporan FIlter PressDwizky WijayaNo ratings yet
- Ji SupplementDocument16 pagesJi SupplementDaniel MontalvoNo ratings yet
- Unit Operations Tutorial 2015-2016Document13 pagesUnit Operations Tutorial 2015-2016hazimraad0% (1)
- Methodology UncertaintyDocument2 pagesMethodology UncertaintyMeluti ArguelloNo ratings yet
- Sepro 1 Appendix PDFDocument45 pagesSepro 1 Appendix PDFMeesaa KbaiiNo ratings yet
- Table of Contents Template Word 05Document26 pagesTable of Contents Template Word 05Ro'a ShehadehNo ratings yet
- Total Energy: International Series in Heating, Ventilation and RefrigerationFrom EverandTotal Energy: International Series in Heating, Ventilation and RefrigerationNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Roller Coaster Lab - Basic: Key ConceptsDocument6 pagesRoller Coaster Lab - Basic: Key ConceptsTeachLABScINo ratings yet
- Put in 'Mustn't' or 'Don't / Doesn't Have To':: 1) We Have A Lot of Work Tomorrow. You Be LateDocument8 pagesPut in 'Mustn't' or 'Don't / Doesn't Have To':: 1) We Have A Lot of Work Tomorrow. You Be LateEmir XHNo ratings yet
- JN2022819 26Document19 pagesJN2022819 26goutham an rajaNo ratings yet
- The Effect of Digitalization On Business Performance An Applied StudyDocument8 pagesThe Effect of Digitalization On Business Performance An Applied StudyFrank MendozaNo ratings yet
- BLEACH Charac Part 1.Document53 pagesBLEACH Charac Part 1.Al LanNo ratings yet
- Suggestive Questions of Isc 2023 BiologyDocument12 pagesSuggestive Questions of Isc 2023 Biologyjahnvipatel2004No ratings yet
- World Pumps Volume 1995 Issue 349 1995 (Doi 10.1016/s0262-1762 (99) 81154-7) - Igor J. Karassik (1911-1995) PDFDocument2 pagesWorld Pumps Volume 1995 Issue 349 1995 (Doi 10.1016/s0262-1762 (99) 81154-7) - Igor J. Karassik (1911-1995) PDFvcockscribdNo ratings yet
- Acr - Lac MPSDocument7 pagesAcr - Lac MPSRAE SEAN CATANNo ratings yet
- Chapter 2 Boolean Algebra Logic GatesDocument29 pagesChapter 2 Boolean Algebra Logic GatesZahra JafarNo ratings yet
- Rwanda's Application For Membership of The Commonwealth: Report and Recommendations of The Commonwealth Human Rights Initiative - Yash GhaiDocument81 pagesRwanda's Application For Membership of The Commonwealth: Report and Recommendations of The Commonwealth Human Rights Initiative - Yash GhaiIntelligentsiya HqNo ratings yet
- 1203.staying Full of GodDocument5 pages1203.staying Full of GodnigelNo ratings yet
- Dabawala in MumbaiDocument12 pagesDabawala in MumbaiRamchandraSangodkarNo ratings yet
- Speech TextDocument4 pagesSpeech Textcici clardianNo ratings yet
- A Brief Biography of Hazrat Khaja Hussian Shah Wali HyderabadDocument17 pagesA Brief Biography of Hazrat Khaja Hussian Shah Wali HyderabadMohammed Abdul Hafeez, B.Com., Hyderabad, IndiaNo ratings yet
- Manila Pavilion v. DeladaDocument2 pagesManila Pavilion v. Deladasitsee watchNo ratings yet
- JFL Volume7 Issue28 Pages121-134Document15 pagesJFL Volume7 Issue28 Pages121-134Karina WedhantiNo ratings yet
- Venturi MetersDocument2 pagesVenturi MetersBala SubrahmanyamNo ratings yet
- Sat Chakra Nirupana: Magnum Opus On Kundalini Chakras by PurnanandaDocument152 pagesSat Chakra Nirupana: Magnum Opus On Kundalini Chakras by Purnanandakrishnarajmd100% (3)
- 2nd Grading Portfolio Pe For PrintDocument9 pages2nd Grading Portfolio Pe For PrintAna Carmela MortelNo ratings yet
- Stories of Sahabah Volume1Document255 pagesStories of Sahabah Volume1shumayounNo ratings yet
- Prophetic ProtocolDocument36 pagesProphetic ProtocolNeil SoansNo ratings yet
- Patriarchy and The Motherhood of GodDocument42 pagesPatriarchy and The Motherhood of GodJason BurrageNo ratings yet
- Clauses WorksheetDocument3 pagesClauses WorksheetSwathi SridharNo ratings yet
- Child Psychology A Contemporary View Point 7th Edition Parke Test BankDocument35 pagesChild Psychology A Contemporary View Point 7th Edition Parke Test Bankaudiblycaribbeeuwiq100% (30)
- Fpga ManualDocument7 pagesFpga ManualRahul SharmaNo ratings yet
- PFT FormDocument1 pagePFT FormConnie Diaz CarmonaNo ratings yet
- PreviewpdfDocument50 pagesPreviewpdfDaniel QuiñonesNo ratings yet
- Cognitive Processing TherapistDocument219 pagesCognitive Processing Therapistdrorgarbi100% (1)