Professional Documents
Culture Documents
Pictorial Essay: Areas of
Pictorial Essay: Areas of
Uploaded by
Safia RehmanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pictorial Essay: Areas of
Pictorial Essay: Areas of
Uploaded by
Safia RehmanCopyright:
Available Formats
813
Pictorial
American Journal of Roentgenology 1995.165:813-816.
CT Mosaic Pattern
Different Causes
Eric
J. Stern1,
Stephen
of Lung Attenuation:
J. Swensen2,
Thomas
E. Hartman2,
Areas of variable lung attenuation in a lobular or multilobular
distribution are occasionally
seen on CT or high-resolution
CT
scans of the lungs [1], although never as a normal finding. This
mosaic pattern of lung attenuation presents a challenge to the
radiologist when deciding which are the abnormal regions of
lung-those
of low attenuation, those of high attenuation, or both.
We have observed three categories
of disease known to cause a
CT mosaic pattern
of lung attenuation: small-airway disease, vascular lung disease, and infiltrative disease. Diseases from each of
these categories
can cause similar patterns
of mosaic lung attenuation on CT scans. However, it is sometimes possible to distinguish among these categories by using additional CT findings. We
illustrate
the known
causes of a CT mosaic pattern of lung attenuation and highlight distinguishing features.
Primary
Small-Airway
Disease
(Fixed
or Reactive)
CT performed
at suspended
full expiration
shows the physiologic consequence
of small-airway
(bronchiolar)
disease:
air
trapping
[1]. Lung regions
that retain air during exhalation
remain more lucent and show less decrease
in volume than
lung supplied
by normal airways
[2]. The distribution
of air
trapping is often patchy and dependent
on the level and sevenity of the airway obstruction.
When the level of airway obstruction is at or near the lobular level, a mosaic pattern of normal
lung and hyperlucent
lung can result (Figs. 1 and 2). Lung
regions that retain air show a decrease
in the caliber and numben of pulmonary
vessels
compared
with normal lung. The
inciting pathologic
processes
can be permanent,
as seen in
patients with obliterative
bronchiolitis
(Fig. 1), or reversible,
as
seen in patients with asthma (Fig. 2). In some instances,
air
Mark
Essay
Distinguishing
Frank1
S.
trapping
can be completely
unsuspected
on routine
suspended
full inspiration
CT scanning
and becomes
evident
only on CT scans obtained
at suspended
full expiration
(Figs.
1 and 2).
Vascular
Lung
Disease
A CT mosaic
pattern of lung attenuation
can result from
thnomboembolic
disease [3] (Figs. 3 and 4) or pulmonany arterial hypertension.
Regions
of hyperemic
(higher
attenuation)
lung mimic ground-glass
infiltrates
when seen
adjacent
to oligemic
(lower attenuation)
regions of lung. This
type of CT pattern
is often called
mosaic
perfusion
on
mosaic oligemia;
the oligemic
lung shows a decrease
in the
caliber
and number
of pulmonary
vessels
compared
with
normal on hypenemic
lung. In one series, the overall accuracy
of CT for detecting
a CT pattern of mosaic lung attenuation
due to chronic pulmonary
thromboembolism
was 72.7% [4].
pulmonary
Primary
Areas
Parenchymal
Disease
Causing
Ground-Glass
of Attenuation
In these lung diseases,
there is a patchy infiltrative
process within the intenstitium
of the lung on partial filling of the
air spaces by fluid, cells, or fibrosis so that the CT attenuation of the affected lung increases
compared
with that of normal parenchyma.
The caliber and number of vessels are not
appreciably
different
between
the normal
and abnormal
regions
of lung [5]. Diseases
that can produce
such a CT
pattern
of mosaic
lung attenuation
include
Pneumocystis
Received March 30, 1995; accepted after revision
Presented
in part at the meeting
of the Society
May 31 . 1995.
of Thoracic
Radiology,
Amelia
Island,
FL, March
1995.
Department of Radiology, Harborview Medical Center (ZA-65), University of Washington, 325 Ninth Ave., Seattle, WA 98104. Address correspondence
2Department of Radiology,
Mayo Clinic, Rochester,
MN 55905.
1
AJR 1995;165:813-816
0361-803X195/1654-813
American
Roentgen
Ray Society
to E. J. Stern.
STERN
814
ET
AL.
AJR:165,
October
1995
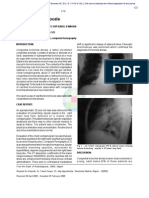
Fig. 1 .-65-year-old
woman
with bronchiolitis obliterans.
A, High-resolution
CT scan at suspended
full inspiration
was interpreted
prospectively
as normal.
B, High-resolution
CT scan at suspended
full expiration
shows multiple focal lobule-size
lucencies,
consistent
with extensive
lobular air
trapping,
a sign
of
severe
small-airway
obstruction.
Note size difference
of centrilobular core
structure
in air trapping
lobule
(straight
arrow)
and normal
lobule
(curved
arrow).
Characteristic
combination
of clinical,
expiratory
CT,
and
physiologic
American Journal of Roentgenology 1995.165:813-816.
(including
no clinical
or spirometric
to bronchodilator
therapy)
suggests
of idiopathic
bronchiolitis
obliterans.
(Case
courtesy
of Chris
Meyer,
Army Medical Center, WA; reproduced
mission from Stern and Frank (1])
features
response
diagnosis
Madlgan
with per-
Fig. 2.-40-year-old
woman with asthma.
A, High-resolution
CT scan obtained
at suspended
full inspiration
shows
B, Repeat
high-resolution
CT scan obtained
at suspended
full expiration
normal
shows
findings,
Including
normal
patchy diffuse air trapping
gradient
of attenuation.
with typical CT mosaic
pattern
of lung
attenuation.
Fig. 3-71-year-old
man with recurrent
and chronic
pulmonary
emboll and resulting
pulmonary
arterial hypertension.
A, Posterior-anterior
chest radiograph
shows apparent
bilateral
perihilar
pulmonary
infiltrates.
B, High-resolution
CT scan shows mosaic pattern of lung attenuation
with perihilar
ground-glass
attenuation
and oligemic
peripheral
lung. Note that
caliber of vessels
in regions of higher attenuation
(straight
arrow) is greater than in lower-attenuation
oligemic lung (curved
arrow).
Apparent
lung infiltrates
on chest radiograph
and high-resolution
CT scan are due to hyperperfused
normal lung. Peripheral
oligemic
hyperlucent
lung
showed no evidence
of air trapping
on expiratory
views. It is oligemic and hyperlucent
because of chronic pulmonary
thromboembolic
disease.
AJR:165,
October
Fig.
4.-65-year-old
arterial
CT MOSAIC
1995
hypertension
woman
PATTERN
OF LUNG
ATTENUATION
815
with pulmonary
due to chronic
pulmonary
thromboembolism.
A and B, Inspiratory
(A) and expiratory
(B)
high-resolution
CT scans show mosaic pattern
of lung attenuation,
so-called
mosaic
perfusion. lnspiratory
image shows caliber
of yessels
is
greater
within
relatively
higherattenuation
lung (straight
arrow)
than lowerattenuation
lung (curved
arrow).
Expiratory
image shows no evidence
of air trapping;
both
hyperperfused
and ollgemic
lung increase
in
attenuation.
CT pattern of mosaic lung attenuation, with larger vessels
in regions of lung haying
relatively
higher
attenuation
and
the
absence of air trapping on expiratory images,
is strong Indication that CT mosaic pattern is
American Journal of Roentgenology 1995.165:813-816.
caused
by pulmonary
vascular
lung
disease.
carinii pneumonia
(Fig. 5), chronic
eosinophilic
pneumonia,
hypersensitivity
pneumonia,
bnonchiolitis
oblitenans
organizing pneumonia,
and pyogenic
pneumonia.
Discussion
The term mosaic pattern of lung attenuation
is nonspecific
and refers to a pattern that occurs in a variety of lung diseases. The terms mosaic perfusion and mosaic oligemia
should be reserved for known cases of primary vascular
lung
disease. Table 1 reviews both the nadiologic and clinical distinguishing
features
of the three different categories
of disease
that can cause a CT mosaic pattern of lung attenuation.
In small-airway
disease and primary vascular
lung disease,
the pulmonary
vessels within the lucent regions of the lung
are small compared
with the vessels in the more opaque
regions of the lung. This discrepancy
in vessel size is likely
due, at least in part, to local hypoxic reflex vasoconstniction
in
small-airway
disease
[2], whereas
the difference
in vessel
size in primary vascular lung disease is due to the underlying
hypoperfusion.
In infiltrative
diseases,
the vessels are more
uniform in size throughout
the different regions of lung attenuation. Thus, analysis
of the size of the pulmonary
vessels
should be an early step in distinguishing
among the causes of
a CT mosaic pattern of lung attenuation.
Using paired inspiratory
and expiratory
CT scans is useful for
distinguishing
small-airway
disease
from a pnmary vascular
lung disease.
In small-airway
disease,
the lucent regions of
lung seen at inspiration
will remain lucent at expiration
because
of air trapping, showing no on minimal increase in lung attenuation and no on minimal
decrease
in volume.
The relatively
opaque normal lung willincrease in attenuation and decrease
in volume, as expected
[6]. In primary vascular
lung disease,
because there is no air trapping or airway disease, the attenuation of both the hyperemic
and oligemic lung at inspiration
will
increase in a similar fashion, and the volume of both will
decrease
uniformly
at expiration.
One can qualitatively
com-
Fig. 5-46-year-old
man with AIDS and Pneumocystis
carlnll pneumonia. High-resolution
CT scan shows pattern of mosaic lung attenuation due to ground-glass
infiltrate
that spares
single
lobular
and
multilobular
regions.
No appreciable
difference
exists
in number
and
size of vessels
in either
region
of lung. No air trapping
would
be
expected
on expiratory
CT.
pare the different
regions
of lung attenuation
for changes
between
inspiration
and expiration,
but occasionally
it will be
helpful to quantitatively
compare the Hounsfield
measurements
of lower-attenuation
and higher-attenuation
areas of lung in
scans obtained at inspiration
and at expiration
(Fig. 6).
A CT mosaic pattern of lung attenuation
may have many
causes.
Just as lung parenchymal
ground-glass
attenuation
is nonspecific,
a CT mosaic
pattern
of lung attenuation
is
STERN
816
TABLE
1 : Distinguishing
Features
of the Three Categories
ET
of Disease
AL.
Causing
AJR:165,
October
1995
a CT Mosaic Pattern of Lung Attenuation
Radiolo gic Features
Disease
Clinical Features
Vessels
Small-airway
disease
Decreased
size
and
CT Scansa
number
in
lucent lung compared with
higher-attenuation
lung
Air trapping
expiratory
Vascular
Infiltrative
lung disease
diseases
Same as small-airway
disease
Similar size and number of
vessels in both regions of lung
present
as evidenced
Dyspnea
by no increase in attenuation or
decrease in volume of lucent lung on
CT scans
Cough
Variable response to bronchodilators
Wheezing
No air trapping
CTscans
seen on expiratory
No air trapping
CT scans
seen on expiratory
No fever
Exertional dyspnea
No cough
No response to bronchodilators
No wheezing
No fever
Dyspnea
Cough
No response
No wheezing
to bronchodilators
Fever
Constitutional
CT scans.
American Journal of Roentgenology 1995.165:813-816.
a Paired inspiration/expiration
symptoms
6-34-year-old
woman with chronic thromboembolic
disease.
A and B, lnsplratory
(A ) and expiratory
(B) high-resolution
CT scans show CT mosaic
pattern of lung attenuation.
Lung attenuation
measured
in
hypoperfused
right upper lobe (region
of interest
(ROl] 1) increased
85 H (from -818 H to -733 H) between
inspiration
and expiration
CT scanning.
Lung attenuation
measured
in hyperperfused
left upper lobe (ROI 2) increased
a similar amount,
79 H (from -721 H to -642 H), between
inspiration
and expiration
CT scanning.
If air trapping
were causing
mosaic
lung attenuation,
differences
between
two regions
would
be much more pronounced
(6]. Note that placements
of ROI are anatomically
within same mosaic regions of lung; slight differences
in positions
of ROI between inspiration and expiration
avoid placing
large vessels
within ROl. Lung attenuation
in oligemic
regions
in left upper lobe (ROl 3) showed
an increase
of
60 H (from -794 H to -734 H), similar to that seen in oligemic
regions
of right lung.
Fig.
also nonspecific.
However,
by using additional
CT and clinical findings
and by obtaining
supplementary
expiratory
CT
scans, it is often possible
to determine
the underlying
disease category
responsible
for the pattern
and, in some
instances,
to suggest
a specific diagnosis.
2. Stern EJ, Webb WR. Dynamic
imaging of lung morphology with ultrafast
J Thorac Imaging 1993:8:273-282
3. Martin KW, Sagel 55, Siegel BA. Mosaic oligemia simulating pulmonary
high-resolution
infiltrates
computed
tomography.
on CT. AJR 1986:147:670-673
4. King MA, Bergin CJ, Yeung DW, et al. Chronic pulmonary thromboembolism:
detection
of regional
hypoperfusion
with CT. Radiologyl994:191
5. Primack SL, MUller NL, Mayo JR, Remy-Jardin
parenchymal
abnormalities
of vascular
RadioGraphics 1994:14:739-746
REFERENCES
1 . Stern EJ, Frank MS. Small-airway
ratory
CT. AJR
1994:163:37-41
diseases
of the lungs: findings at expi-
origin:
:359-363
M, Remy J. Pulmonary
high-resolution
CT findings.
6. Webb WR, Stern EJ, Kanth N, Gamsu G. Dynamic pulmonary
ings in healthy
adult
men.
Radiology
1993:186:117-124
CT: find-
American Journal of Roentgenology 1995.165:813-816.
This article has been cited by:
1. Ali Ataya, Sheylan Patel, Jessica Cope, Hassan Alnuaimat. 2016. Pulmonary arterial hypertension and associated conditions.
Disease-a-Month 62:11, 382-405. [CrossRef]
2. Xiaohua Wu, Dawei Dong, Daqing Ma. 2016. Thin-Section Computed Tomography Manifestations During Convalescence and
Long-Term Follow-Up of Patients with Severe Acute Respiratory Syndrome (SARS). Medical Science Monitor 22, 2793-2799.
[CrossRef]
3. Seth J. Kligerman, Travis Henry, Cheng T. Lin, Teri J. Franks, Jeffrey R. Galvin. 2015. Mosaic Attenuation: Etiology, Methods
of Differentiation, and Pitfalls. RadioGraphics 35:5, 1360-1380. [CrossRef]
4. Kamonpun Ussavarungsi, Augustine Lee, Charles Burger. 2015. Mosaic Pattern of Lung Attenuation on Chest CT in Patients
with Pulmonary Hypertension. Diseases 3:3, 205-212. [CrossRef]
5. Alicja Sieminska, Krzysztof Kuziemski. 2014. Respiratory bronchiolitis-interstitial lung disease. Orphanet Journal of Rare Diseases
9:1. . [CrossRef]
6. Shalini Wijesuriya, Ladli Chandratreya, Andrew R. Medford. 2013. Chronic Pulmonary Emboli and Radiologic Mimics on CT
Pulmonary Angiography. Chest 143:5, 1460-1471. [CrossRef]
7. Aletta Ann Frazier, Allen P. Burke. 2012. The Imaging of Pulmonary Hypertension. Seminars in Ultrasound, CT and MRI 33:6,
535-551. [CrossRef]
8. Bo Hyun Kim, Joon Beom Seo, Eun Jin Chae, Hyun Joo Lee, Hye Jeon Hwang, Chaehun Lim. 2012. Analysis of perfusion defects
by causes other than acute pulmonary thromboembolism on contrast-enhanced dual-energy CT in consecutive 537 patients.
European Journal of Radiology 81:4, e647-e652. [CrossRef]
9. A. Rossi, D. Attin, A. Borgonovi, F. Buia, F. De Luca, P.L. Guidalotti, P. Fughelli, N. Gali, M. Zompatori. 2012. Evaluation
of mosaic pattern areas in HRCT with Min-IP reconstructions in patients with pulmonary hypertension: Could this evaluation
replace lung perfusion scintigraphy?. European Journal of Radiology 81:1, e1-e6. [CrossRef]
10. Carole A. Ridge, Alexander A. Bankier, Ronald L. Eisenberg. 2011. Mosaic Attenuation. American Journal of Roentgenology 197:6,
W970-W977. [Citation] [Full Text] [PDF] [PDF Plus]
11. Toms Franquet. 2011. Imaging of Pulmonary Viral Pneumonia. Radiology 260:1, 18-39. [CrossRef]
12. Yasunobu Hayabuchi, Miki Inoue, Noriko Watanabe, Miho Sakata, Manal Mohamed Helmy Nabo, Shoji Kagami. 2011.
Minimum-intensity projection of multidetector-row computed tomography for assessment of pulmonary hypertension in children
with congenital heart disease. International Journal of Cardiology 149:2, 192-198. [CrossRef]
13. Teresa Bandeira, Filipa Negreiro, Rosrio Ferreira, Marisa Salgueiro, Lusa Lobo, Pedro Aguiar, J.C. Trindade. 2011. Clinical,
radiological, and physiological differences between obliterative bronchiolitis and problematic severe asthma in adolescents and
young adults: The early origins of the overlap syndrome?. Pediatric Pulmonology 46:6, 573-580. [CrossRef]
14. Irene M. Lang, Christina Plank, Roela Sadushi-Kolici, Johannes Jakowitsch, Walter Klepetko, Gerald Maurer. 2010. Imaging in
Pulmonary Hypertension. JACC: Cardiovascular Imaging 3:12, 1287-1295. [CrossRef]
15. Franois Pontana, Martine Remy-Jardin, Alain Duhamel, Jean-Baptiste Faivre, Benoit Wallaert, Jacques Remy. 2010. Lung
Perfusion with Dual-Energy Multi-detector Row CT. Academic Radiology 17:5, 587-594. [CrossRef]
16. David M Hansell, David A Lynch, H Page McAdams, Alexander A BankierBasic HRCT patterns of lung disease 153-204.
[CrossRef]
17. Toms FranquetNonneoplastic Parenchymal Lung Disease 863-926. [CrossRef]
18. Eun-Young Kang, Ok Hee Woo, Bong Kyung Shin, Hwan Seok Yong, Yu-Whan Oh, Han Kyeom Kim. 2009. Bronchiolitis.
Journal of Computer Assisted Tomography 33:1, 32-41. [CrossRef]
19. Tommaso Bartalena, Devil Oboldi, Pier Luigi Guidalotti, Maria Francesca Rinaldi, Paola Bertaccini, Gabriella Napoli, Giampaolo
Gavelli. 2008. Lung Perfusion in Patients With Pulmonary Hypertension: Comparison Between MDCT Pulmonary Angiography
With minIP Reconstructions and 99mTc-MAA Perfusion Scan. Investigative Radiology 43:6, 368-373. [CrossRef]
20. Chih-Yung Chiu, Kin-Sun Wong, Yhu-Chering Huang, Tzou-Yien Lin. 2008. Bronchiolitis obliterans in children: Clinical
presentation, therapy and long-term follow-up. Journal of Paediatrics and Child Health 44:3, 129-133. [CrossRef]
21. Rex C. Yung, Leo Patrick LawlerAdvances in Diagnostic Bronchoscopy: Virtual Bronchoscopy and Advanced Airway Imaging
44-75. [CrossRef]
22. Nicholas John Screaton, Deepa GopalanPulmonary Arterial Hypertension 936-963. [CrossRef]
American Journal of Roentgenology 1995.165:813-816.
23. David Z. Tzeng, Kevin O. Leslie, David Shelton, Andrew Chan. 2008. Unusual Dyspnea in a Woman With CREST Syndrome.
Chest 133:1, 286-290. [CrossRef]
24. Susan J. Copley, Y. C. Gary Lee, David M. Hansell, Pathmanathan Sivakumaran, Michael B. Rubens, Anthony J. Newman
Taylor, Robin M. Rudd, A. William Musk, Athol U. Wells. 2007. Asbestos-induced and Smoking-related Disease: Apportioning
Pulmonary Function Deficit by Using Thin-Section CT. Radiology 242:1, 258-266. [CrossRef]
25. Nicolas Sans, Jacques Giron, Pierre Fajadet, Denise Galy-Fourcade, Patrice Laborde, Jean-Jacques Railhac, Grard Durand, JeanPaul Senac. 2006. Syndrome bronchique. EMC - Radiologie et imagerie mdicale - Cardiovasculaire - Thoracique - Cervicale 1:1,
1-17. [CrossRef]
26. Rosita M. Shah, Wallace Miller. 2005. Pulmonary Complications of Transplantation: Radiographic Considerations. Clinics in
Chest Medicine 26:4, 545-560. [CrossRef]
27. Arnaud Resten, Sophie Maitre, Dominique Musset. 2005. CT imaging of peripheral pulmonary vessel disease. European Radiology
15:10, 2045-2056. [CrossRef]
28. Christian Lohrmann, Markus Uhl, Klaus Warnatz, Nadir Ghanem, Elmar Kotter, Oliver Schaefer, Mathias Langer. 2004. Highresolution CT imaging of the lung for patients with primary Sjgrens syndrome. European Journal of Radiology 52:2, 137-143.
[CrossRef]
29. Z.A. Aziz, S.P. Padley, D.M. Hansell. 2004. CT techniques for imaging the lung: recommendations for multislice and single slice
computed tomography. European Journal of Radiology 52:2, 119-136. [CrossRef]
30. Arnaud Resten, Sophie Maitre, Marc Humbert, Anne Rabiller, Olivier Sitbon, Frdrique Capron, Grald Simonneau, Dominique
Musset. 2004. Pulmonary Hypertension: CT of the Chest in Pulmonary Venoocclusive Disease. American Journal of Roentgenology
183:1, 65-70. [Abstract] [Full Text] [PDF] [PDF Plus]
31. Masanori Akira, Satoru Yamamoto, Yoshikazu Inoue, Mitsunori Sakatani. 2003. High-Resolution CT of Asbestosis and Idiopathic
Pulmonary Fibrosis. American Journal of Roentgenology 181:1, 163-169. [Abstract] [Full Text] [PDF] [PDF Plus]
32. Nobuyuki Tanaka, Tsuneo Matsumoto, Gouji Miura, Takuya Emoto, Naofumi Matsunaga, Katsuhiko Ueda, David A. Lynch.
2003. Air Trapping at CT: High Prevalence in Asymptomatic Subjects with Normal Pulmonary Function. Radiology 227:3,
776-785. [CrossRef]
33. David M. Hansell. 2002. Small-Vessel Diseases of the Lung: CT-Pathologic Correlates. Radiology 225:3, 639-653. [CrossRef]
34. Arnaud Resten, Sophie Matre, Marc Humbert, Olivier Sitbon, Frdrique Capron, Grald Simoneau, Dominique Musset. 2002.
Pulmonary Arterial Hypertension: Thin-Section CT Predictors of Epoprostenol Therapy Failure. Radiology 222:3, 782-788.
[CrossRef]
35. Sujal R. Desai, David M. HansellImaging 465-480. [CrossRef]
36. John R. Mayo, Michael E. Hayden. 2002. Hyperpolarized Helium 3 Diffusion Imaging of the Lung. Radiology 222:1, 8-11.
[CrossRef]
37. David M. Hansell. 2001. HIGH-RESOLUTION CT OF DIFFUSE LUNG DISEASE. Radiologic Clinics of North America 39:6,
1091-1113. [CrossRef]
38. Martin Uffmann, Hans P. Kiener, Alexander A. Bankier, Manfred M. Baldt, Thomas Zontsich, Christian J. Herold. 2001. Lung
Manifestation in Asymptomatic Patients with Primary Sjgren Syndrome: Assessment with High Resolution CT and Pulmonary
Function Tests. Journal of Thoracic Imaging 16:4, 282-289. [CrossRef]
39. Ella A. Kazerooni. 2001. High-Resolution CT of the Lungs. American Journal of Roentgenology 177:3, 501-519. [Citation] [Full
Text] [PDF] [PDF Plus]
40. Noriaki Kubo, Hideshi Tomita, Shigeto Fuse, Naomi Abe, Kinnya Hatakeyama, Shunzo Chiba. 2001. Helical Computer Assisted
Tomography in Pulmonary Hypertension Complicating Left-to-Right Shunts. Japanese Circulation Journal 65:3, 188-192.
[CrossRef]
41. David M. Hansell. 2001. HRCT of obliterative bronchiolitis and other small airways diseases. Seminars in Roentgenology 36:1,
51-65. [CrossRef]
42. Kazuya Ichikado, Moritaka Suga, Yasuhiro Gushima, Takeshi Johkoh, Kazuhiro Iyonaga, Toshimi Yokoyama, Osamu Honda,
Yoshihisa Shigeto, Seiji Tomiguchi, Mutsumasa Takahashi, Harumi Itoh, Junpei Ikezoe, Nestor L. Mller, Masayuki Ando.
2000. Hyperoxia-induced Diffuse Alveolar Damage in Pigs: Correlation between Thin-Section CT and Histopathologic Findings.
Radiology 216:2, 531-538. [CrossRef]
43. Keith H. Wittenberg, Stephen J. Swensen, Jeffrey L. Myers. 2000. Pulmonary Involvement with Erdheim-Chester Disease.
American Journal of Roentgenology 174:5, 1327-1331. [Abstract] [Full Text] [PDF] [PDF Plus]
American Journal of Roentgenology 1995.165:813-816.
44. Ritu Madan, Thomas J. Donnelly. 2000. A 69-Year-Old Woman With CREST Syndrome, Dyspnea, and a Mosaic CT
Attenuation Pattern. Chest 117:2, 584-587. [CrossRef]
45. Sujal R. Desai, Athol U. Wells, Michael B. Rubens, Timothy W. Evans, David M. Hansell. 1999. Acute Respiratory Distress
Syndrome: CT Abnormalities at Long-term Follow-up. Radiology 210:1, 29-35. [CrossRef]
46. Tae Kyoung Kim, Jung-Gi Im, Sung Hyun Kim, Hyung Jin Won, Joon Beom Seo, Kyung Mo Yeon, Man Chung Han. 1998.
Experimentally induced pulmonary arterial occlusion with detachable balloon in pigs: Thin-section CT findings. Academic
Radiology 5:12, 822-831. [CrossRef]
47. C. Wittram, D.C. Rapparort. 1998. Case report: Expiratory helical CT scan minimum intensity projection imaging in cystic
fibrosis. Clinical Radiology 53:8, 615-616. [CrossRef]
48. Richard Long, Bruce Maycher, Anil Dhar, Jure Manfreda, Earl Hershfield, Nicholas Anthonisen. 1998. Pulmonary Tuberculosis
Treated With Directly Observed Therapy. Chest 113:4, 933-943. [CrossRef]
49. Ann N. Leung, Kendra Fisher, Vincent Valentine, Reda E. Girgis, Gerald J. Berry, Robert C. Robbins, James Theodore. 1998.
Bronchiolitis Obliterans After Lung Transplantation. Chest 113:2, 365-370. [CrossRef]
50. S.Melanie Greaves, Eric M Hart, Denise R Aberle. 1997. CT of pulmonary thromboembolism. Seminars in Ultrasound, CT and
MRI 18:5, 323-337. [CrossRef]
51. S.R. Desai, D.M. Hansell. 1997. Small airways disease: Expiratory computed tomography comes of age. Clinical Radiology 52:5,
332-337. [CrossRef]
You might also like
- PT Management of Restrictive Lung DiseaseDocument16 pagesPT Management of Restrictive Lung DiseaseSiva Shanmugam25% (4)
- Upper Respiratory Tract InfectionsDocument25 pagesUpper Respiratory Tract Infectionsanjitha100% (1)
- Chronic Obstructive Pulmonary Disease: Radiology-Pathology CorrelationDocument10 pagesChronic Obstructive Pulmonary Disease: Radiology-Pathology CorrelationNetii FarhatiNo ratings yet
- Journal of Thoracic ImagingDocument11 pagesJournal of Thoracic Imagingestues2No ratings yet
- Journal Radiologi 3Document14 pagesJournal Radiologi 3WinayNayNo ratings yet
- Approach To Interstitial Lung DiseasesDocument38 pagesApproach To Interstitial Lung DiseasesmeawchiNo ratings yet
- RespiDocument58 pagesRespiIshani PatelNo ratings yet
- Copd 1Document4 pagesCopd 1Kavesha KarunakaranNo ratings yet
- Pulmonary Imaging: Xie Xin Li Department of Nuclear Medicine, First Affiliated Hospital, Zhengzhou UniversityDocument38 pagesPulmonary Imaging: Xie Xin Li Department of Nuclear Medicine, First Affiliated Hospital, Zhengzhou Universityapi-19916399No ratings yet
- Imaging - Chest RadiologyDocument78 pagesImaging - Chest Radiologymoggs7No ratings yet
- Pitfalls of EmphysemaDocument7 pagesPitfalls of EmphysemaWahyu Puspita IrjayantiNo ratings yet
- Respiratory Step 2 CK NoteDocument41 pagesRespiratory Step 2 CK NoteXboyx MahdiNo ratings yet
- Journal Reading RadiologiDocument49 pagesJournal Reading RadiologiPhilipusHendryHartonoNo ratings yet
- Pulmonary Emphysema: EpidemiologyDocument4 pagesPulmonary Emphysema: EpidemiologyAnonymous 835s2sxNo ratings yet
- Jurnal RadiologiDocument5 pagesJurnal RadiologiKen FcNo ratings yet
- Radiology RoundsDocument30 pagesRadiology RoundsKelum BuddhikaNo ratings yet
- Imaging of EmphysemaDocument8 pagesImaging of EmphysemaWijayanti EfendiNo ratings yet
- Cor PulmonaleDocument27 pagesCor PulmonaleumapathisivanNo ratings yet
- Pulmonary Infarction - Radiology Reference ArticleDocument7 pagesPulmonary Infarction - Radiology Reference ArticleHieu PhanNo ratings yet
- Mikroorganisme Penyebab ISPA Pada AnakDocument19 pagesMikroorganisme Penyebab ISPA Pada AnakVania TobingNo ratings yet
- AtelectasisDocument12 pagesAtelectasisAureliaNo ratings yet
- X Ray InterprationDocument6 pagesX Ray InterprationMelvita KurniawanNo ratings yet
- Pulmonary Embolism (PE) - Pulmonary Disorders - MSD Manual Professional EditionDocument25 pagesPulmonary Embolism (PE) - Pulmonary Disorders - MSD Manual Professional Editionpeterpavel112No ratings yet
- High Resolution CT of The Lung Patterns of Disease and Differential DiagnosesDocument30 pagesHigh Resolution CT of The Lung Patterns of Disease and Differential DiagnosesNicolai Babalici100% (1)
- Imaging Pulmonary Infection, Classic Sign and Pattern PRESENTASIDocument84 pagesImaging Pulmonary Infection, Classic Sign and Pattern PRESENTASIMark Brown100% (1)
- Jurnal 2Document0 pagesJurnal 2Seftiana SaftariNo ratings yet
- Cor Pulmonale (Pneumoconiosis)Document23 pagesCor Pulmonale (Pneumoconiosis)Sai charithaNo ratings yet
- Lobar AtelectasisDocument5 pagesLobar AtelectasisRachman ⎝⏠⏝⏠⎠ BuyungNo ratings yet
- Emad Efat Chest CT BestDocument260 pagesEmad Efat Chest CT Bestdr Mohit TaylorNo ratings yet
- Chest X-Ray ReviewDocument145 pagesChest X-Ray ReviewHamid ShaalanNo ratings yet
- 2.2 Pulmonary Radiology 2Document41 pages2.2 Pulmonary Radiology 2Casey Rae YanoNo ratings yet
- Case Report: Transient Interruption of ContrastDocument3 pagesCase Report: Transient Interruption of ContrastGordana PuzovicNo ratings yet
- Emphysema ImagingDocument6 pagesEmphysema Imagingestues2No ratings yet
- A Case of Tracheal Tumor Masquerading As AsthmaDocument4 pagesA Case of Tracheal Tumor Masquerading As Asthmamastansk84No ratings yet
- GgoDocument6 pagesGgoAnupam ShrivastavaNo ratings yet
- Bronchiectasis. Tramline Shadows Are Visible Through The Heart ShadowDocument5 pagesBronchiectasis. Tramline Shadows Are Visible Through The Heart ShadowHardianti AgriNo ratings yet
- High Resolution Chest CT (HRCT) : Protocol, Indications, and PathologiesDocument36 pagesHigh Resolution Chest CT (HRCT) : Protocol, Indications, and PathologiesAashishNo ratings yet
- Diseases of The Respiratory SystemDocument71 pagesDiseases of The Respiratory SystemZafir SharifNo ratings yet
- Pictorial Essay: Bronchiectasis: CT CriteriaDocument7 pagesPictorial Essay: Bronchiectasis: CT CriteriadarendraabimayuNo ratings yet
- PDF To WordDocument17 pagesPDF To Wordanisa ghinaNo ratings yet
- Tension PneumothoraxDocument40 pagesTension Pneumothoraxbemi lestariNo ratings yet
- PneumothoraxDocument36 pagesPneumothoraxEira Lopez50% (6)
- Chronic Cor Pulmonale: General CardiologyDocument7 pagesChronic Cor Pulmonale: General CardiologyAnastasia Eka PuteriNo ratings yet
- Interstitial Lung Diseases Radiology 22222Document26 pagesInterstitial Lung Diseases Radiology 22222Daniel AshooriNo ratings yet
- Radiografie Del ToraceDocument33 pagesRadiografie Del Toraceblablabla25100% (1)
- High Resolution CT of The Lungs: Technique, Indications and FindingsDocument9 pagesHigh Resolution CT of The Lungs: Technique, Indications and FindingsHari Baskar SNo ratings yet
- Practice Essentials of Pulmonary ThromboembolismDocument39 pagesPractice Essentials of Pulmonary ThromboembolismEzzat Abdelhafeez SalemNo ratings yet
- Radiology 111Document51 pagesRadiology 111Sherika A. BurgessNo ratings yet
- Interstitial Lung Diseases RadiologyDocument26 pagesInterstitial Lung Diseases RadiologyDaniel AshooriNo ratings yet
- Cor Pulmonale ReferensiDocument6 pagesCor Pulmonale ReferensiMiranty Aditya HadiniNo ratings yet
- CT Halo SignDocument4 pagesCT Halo Signmitmat4uNo ratings yet
- Jurnal Radiologi TBDocument7 pagesJurnal Radiologi TBrian00019No ratings yet
- Imaging of Chest DiseasesDocument184 pagesImaging of Chest DiseasesGembong Putro100% (1)
- Radio Logical Chest SignsDocument15 pagesRadio Logical Chest SignsSamuel InbarajaNo ratings yet
- Radiology AssignmentDocument27 pagesRadiology AssignmentRoyce GonzagaNo ratings yet
- Section II - Chest Radiology: Figure 1ADocument35 pagesSection II - Chest Radiology: Figure 1AHaluk Alibazoglu100% (1)
- Chest Radiography Diagnostic Value and Interpretation: Imaging ModalitiesDocument12 pagesChest Radiography Diagnostic Value and Interpretation: Imaging Modalitiesdoctor evanNo ratings yet
- CT Chest New 2Document57 pagesCT Chest New 2subha95No ratings yet
- Radiological Chest SignsDocument15 pagesRadiological Chest SignsEzekiel ArtetaNo ratings yet
- Medical Mnemonic Sketches : Pulmonary DiseasesFrom EverandMedical Mnemonic Sketches : Pulmonary DiseasesNo ratings yet
- A Simple Guide to Pulmonary Infarction, Diagnosis, Treatment and Related ConditionsFrom EverandA Simple Guide to Pulmonary Infarction, Diagnosis, Treatment and Related ConditionsNo ratings yet
- Runny Nose in The Child Care SettingDocument2 pagesRunny Nose in The Child Care Settingisaac abbanNo ratings yet
- Cystic Fibrosis - Management of Pulmonary Exacerbations - UpToDateDocument31 pagesCystic Fibrosis - Management of Pulmonary Exacerbations - UpToDateDylanNo ratings yet
- COPD Case Study ResearchDocument5 pagesCOPD Case Study ResearchErica MotoNo ratings yet
- BronchiolitisDocument12 pagesBronchiolitisEz BallNo ratings yet
- Grade 7 3rd Term English Question 2078Document8 pagesGrade 7 3rd Term English Question 2078Deependra SilwalNo ratings yet
- Group 1 Case Study Chapter 24Document10 pagesGroup 1 Case Study Chapter 24Doneva Lyn MedinaNo ratings yet
- Common ColdDocument6 pagesCommon ColdRAM SOFTWARENo ratings yet
- PTS - Soal Bahasa Inggris Kelas IXDocument6 pagesPTS - Soal Bahasa Inggris Kelas IXRini YusmayantiNo ratings yet
- Polio: A PR Case Study.Document73 pagesPolio: A PR Case Study.Karunya VkNo ratings yet
- Disorder of Respiratory SystemDocument89 pagesDisorder of Respiratory SystemDarine Nasr100% (1)
- NCP - Ineffective Airway ClearanceDocument4 pagesNCP - Ineffective Airway ClearanceJet BautistaNo ratings yet
- Nursing Care Plan Assessment Diagnosis Inference Planning Intervention Rationale EvaluationDocument5 pagesNursing Care Plan Assessment Diagnosis Inference Planning Intervention Rationale Evaluationkrish_tartNo ratings yet
- Influenza 1918.0910Document19 pagesInfluenza 1918.0910RafaelNo ratings yet
- Nebulization TherapyDocument3 pagesNebulization TherapyGemalie KadilNo ratings yet
- Lung ExaminationDocument14 pagesLung Examinationსალომე მუმლაძე “Slay” TMANo ratings yet
- Who Influenza SurveillanceDocument153 pagesWho Influenza SurveillanceRidho Al FiqriNo ratings yet
- 2019-11-09 PPT PpokDocument26 pages2019-11-09 PPT PpokAmha ViethreeNo ratings yet
- Bronchiectasis 1Document22 pagesBronchiectasis 1Imam MardaniNo ratings yet
- Influenza: DR.T .V.R Ao MDDocument81 pagesInfluenza: DR.T .V.R Ao MDtummalapalli venkateswara raoNo ratings yet
- Distress FailureDocument23 pagesDistress Failureananda febriani auliaNo ratings yet
- 5 6226231896498503742Document62 pages5 6226231896498503742dessypoerwantoNo ratings yet
- Pathophysiology of Koch's Disease (Tuberculosis) : Primary InfectionDocument4 pagesPathophysiology of Koch's Disease (Tuberculosis) : Primary InfectionbijelNo ratings yet
- Maklumat Vaksinasi: Vaccination DetailsDocument1 pageMaklumat Vaksinasi: Vaccination DetailsFahrin ZikriNo ratings yet
- Announcement BIRCDocument8 pagesAnnouncement BIRCHendarsyah SuryadinataNo ratings yet
- NCP - CopdDocument3 pagesNCP - CopdhystericoNo ratings yet
- Types of PneumoniaDocument6 pagesTypes of PneumoniasakuraleeshaoranNo ratings yet
- PDF Ers Handbook of Respiratory Medicine Paolo Palange Ebook Full ChapterDocument53 pagesPDF Ers Handbook of Respiratory Medicine Paolo Palange Ebook Full Chapterdoris.stancliff777100% (3)
- Community-Acquired Pneumonia in Children - Clinical Features and Diagnosis - UpToDateDocument48 pagesCommunity-Acquired Pneumonia in Children - Clinical Features and Diagnosis - UpToDateAlejandra AubadNo ratings yet