Professional Documents
Culture Documents
Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)
Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)
Uploaded by
Vinoth KumarCopyright:
Available Formats
You might also like
- 12 Chemistry Chemical Kinetics Test 01Document1 page12 Chemistry Chemical Kinetics Test 01Minhaz AlamNo ratings yet
- Chemical Kinetics AssinmentDocument9 pagesChemical Kinetics AssinmentKhushi TiwariNo ratings yet
- CHEMICAL KINETIC-worksheet - Module - 1Document2 pagesCHEMICAL KINETIC-worksheet - Module - 1mathesh0000007No ratings yet
- 12 Chemistry Chemical Kinetics Test 01 Answer 7d4lDocument2 pages12 Chemistry Chemical Kinetics Test 01 Answer 7d4lHari krishnaNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Document2 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Hari krishnaNo ratings yet
- Rate of ReactionDocument44 pagesRate of Reactionpokyik cheungNo ratings yet
- Chemical KineticsDocument13 pagesChemical KineticsOM SableNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Document2 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Shreyash KolekarNo ratings yet
- Chap - 13achemical KineticsDocument27 pagesChap - 13achemical Kineticstalakhaseeb05No ratings yet
- Rate of Reaction: With Sir Manny P. Vista Grade 12 Stem Gen Chem 2 Subject TeacherDocument8 pagesRate of Reaction: With Sir Manny P. Vista Grade 12 Stem Gen Chem 2 Subject TeacherJack Daniel CandelarioNo ratings yet
- Chemistry Booklet No 4 EngineeringDocument382 pagesChemistry Booklet No 4 EngineeringVarad BhosaleNo ratings yet
- Sk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsDocument2 pagesSk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsNeil8353 GgNo ratings yet
- SHCC - 2023 - Chem Paper2 - AnnaDocument8 pagesSHCC - 2023 - Chem Paper2 - AnnaOof GucciNo ratings yet
- 5.4 Elementary Reactions StudentDocument3 pages5.4 Elementary Reactions Studenthoàng michelleNo ratings yet
- Chemistry Worksheet 2Document3 pagesChemistry Worksheet 2LemontNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics)Document2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics)Shreyash KolekarNo ratings yet
- OCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelDocument10 pagesOCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelSigourney MarshNo ratings yet
- P2 Memo 2015Document10 pagesP2 Memo 2015Bonga DubeNo ratings yet
- Chm271 - Tutorial 5 - Chemical KineticsDocument6 pagesChm271 - Tutorial 5 - Chemical Kineticsfiefy zmrNo ratings yet
- CHM271 - Tutorial 5 - Chemical KineticsDocument6 pagesCHM271 - Tutorial 5 - Chemical KineticsisfaNo ratings yet
- Chemical Kinetics - IITDocument24 pagesChemical Kinetics - IITSiddu GowdaNo ratings yet
- 08a. Chemical Kinetics SheetDocument33 pages08a. Chemical Kinetics SheetVIKRANTH KUMAR JAKKOJUNo ratings yet
- Chemical KineticsDocument11 pagesChemical KineticsNishant Raj KhuraNo ratings yet
- DPP-1 (Chemical Reaction & Equations)Document2 pagesDPP-1 (Chemical Reaction & Equations)Sujal paliwalNo ratings yet
- Chemical Kinetics-1Document50 pagesChemical Kinetics-1telangtanushreeNo ratings yet
- Unit 4 CHEMICAL KINETICS 2017Document10 pagesUnit 4 CHEMICAL KINETICS 2017Gaurav SharmaNo ratings yet
- Tugas IX 1718Document2 pagesTugas IX 1718wahyudinsysNo ratings yet
- CHEMISTRY (Theory) Set 1 (12!03!2019) SolutionsDocument17 pagesCHEMISTRY (Theory) Set 1 (12!03!2019) Solutions8D Music KingNo ratings yet
- Latihan Soal Laju Reaksi Part 1Document4 pagesLatihan Soal Laju Reaksi Part 1kamallemu2No ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- FMT Chemistry Class-12 (17 May 2020) PDFDocument2 pagesFMT Chemistry Class-12 (17 May 2020) PDFdislikeNo ratings yet
- Mock Board 1Document5 pagesMock Board 1Arjun PasrichaNo ratings yet
- Cbse Xii - Chemistry: Board Paper - 2017Document6 pagesCbse Xii - Chemistry: Board Paper - 2017Gargi SharmaNo ratings yet
- Physical Sciences P2 Memo May 2022Document8 pagesPhysical Sciences P2 Memo May 2022thabangaphanebusiness1No ratings yet
- Question On Chemical Kinetics-MA 2022Document12 pagesQuestion On Chemical Kinetics-MA 2022Sangay ChodenNo ratings yet
- Reaction Notes-ChemistryDocument19 pagesReaction Notes-ChemistrySirupyEwe GamerNo ratings yet
- Please Note The Video Questions Are On Page 3 of This DocumentDocument11 pagesPlease Note The Video Questions Are On Page 3 of This DocumentGiovanna GonçalvesNo ratings yet
- Equilibria Energetics and Transition ElementsDocument19 pagesEquilibria Energetics and Transition ElementsCocoNo ratings yet
- 12 2008 Chemistry 1 PDFDocument18 pages12 2008 Chemistry 1 PDFanush JainNo ratings yet
- CYC 01 20-21 Even QuestionDocument3 pagesCYC 01 20-21 Even QuestionSaikat LayekNo ratings yet
- PHYS CE Tutorial QuestionsDocument3 pagesPHYS CE Tutorial QuestionsMel SalazarNo ratings yet
- By Einar Helland Berger (Own Work) (CC BY SA 2.5 ), Via Wikimedia CommonsDocument49 pagesBy Einar Helland Berger (Own Work) (CC BY SA 2.5 ), Via Wikimedia CommonsHoàng Anh DbbyNo ratings yet
- Bài tập Động hóa học chương 2Document2 pagesBài tập Động hóa học chương 2Thảo PhươngNo ratings yet
- Answer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1Document5 pagesAnswer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1kjjkimkmkNo ratings yet
- General Chemistry I Final Exam Sem 1 2009Document4 pagesGeneral Chemistry I Final Exam Sem 1 2009John BrownNo ratings yet
- CBSE Chemistry Class 11 (Mid Term Exam Model Paper)Document3 pagesCBSE Chemistry Class 11 (Mid Term Exam Model Paper)RounakNo ratings yet
- 14 Chemical KineticsDocument6 pages14 Chemical KineticsPriyadharshni RaviNo ratings yet
- IIT-JEE Syllabus: RSM79 Ph-II CK CH 1Document69 pagesIIT-JEE Syllabus: RSM79 Ph-II CK CH 1Kushagra SinghNo ratings yet
- Strategic Intervention Material in Chemical ReactionsDocument15 pagesStrategic Intervention Material in Chemical ReactionsLorna Aggabao100% (1)
- Workout No. 4.5Document2 pagesWorkout No. 4.5Renamae IgnacioNo ratings yet
- Chem 1110 Midterm Test Winter Term 11Document12 pagesChem 1110 Midterm Test Winter Term 11sanaassaf19No ratings yet
- 01 Redox ReactionDocument28 pages01 Redox Reactionprashantyadavpky07No ratings yet
- Hsslive-Xii-Chemistry-Qb-Anil-4. CHEMICAL KINETICSDocument4 pagesHsslive-Xii-Chemistry-Qb-Anil-4. CHEMICAL KINETICSpremathangam807No ratings yet
- CBSE Class 12 Question Paper 2019 Chemistry Set 1Document15 pagesCBSE Class 12 Question Paper 2019 Chemistry Set 1Saran.kNo ratings yet
- Class X Science WatermarkDocument103 pagesClass X Science WatermarkMRT VoiceNo ratings yet
- CBSE 12 Chemistry Question Paper 2009 Set 2Document6 pagesCBSE 12 Chemistry Question Paper 2009 Set 2AkhilNo ratings yet
- Sample Paper Chemistry Clas Xi Set 5Document9 pagesSample Paper Chemistry Clas Xi Set 5abhijeetkumar12345trNo ratings yet
- Chemical Kinetics: Dr. Mohamed FadelDocument52 pagesChemical Kinetics: Dr. Mohamed Fadelعبدالحميد العرفيNo ratings yet
- Books: Physical Chemistry by Chemical Kinetics byDocument11 pagesBooks: Physical Chemistry by Chemical Kinetics byBB TestNo ratings yet
- 9th ReasoningDocument4 pages9th ReasoningVinoth KumarNo ratings yet
- TDocument2 pagesTVinoth KumarNo ratings yet
- Test1 Ch15 Kinetics Practice ProblemsDocument15 pagesTest1 Ch15 Kinetics Practice ProblemsVinoth KumarNo ratings yet
- Measurement of Temperature: Precision: Accuracy: RulesDocument3 pagesMeasurement of Temperature: Precision: Accuracy: RulesVinoth KumarNo ratings yet
Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)
Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)
Uploaded by
Vinoth KumarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)
Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics)
Uploaded by
Vinoth KumarCopyright:
Available Formats
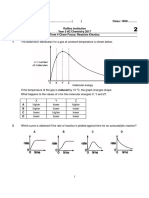
CBSE TEST PAPER-01
CLASS - XII CHEMISTRY (Chemical Kinetics)
Topic: -Rate of reaction Average and instantaneous rates
1. Define the term chemical kinetics? [1]
2. Define Rate of reaction? [1]
3. What is average rate of a reaction? How is it determined? [2]
4. What are the units of rate of a reaction? [1]
5. What is instantaneous rate of a reaction? How is it determined? [2]
6. For the following reactions, write the rate of reaction expression in terms
of reactants and products? [2]
i) 4NH3 (g) + 5O2 (g) 4NO (g) + 6H2O (g)
ii) 2N2O5 2NO2 + O2
7. For the chemical decomposition of SO2Cl2, its initial concentration is
0.8420 mol/L and final concentration is 0.215 molL-1 in 2 hours.
What is the average rate of this reaction? [2]
8. In the expression of rate of reaction in terms of reactants, what is the
significance of negative sign? [2]
[ O3 ]
9.
3O2 (g), -
For the reaction 2O3 (g)
was found to be 5.0 X 10-4 at m/s.
t
[ O2 ]
Determine the value of in atm /s during this period of time? [2]
t
10. A chemical reaction 2A 4B+C in gas phase occurs in a closed vessel.
The concentration of B is found to be increased by 5 X 10-3 mole L-1 in 10 second.
Calculate (i) the rate of appearance of B (ii) the rate of disappearance of A? [2]
Material downloaded from http://myCBSEguide.com and http://onlineteachers.co.in
Portal for CBSE Notes, Test Papers, Sample Papers, Tips and Tricks
You might also like
- 12 Chemistry Chemical Kinetics Test 01Document1 page12 Chemistry Chemical Kinetics Test 01Minhaz AlamNo ratings yet
- Chemical Kinetics AssinmentDocument9 pagesChemical Kinetics AssinmentKhushi TiwariNo ratings yet
- CHEMICAL KINETIC-worksheet - Module - 1Document2 pagesCHEMICAL KINETIC-worksheet - Module - 1mathesh0000007No ratings yet
- 12 Chemistry Chemical Kinetics Test 01 Answer 7d4lDocument2 pages12 Chemistry Chemical Kinetics Test 01 Answer 7d4lHari krishnaNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Document2 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Hari krishnaNo ratings yet
- Rate of ReactionDocument44 pagesRate of Reactionpokyik cheungNo ratings yet
- Chemical KineticsDocument13 pagesChemical KineticsOM SableNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Document2 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)Shreyash KolekarNo ratings yet
- Chap - 13achemical KineticsDocument27 pagesChap - 13achemical Kineticstalakhaseeb05No ratings yet
- Rate of Reaction: With Sir Manny P. Vista Grade 12 Stem Gen Chem 2 Subject TeacherDocument8 pagesRate of Reaction: With Sir Manny P. Vista Grade 12 Stem Gen Chem 2 Subject TeacherJack Daniel CandelarioNo ratings yet
- Chemistry Booklet No 4 EngineeringDocument382 pagesChemistry Booklet No 4 EngineeringVarad BhosaleNo ratings yet
- Sk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsDocument2 pagesSk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsNeil8353 GgNo ratings yet
- SHCC - 2023 - Chem Paper2 - AnnaDocument8 pagesSHCC - 2023 - Chem Paper2 - AnnaOof GucciNo ratings yet
- 5.4 Elementary Reactions StudentDocument3 pages5.4 Elementary Reactions Studenthoàng michelleNo ratings yet
- Chemistry Worksheet 2Document3 pagesChemistry Worksheet 2LemontNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics)Document2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics)Shreyash KolekarNo ratings yet
- OCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelDocument10 pagesOCR - Chemistry - Module 5 Part 1 - GraspIT ANSWERS - A LevelSigourney MarshNo ratings yet
- P2 Memo 2015Document10 pagesP2 Memo 2015Bonga DubeNo ratings yet
- Chm271 - Tutorial 5 - Chemical KineticsDocument6 pagesChm271 - Tutorial 5 - Chemical Kineticsfiefy zmrNo ratings yet
- CHM271 - Tutorial 5 - Chemical KineticsDocument6 pagesCHM271 - Tutorial 5 - Chemical KineticsisfaNo ratings yet
- Chemical Kinetics - IITDocument24 pagesChemical Kinetics - IITSiddu GowdaNo ratings yet
- 08a. Chemical Kinetics SheetDocument33 pages08a. Chemical Kinetics SheetVIKRANTH KUMAR JAKKOJUNo ratings yet
- Chemical KineticsDocument11 pagesChemical KineticsNishant Raj KhuraNo ratings yet
- DPP-1 (Chemical Reaction & Equations)Document2 pagesDPP-1 (Chemical Reaction & Equations)Sujal paliwalNo ratings yet
- Chemical Kinetics-1Document50 pagesChemical Kinetics-1telangtanushreeNo ratings yet
- Unit 4 CHEMICAL KINETICS 2017Document10 pagesUnit 4 CHEMICAL KINETICS 2017Gaurav SharmaNo ratings yet
- Tugas IX 1718Document2 pagesTugas IX 1718wahyudinsysNo ratings yet
- CHEMISTRY (Theory) Set 1 (12!03!2019) SolutionsDocument17 pagesCHEMISTRY (Theory) Set 1 (12!03!2019) Solutions8D Music KingNo ratings yet
- Latihan Soal Laju Reaksi Part 1Document4 pagesLatihan Soal Laju Reaksi Part 1kamallemu2No ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- FMT Chemistry Class-12 (17 May 2020) PDFDocument2 pagesFMT Chemistry Class-12 (17 May 2020) PDFdislikeNo ratings yet
- Mock Board 1Document5 pagesMock Board 1Arjun PasrichaNo ratings yet
- Cbse Xii - Chemistry: Board Paper - 2017Document6 pagesCbse Xii - Chemistry: Board Paper - 2017Gargi SharmaNo ratings yet
- Physical Sciences P2 Memo May 2022Document8 pagesPhysical Sciences P2 Memo May 2022thabangaphanebusiness1No ratings yet
- Question On Chemical Kinetics-MA 2022Document12 pagesQuestion On Chemical Kinetics-MA 2022Sangay ChodenNo ratings yet
- Reaction Notes-ChemistryDocument19 pagesReaction Notes-ChemistrySirupyEwe GamerNo ratings yet
- Please Note The Video Questions Are On Page 3 of This DocumentDocument11 pagesPlease Note The Video Questions Are On Page 3 of This DocumentGiovanna GonçalvesNo ratings yet
- Equilibria Energetics and Transition ElementsDocument19 pagesEquilibria Energetics and Transition ElementsCocoNo ratings yet
- 12 2008 Chemistry 1 PDFDocument18 pages12 2008 Chemistry 1 PDFanush JainNo ratings yet
- CYC 01 20-21 Even QuestionDocument3 pagesCYC 01 20-21 Even QuestionSaikat LayekNo ratings yet
- PHYS CE Tutorial QuestionsDocument3 pagesPHYS CE Tutorial QuestionsMel SalazarNo ratings yet
- By Einar Helland Berger (Own Work) (CC BY SA 2.5 ), Via Wikimedia CommonsDocument49 pagesBy Einar Helland Berger (Own Work) (CC BY SA 2.5 ), Via Wikimedia CommonsHoàng Anh DbbyNo ratings yet
- Bài tập Động hóa học chương 2Document2 pagesBài tập Động hóa học chương 2Thảo PhươngNo ratings yet
- Answer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1Document5 pagesAnswer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1kjjkimkmkNo ratings yet
- General Chemistry I Final Exam Sem 1 2009Document4 pagesGeneral Chemistry I Final Exam Sem 1 2009John BrownNo ratings yet
- CBSE Chemistry Class 11 (Mid Term Exam Model Paper)Document3 pagesCBSE Chemistry Class 11 (Mid Term Exam Model Paper)RounakNo ratings yet
- 14 Chemical KineticsDocument6 pages14 Chemical KineticsPriyadharshni RaviNo ratings yet
- IIT-JEE Syllabus: RSM79 Ph-II CK CH 1Document69 pagesIIT-JEE Syllabus: RSM79 Ph-II CK CH 1Kushagra SinghNo ratings yet
- Strategic Intervention Material in Chemical ReactionsDocument15 pagesStrategic Intervention Material in Chemical ReactionsLorna Aggabao100% (1)
- Workout No. 4.5Document2 pagesWorkout No. 4.5Renamae IgnacioNo ratings yet
- Chem 1110 Midterm Test Winter Term 11Document12 pagesChem 1110 Midterm Test Winter Term 11sanaassaf19No ratings yet
- 01 Redox ReactionDocument28 pages01 Redox Reactionprashantyadavpky07No ratings yet
- Hsslive-Xii-Chemistry-Qb-Anil-4. CHEMICAL KINETICSDocument4 pagesHsslive-Xii-Chemistry-Qb-Anil-4. CHEMICAL KINETICSpremathangam807No ratings yet
- CBSE Class 12 Question Paper 2019 Chemistry Set 1Document15 pagesCBSE Class 12 Question Paper 2019 Chemistry Set 1Saran.kNo ratings yet
- Class X Science WatermarkDocument103 pagesClass X Science WatermarkMRT VoiceNo ratings yet
- CBSE 12 Chemistry Question Paper 2009 Set 2Document6 pagesCBSE 12 Chemistry Question Paper 2009 Set 2AkhilNo ratings yet
- Sample Paper Chemistry Clas Xi Set 5Document9 pagesSample Paper Chemistry Clas Xi Set 5abhijeetkumar12345trNo ratings yet
- Chemical Kinetics: Dr. Mohamed FadelDocument52 pagesChemical Kinetics: Dr. Mohamed Fadelعبدالحميد العرفيNo ratings yet
- Books: Physical Chemistry by Chemical Kinetics byDocument11 pagesBooks: Physical Chemistry by Chemical Kinetics byBB TestNo ratings yet
- 9th ReasoningDocument4 pages9th ReasoningVinoth KumarNo ratings yet
- TDocument2 pagesTVinoth KumarNo ratings yet
- Test1 Ch15 Kinetics Practice ProblemsDocument15 pagesTest1 Ch15 Kinetics Practice ProblemsVinoth KumarNo ratings yet
- Measurement of Temperature: Precision: Accuracy: RulesDocument3 pagesMeasurement of Temperature: Precision: Accuracy: RulesVinoth KumarNo ratings yet