Professional Documents
Culture Documents
Standard Reduction Potentials Data Extended PDF
Standard Reduction Potentials Data Extended PDF
Uploaded by
Ace0 ratings0% found this document useful (0 votes)
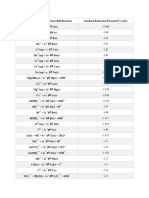
383 views2 pagesThe document lists standard reduction potentials (ε0) for various half-reactions. It contains over 60 reduction half-reactions ranging from highly reducing reactions like Li+/e- → Li with an ε0 of -3.04 V to strongly oxidizing reactions like F2/2e- → 2F- with an ε0 of 2.87 V. The half-reactions cover a wide variety of metals, nonmetals, and polyatomic ions. Many common redox couples are included such as Fe3+/Fe2+, Cu2+/Cu+, Ag+/Ag, and O2/H2O.
Original Description:
This table contains the Standard electrode reduction potentials values of some half-reactions.
Original Title
Standard Reduction Potentials Data Extended pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document lists standard reduction potentials (ε0) for various half-reactions. It contains over 60 reduction half-reactions ranging from highly reducing reactions like Li+/e- → Li with an ε0 of -3.04 V to strongly oxidizing reactions like F2/2e- → 2F- with an ε0 of 2.87 V. The half-reactions cover a wide variety of metals, nonmetals, and polyatomic ions. Many common redox couples are included such as Fe3+/Fe2+, Cu2+/Cu+, Ag+/Ag, and O2/H2O.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
383 views2 pagesStandard Reduction Potentials Data Extended PDF
Standard Reduction Potentials Data Extended PDF
Uploaded by
AceThe document lists standard reduction potentials (ε0) for various half-reactions. It contains over 60 reduction half-reactions ranging from highly reducing reactions like Li+/e- → Li with an ε0 of -3.04 V to strongly oxidizing reactions like F2/2e- → 2F- with an ε0 of 2.87 V. The half-reactions cover a wide variety of metals, nonmetals, and polyatomic ions. Many common redox couples are included such as Fe3+/Fe2+, Cu2+/Cu+, Ag+/Ag, and O2/H2O.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
Reduction Half-Reaction 0 Reduction Half-Reaction 0
Li+ + e- Li(s) -3.04 Co2+ + 2e- Co(s) -0.28
Cs+ + e- Cs(s) -3.02 V3+ + e- V2+ -0.26
Ca(OH)2 + 2e- Ca + 2OH- -3.02 Ni2+ + 2e- Ni(s) -0.23
Ba(OH)2 + 2e- Ba + 2OH- -2.99 Si(s) + 4H+ + 4e- SiH4(g) -0.14
Rb+ + e- Rb(s) -2.98 Sn2+ + 2e- Sn(s) -0.13
K+ + e- K(s) -2.93 Pb2+ + 2e- Pb(s) -0.12
Ba2+ + 2e- Ba(s) -2.91 P(red) + 3H+ + 3e- PH3(g) -0.11
Fr+ + e- Fr s) -2.9 Se(s) + 2H+ + 2e- H2Se(g) -0.11
Sr2+ + 2e- Sr(s) -2.89 CO2(g) + 2H+ + 2e- CO(g) + H2O -0.11
Ca2+ + 2e- Ca(s) -2.86 SnO(s) + 2H+ + 2e- Sn(s) + H2O -0.10
Ra2+ + 2e- Ra(s) -2.8 SnO2(s)+ 4H++4e- SnO(s) + 2H2O -0.09
Na+ + e- Na(s) -2.71 P(white) + 3H+ + 3e- PH3(g) -0.06
Mg+ + e- Mg(s) -2.70 Fe3+ + 3e- Fe(s) -0.04
Mg(OH)2 + 2e- Mg(s) + 2OH- -2.69 2H+ + 2e- H2(g) 0.00
Mg2+ + 2e- Mg(s) -2.37 AgBr(s) + e- Ag(s) + Br- 0.07
Al(OH)3(s) + 3e- Al(s) + 3OH- -2.31 Fe3O4 + 8H+ + 8e-3Fe(s) + 4H2O 0.08
H2 + 2e- 2H- -2.23 HgO(s) + H2O + 2e- Hg(l) +2OH- 0.09
Sc3+ + 3e- Sc(s) -2.07 C(s) + 4H+ + 4e- CH4(g) 0.13
Be2+ + 2e- Be(s) -1.84 S(s) + 2H+ + 2e- H2S(g) 0.14
Al3+ + 3e- Al(s) -1.66 Sn4+ + 2e- Sn2+ 0.15
Ti3+ + 3e- Ti(s) -1.37 Cu2+ + e- Cu+ 0.15
ZnO(s) + H2O +2e-Zn(s) + 2OH- -1.22 SO42- + 4H+ + 2e-SO2(aq) + 2H2O 0.17
Mn2+ + 2e- Mn(s) -1.18 ClO4- + H2O + 2e- ClO3- + 2OH- 0.17
V2+ + 2e- V(s) -1.13 AgCl(s) + e- Ag(s) + Cl- 0.22
Sn(s) + 4H+ + 4e- SnH4(g) -1.07 H3AsO3 + 3H+ +3e-As(s) + 3H2O 0.24
SiO2(s) + 4H+ + 4e-Si(s) + 2H2O -0.91 Cu2+ + 2e- Cu(s) 0.34
Fe(OH)2(s) + 2e- Fe(s) + 2OH- -0.89 ClO3- + H2O + 2e- ClO2- + 2OH- 0.35
Fe2O3+3H2O 2Fe(OH)2+2OH- -0.86 O2(g) + 2H2O + 4e- 4OH-(aq) 0.40
2H2O + 2e- H2(g) + 2OH- -0.82 IO- + H2O + 2e- I- + 2OH- 0.49
Zn2+ + 2e- Zn(s) -0.76 CH3OH(aq)+2H++2e-CH4(g)+H2O 0.50
Cr3+ + 3e- Cr(s) -0.74 SO2(aq) + 4H+ + 4e- S(s) + 2H2O 0.50
Ag2S(s) + 2e- 2Ag(s) + S2-(aq) -0.69 Cu+ + e- Cu(s) 0.52
PbO(s) + H2O + 2e-Pb(s) +2OH- -0.58 CO(g) + 2H+ + 2e- C(s) + H2O 0.52
Fe2+ + 2e- Fe(s) -0.44 I2(s) + 2e- 2I- 0.54
Cr3+ + e- Cr2+ -0.42 ClO2- + H2O + 2e- ClO- + 2OH- 0.59
Cd2+ + 2e- Cd(s) -0.40 O2(g) + 2H+ + 2e- H2O2(aq) 0.70
Cu2O(s)+H2O +2e-2Cu(s) +2OH- -0.36 Tl3+ + 3e- Tl(s) 0.72
PbSO4(s) + 2e- Pb(s) + SO42- -0.35 H2SeO3(aq)+4H+ +4e-Se(s) +3H2O 0.74
Fe3+ + e- Fe2+ 0.77 BrO4- + 2H+ + 2e-BrO3- +H2O 1.85
Ag+ + e- Ag(s) 0.80 Ag2+ + e- Ag+ 1.98
Hg22+ + 2e- 2Hg(l) 0.80 S2O82- + 2e- 2SO42- 2.01
NO3- + 2H+ + e- NO2(g) + H2O 0.80 F2(g) + 2e- 2F- 2.87
Hg2+ + 2e- Hg(l) 0.85 F2(g) + 2H+ + 2e- 2HF(aq) 3.05
ClO- + H2O + 2e- Cl- + 2OH- 0.90
2Hg2+ + 2e- Hg22+ 0.91
Pd2+ + 2e- Pd(s) 0.91
MnO2(s) +4H+ + e-Mn3+ +2H2O 0.95
NO3- + 4H+ + 3e-NO(g) + 2H2O 0.95
Br2(aq) + 2e- 2Br- 1.08
IO3- + 5H+ +4e-HIO(aq) + 2H2O 1.13
HSeO4- +3H++2e-H2SeO3+H2O 1.15
Ag2O(s) +2H+ +2e-2Ag(s) +H2O 1.17
Pt2+ + 2e- Pt(s) 1.18
2IO3- +12H+ +10e-I2(s) + 6H2O 1.20
ClO4- + 2H+ +2e- ClO3- + H2O 1.20
O2(g) + 4H+ + 4e- 2H2O 1.22
MnO2(s)+4H+ +2e-Mn2+ +2H2O 1.23
Cr2O72-+14H++6e-2Cr3++7H2O 1.33
Cl2(g) + 2e- 2Cl- 1.36
CoO2(s) +4H+ +e- Co3+ + 2H2O 1.42
2HIO(aq) +2H+ +2e-I2(s) + 2H2O 1.44
BrO3-+5H++4e-HBrO(aq)+2H2O 1.45
2BrO3-+12H++10e-Br2(l)+6H2O 1.48
2ClO3-+12H++10e-Cl2(g)+6H2O 1.49
HClO(aq) + H+ +2e-Cl-(aq) +H2O 1.49
Au3+ + 3e- Au(s) 1.50
MnO4- + 8H+ + 5e-Mn2++4H2O 1.51
NiO2(s) + 4H+ + 2e-Ni2+ + 2OH- 1.59
2HClO(aq)+2H++2eCl2(g)+2H2O 1.63
Ag2O3(s)+6H++4e-2Ag+ +3H2O 1.67
HClO2 + 2H+ +2e-HClO + H2O 1.67
Pb4+ + 2e- Pb2+ 1.69
MnO4-+2H2O+3e-MnO2+4OH- 1.69
MnO4-+4H++3e-MnO2(s)+2H2O 1.70
AgO(s) + 2H+ + e- Ag+ + H2O 1.77
H2O2(aq) + 2H+ + 2e- 2H2O 1.78
Co3+ + e- Co2+ 1.82
Au+ + e- Au(s) 1.83
You might also like
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjaverfrivNo ratings yet
- Standard Reduction PotentialDocument7 pagesStandard Reduction Potentialyoyotoonzone1No ratings yet
- Chapter 3 Oxidation ReductionDocument68 pagesChapter 3 Oxidation Reductionlong.vuongbz188No ratings yet
- ElectrodeDocument2 pagesElectrodeThatcher PanchoNo ratings yet
- SRP Table Chem DataDocument1 pageSRP Table Chem Dataapi-222503660No ratings yet
- Chapter 4 Oxidation-ReductionDocument68 pagesChapter 4 Oxidation-ReductionPHƯƠNG ĐẶNG YẾNNo ratings yet
- DebateDocument3 pagesDebatebbangeles1No ratings yet
- Appendix EDocument1 pageAppendix EYrhon IbanezNo ratings yet
- Standard Reduction Potentials at 25Document1 pageStandard Reduction Potentials at 25Beverly RamosNo ratings yet
- Standard Reduction PotentialsDocument5 pagesStandard Reduction PotentialsnathaloaNo ratings yet
- Standard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Document2 pagesStandard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Alexander RodriguezNo ratings yet
- Standard Electrode and Reduction Potentials at 298 K PrintableDocument3 pagesStandard Electrode and Reduction Potentials at 298 K Printablecarina_yii9690100% (1)
- P2 Standard Reduction Potentials by ValueDocument6 pagesP2 Standard Reduction Potentials by ValueASTRID ELIZABET CUEVA GUTIERREZNo ratings yet
- Standard Reduction PotentialDocument8 pagesStandard Reduction PotentialMateus CostaNo ratings yet
- Standard Reduction Potential TablesDocument1 pageStandard Reduction Potential TablesJenver NanquiladaNo ratings yet
- EMF SeriesDocument5 pagesEMF Seriesmike rosaNo ratings yet
- Standardreductionpotentials PDFDocument1 pageStandardreductionpotentials PDFBadrus SyamsiNo ratings yet
- CHEM1 Datasheet May 2020Document4 pagesCHEM1 Datasheet May 2020Miku HatsuneNo ratings yet
- Standard Reduction Potentials (At 25 C, 101.325 Kpa, 1M) Half-Reaction E (Volts)Document1 pageStandard Reduction Potentials (At 25 C, 101.325 Kpa, 1M) Half-Reaction E (Volts)sena_chem6706No ratings yet
- Standard Electrode Potential SeriesDocument1 pageStandard Electrode Potential SeriesWONG KEE PING MoeNo ratings yet
- The Dien Cuc ChuanDocument9 pagesThe Dien Cuc Chuanvinasat1108No ratings yet
- STD Electrode PontentialsDocument3 pagesSTD Electrode PontentialsVishal PamnaniNo ratings yet
- Chemistry Stage 2 and 3 Data Sheet 2010Document4 pagesChemistry Stage 2 and 3 Data Sheet 2010Edy LiewNo ratings yet
- Standard Electrode PotentialDocument11 pagesStandard Electrode PotentialRSLNo ratings yet
- Potenciales Estandar Del ElectrodoDocument3 pagesPotenciales Estandar Del ElectrododavidNo ratings yet
- E ValuesDocument1 pageE ValuesShania LoveresNo ratings yet
- Reduction Half-Reaction E (V) : Neutral or Acid SolutionDocument3 pagesReduction Half-Reaction E (V) : Neutral or Acid SolutionEric FernandoNo ratings yet
- 233 SolutionsDocument11 pages233 Solutionsestellasr00No ratings yet
- Edexecel IAL Lesson 1Document20 pagesEdexecel IAL Lesson 1Pevin De silvaNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25°C: TablesDocument2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: TablesLouie G NavaltaNo ratings yet
- SOA and SRA TableDocument1 pageSOA and SRA TableAhhhhhhhhhhhNo ratings yet
- A - Oxidation - ReductionDocument2 pagesA - Oxidation - ReductionAlyasin FrougaNo ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFAlexander Salado IbrahimNo ratings yet
- Chem 2Document8 pagesChem 22021302095No ratings yet
- Red Ox AnswersDocument2 pagesRed Ox Answerspaulmutambo509No ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFalbi veshiNo ratings yet
- Tablas Redox 2146Document15 pagesTablas Redox 2146TshikoNo ratings yet
- High School Science - Redox ReactionsDocument12 pagesHigh School Science - Redox ReactionsPort of Long BeachNo ratings yet
- Elc STD PotentialsDocument1 pageElc STD PotentialsArchita VNo ratings yet
- Sap 5Document22 pagesSap 5reza noviyantiNo ratings yet
- Nguyên tố Dạng oxi hoá +ne Dạng khử E, VDocument12 pagesNguyên tố Dạng oxi hoá +ne Dạng khử E, VNhat KhanhNo ratings yet
- Problems For Balancing of Redox ReactionsDocument1 pageProblems For Balancing of Redox ReactionsUtsavNo ratings yet
- Topic 13 Exercise 3 - Spontaneous ReactionsDocument1 pageTopic 13 Exercise 3 - Spontaneous ReactionsdenisNo ratings yet
- © Ncert Not To Be Republished: A I E, A N M MDocument10 pages© Ncert Not To Be Republished: A I E, A N M MbnkjayaNo ratings yet
- Chemical Reactions Class XDocument5 pagesChemical Reactions Class Xaprajita royNo ratings yet
- UNIT 2 Electrochemistry FinalDocument26 pagesUNIT 2 Electrochemistry FinalA HNo ratings yet
- Chapter-Wise Important Chemical Reactions For Class 10Document9 pagesChapter-Wise Important Chemical Reactions For Class 10Manish SainNo ratings yet
- UNIT 2 Electrochemistry FinalDocument25 pagesUNIT 2 Electrochemistry FinalPisces SandNo ratings yet
- Grade 11 Paper 2 Notes LearnersDocument149 pagesGrade 11 Paper 2 Notes Learnerslethabo.mokoenaNo ratings yet
- Clasifiacion de Reacciones QuimicasDocument5 pagesClasifiacion de Reacciones QuimicasMONTSERRAT MURILLO SERRANONo ratings yet
- HalfCellPoten TableDocument1 pageHalfCellPoten TableJeisson David Sanchez PInzonNo ratings yet
- Chemical Equation (2019)Document1 pageChemical Equation (2019)Ismalinda AbdullahNo ratings yet
- Balancing Redox Reactions WorksheetDocument2 pagesBalancing Redox Reactions Worksheetsai sreerama mNo ratings yet
- E° HBCPDocument10 pagesE° HBCPFelipe FariaNo ratings yet
- Chemistry: Written Examination 2Document3 pagesChemistry: Written Examination 2Mohamed MawasNo ratings yet
- Extract 10 PagesDocument10 pagesExtract 10 PageskuoklukeNo ratings yet
- Oxidation-Reduction Practice Problems: BonusDocument2 pagesOxidation-Reduction Practice Problems: BonusMandy HitaNo ratings yet
- ReaksiDocument2 pagesReaksiherna watiNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Composition and Rhetoric: English 101Document1 pageComposition and Rhetoric: English 101AceNo ratings yet
- English Rubric: EssayDocument1 pageEnglish Rubric: EssayAceNo ratings yet
- Acidity Constants Data Part 1Document2 pagesAcidity Constants Data Part 1AceNo ratings yet
- Eng 0101 E2Document1 pageEng 0101 E2AceNo ratings yet
- Basicity Constants Data Part 1Document1 pageBasicity Constants Data Part 1AceNo ratings yet
- Conjugation of Verbs Be, Have, Do and WriteDocument2 pagesConjugation of Verbs Be, Have, Do and WriteAceNo ratings yet
- Test Sample 7ADocument6 pagesTest Sample 7AAce50% (2)
- Bb4 Int Test 5BDocument5 pagesBb4 Int Test 5BDiaconu Andreea EmanuelaNo ratings yet
- Test Sample 5ADocument5 pagesTest Sample 5AAce100% (1)
- Bb4 Int Test 3ADocument5 pagesBb4 Int Test 3AAceNo ratings yet