Professional Documents
Culture Documents
Carnot Cycle PDF
Carnot Cycle PDF
Uploaded by
Sheikh AzizOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carnot Cycle PDF
Carnot Cycle PDF
Uploaded by
Sheikh AzizCopyright:
Available Formats
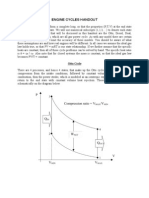
Efficiency of the Carnot cycle
In this derivation we shall show that the efficiency of the Carnot cycle only depends on the
temperatures of the heat source and the heat sink, regardless of the type of gas used in the
process. (You can do this on your own if you follow the steps in the supplemental WS TS2, the
easy way part)
Lets say a heat engine goes through the Carnot cycle in 4 steps as follows:
(i) Isothermal expansion from V1 to V2 at constant T1
(ii) Adiabatic expansion from V2 to V3. The temperature goes from T1 to T3.
(iii) Isothermal compression from V3 to V4 at constant T3
(iv) Adiabatic compression from V4 to V1. The temperature goes from T3 to T1.
Using first law, we see:
(i)
(ii)
(iii)
(iv)
We also know that the total change in internal energy for the whole cycle has to vanish:
Thus:
So the efficiency is:
(with ). Now, we use the following formula to have Q in terms of T:
where S is entropy. Since , (because the temperatures are
nonzero). We also know that the Carnot cycle is a reversible process, so . That means:
Therefore:
Notice we did not have to use the ideal gas law at all. So this formula applies for all gases.
You might also like
- ENSC3007 Lab Write UpDocument9 pagesENSC3007 Lab Write UpammtstNo ratings yet
- Carnot CycleDocument9 pagesCarnot CycleVipin TitariyaNo ratings yet
- Formal Report 5 Heat EngineDocument8 pagesFormal Report 5 Heat EngineRichmond L. CrisostomoNo ratings yet
- Dr. Ranojit Kumar Dutta (DRD)Document27 pagesDr. Ranojit Kumar Dutta (DRD)KRZ. Arpon Root HackerNo ratings yet
- Carnot Cycle NotesDocument7 pagesCarnot Cycle NotesDigvijay JadejaNo ratings yet
- Gas Power CyclesDocument17 pagesGas Power Cycleskimosave99No ratings yet
- Carnot CycleDocument2 pagesCarnot CycleRaj SathwaraNo ratings yet
- Thermodynamic Cycles: Wesleyan University-Philippines Mabini Extension, Cabanatuan CityDocument9 pagesThermodynamic Cycles: Wesleyan University-Philippines Mabini Extension, Cabanatuan CityRoss Sonny CruzNo ratings yet
- Lecture 5Document9 pagesLecture 5Shakil AhmedNo ratings yet
- Applications of TDsDocument15 pagesApplications of TDsReddyvari VenugopalNo ratings yet
- Kelompok 1: Andari Yuta Palwa Intan Meidita Wulandari Lian Elvani Hafifah Marza Nyayu Halimah Tussakdiah Optimisma Situngkir Siti Rahma YantiDocument11 pagesKelompok 1: Andari Yuta Palwa Intan Meidita Wulandari Lian Elvani Hafifah Marza Nyayu Halimah Tussakdiah Optimisma Situngkir Siti Rahma YantiHalimahNo ratings yet
- Carnot CycleDocument26 pagesCarnot CycleNafisa AnikaNo ratings yet
- Lecture 7Document30 pagesLecture 7ansat5.ansatNo ratings yet
- Heat Engine CycleDocument9 pagesHeat Engine CycleLewis Katongo KabwitaNo ratings yet
- Carnot EngineDocument5 pagesCarnot EngineIan Jonathan OmbaoNo ratings yet
- The Stirling Engine: Gunther Cronenberg March 28, 2005Document12 pagesThe Stirling Engine: Gunther Cronenberg March 28, 2005Rachmat Cahaya PutraNo ratings yet
- Lec9 - Air Cycle Refrigeration SystemsDocument38 pagesLec9 - Air Cycle Refrigeration SystemsSantanu DattaNo ratings yet
- ThermodynamicsDocument12 pagesThermodynamicsSoham NagNo ratings yet
- Carnot CycleDocument6 pagesCarnot CyclechillnessNo ratings yet
- Carnot's EngineDocument6 pagesCarnot's Enginem54mohtashimNo ratings yet
- PHY 103 Lecture NoteDocument28 pagesPHY 103 Lecture Notetaiwodamola789No ratings yet
- Advanced Thermodynamics and Combustion (Meng6102) by Dinku Seyoum (PHD) Mr. MereraDocument55 pagesAdvanced Thermodynamics and Combustion (Meng6102) by Dinku Seyoum (PHD) Mr. Mererarobelassefa708No ratings yet
- City and Guilds 9210 Level 6 Module - Unit 128 Applied ThermodynamicsDocument13 pagesCity and Guilds 9210 Level 6 Module - Unit 128 Applied ThermodynamicskhumisoNo ratings yet
- Carnot Cycle: On The Motive Power of Fire in 1824. The Book Proposed A Generalized Theory ofDocument2 pagesCarnot Cycle: On The Motive Power of Fire in 1824. The Book Proposed A Generalized Theory ofLucho BenottoNo ratings yet
- Thermodynamics Question Solve 2010Document10 pagesThermodynamics Question Solve 2010MD SR ShantoNo ratings yet
- SME1207Document201 pagesSME1207OM TAPARENo ratings yet
- BTD Question Bank1Document16 pagesBTD Question Bank1Mahantesh ChulakiNo ratings yet
- Question PART 4 (2023)Document7 pagesQuestion PART 4 (2023)Phong ĐặngNo ratings yet
- Lecture 2 Thermodynamic LawsDocument27 pagesLecture 2 Thermodynamic LawsRalph SotoNo ratings yet
- Carnot CycleDocument2 pagesCarnot CyclerafiaghaNo ratings yet
- Thermodynamics - part 4Document14 pagesThermodynamics - part 4Abhishek ChowdhuryNo ratings yet
- Gas Power Cycles Sivakumar.E VITDocument47 pagesGas Power Cycles Sivakumar.E VITmohan govindasamyNo ratings yet
- Lec 6 Entropy and The Second Law of ThermodynamicsDocument77 pagesLec 6 Entropy and The Second Law of ThermodynamicsACCOUNT ONENo ratings yet
- Thermal Physics Material - KRDocument54 pagesThermal Physics Material - KRAqsa RaffaqNo ratings yet
- Assignment 1 Thermodynamics2022-2023Document1 pageAssignment 1 Thermodynamics2022-2023Ashish OraonNo ratings yet
- 017 Heat EnginesDocument88 pages017 Heat EnginesDean SchoemanNo ratings yet
- Carnot Heat EngineDocument10 pagesCarnot Heat EngineSheraz Ali100% (1)
- 8.3 The Carnot Cycle As A Two-Phase Power Cycle: (Cycle in - Coordinates) (Cycle inDocument26 pages8.3 The Carnot Cycle As A Two-Phase Power Cycle: (Cycle in - Coordinates) (Cycle inAkatew Haile MebrahtuNo ratings yet
- Tugas Termodinamika Teknik Kimia I "Power Cycle": Dosen Pembimbing: Dr. Ir. Renita Manurung, MTDocument17 pagesTugas Termodinamika Teknik Kimia I "Power Cycle": Dosen Pembimbing: Dr. Ir. Renita Manurung, MT17-154 / Wahdi Hidayat SiagianNo ratings yet
- Thermodynamics Question BankDocument4 pagesThermodynamics Question BankOyedotun TundeNo ratings yet
- Thermodynamics.: Made by Engr. Ayesha AliDocument14 pagesThermodynamics.: Made by Engr. Ayesha Alihamza12No ratings yet
- 2nd Law of ThermodynamicsDocument29 pages2nd Law of ThermodynamicsMayday BomhNo ratings yet
- Carnot Engine: by (Khlood Salim Al-Kafajy)Document11 pagesCarnot Engine: by (Khlood Salim Al-Kafajy)Ali Mohammed Alkafajy100% (1)
- TermodinamikaDocument35 pagesTermodinamikaaji wiranegaraNo ratings yet
- gasTurbinesFullVersion PDFDocument27 pagesgasTurbinesFullVersion PDFinvincbleNo ratings yet
- ThermodynamicsDocument22 pagesThermodynamicssachiandmarshneelNo ratings yet
- Carnot Cycle 3Document12 pagesCarnot Cycle 3Tamo JitNo ratings yet
- AE321 Tut1Document4 pagesAE321 Tut1Prabhash singhNo ratings yet
- Second Law of Thermodynamics BME IIDocument37 pagesSecond Law of Thermodynamics BME IINIRUPAN KARKINo ratings yet
- Thermo CyclesDocument3 pagesThermo CyclesRhandi MuliaNo ratings yet
- Cyclic Process Second Law EnginesDocument18 pagesCyclic Process Second Law EnginesM Khaidiz RafiNo ratings yet
- Class 11 - Physics - ThermodynamicsDocument7 pagesClass 11 - Physics - ThermodynamicsSha HNo ratings yet
- Diesel CycleDocument52 pagesDiesel CycleTyler O'connor100% (4)
- Handling and Storage of Flammable MaterialsDocument13 pagesHandling and Storage of Flammable MaterialsSheikh AzizNo ratings yet
- Handling and Storage of Flammable Materials PDFDocument20 pagesHandling and Storage of Flammable Materials PDFSheikh AzizNo ratings yet
- Asrar E Khudi by Allama Muhammad Iqbal PDFDocument87 pagesAsrar E Khudi by Allama Muhammad Iqbal PDFSheikh AzizNo ratings yet
- Bal-e-Jibril-071 Ki Haq Se Farishton Ne Iqbal Ki GhamaziDocument1 pageBal-e-Jibril-071 Ki Haq Se Farishton Ne Iqbal Ki GhamaziSheikh AzizNo ratings yet
- Rahman BabaDocument155 pagesRahman BabaSheikh AzizNo ratings yet
- Asrar E Khudi by Allama Muhammad Iqbal PDFDocument87 pagesAsrar E Khudi by Allama Muhammad Iqbal PDFSheikh AzizNo ratings yet
- Quality Control Solutions Manual Dale H. Besterfield 9eDocument20 pagesQuality Control Solutions Manual Dale H. Besterfield 9eAubrey Alvarez55% (11)
- Book 6th EditionDocument991 pagesBook 6th EditionSheikh AzizNo ratings yet
- Gazette 5th Pec 2017 KasurDocument192 pagesGazette 5th Pec 2017 KasurSheikh AzizNo ratings yet
- Pro - ENGINEER Wildfire 5.0 - Tutorial and Multimedia CD (Book, 2009) (WorldCatDocument3 pagesPro - ENGINEER Wildfire 5.0 - Tutorial and Multimedia CD (Book, 2009) (WorldCatSheikh AzizNo ratings yet
- 7 - Diesel - Cycle PDFDocument5 pages7 - Diesel - Cycle PDFLalith KrishnanNo ratings yet
- Carnot CycleyttyrtDocument1 pageCarnot CycleyttyrtSheikh AzizNo ratings yet