Professional Documents
Culture Documents
SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-15) Date: Topic:Ketones
SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-15) Date: Topic:Ketones
Uploaded by
Sachin DedhiaCopyright:
Available Formats
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Christa Sommerer, Laurent Mignonneau (Eds.) - The Art and Science of Interface and Interaction DesignDocument199 pagesChrista Sommerer, Laurent Mignonneau (Eds.) - The Art and Science of Interface and Interaction DesignJuan Jose TirigallNo ratings yet
- Stargate CIA-RDP96-00787R000100200002-9Document19 pagesStargate CIA-RDP96-00787R000100200002-9taabenaNo ratings yet
- Aldehydes & KetonesDocument9 pagesAldehydes & Ketoneskrishna janamNo ratings yet
- Alcohol Phenol Ether and Carbonyl Compounds. Assignment Q. (Adv) .Document8 pagesAlcohol Phenol Ether and Carbonyl Compounds. Assignment Q. (Adv) .Anurag RamachandranNo ratings yet
- Test - A: BR (1) CH BR (2) (4) BRH C - H CDocument5 pagesTest - A: BR (1) CH BR (2) (4) BRH C - H CVansh ChauhanNo ratings yet
- IIT JAM Chemistry Test PaperDocument15 pagesIIT JAM Chemistry Test PaperAnil Kumar100% (1)
- Structure Identification & POCDocument8 pagesStructure Identification & POCHarshil rawal100% (1)
- Unit-12-Aldehydes, Ketones-MCQDocument5 pagesUnit-12-Aldehydes, Ketones-MCQArsenal Exploiter RepotsNo ratings yet
- SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-12) Date: Topic: Halogen DerivativesDocument7 pagesSECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-12) Date: Topic: Halogen DerivativesSachin DedhiaNo ratings yet
- Me Me CL BR CH-CH CH Oh: O PCLDocument3 pagesMe Me CL BR CH-CH CH Oh: O PCLAkhil JamwalNo ratings yet
- ALD and AMineDocument3 pagesALD and AMineAnubrata SarkarNo ratings yet
- Carbonyl Compund Subjective QuestionsDocument11 pagesCarbonyl Compund Subjective QuestionsVinod AgrawalNo ratings yet
- Guided Plan-6 (E)Document7 pagesGuided Plan-6 (E)abhiraw30062005No ratings yet
- GujCET - D26 Mar 2023Document34 pagesGujCET - D26 Mar 2023aadityabhagchandaniNo ratings yet
- 12th Chemistry Compulsory Problems English (Document34 pages12th Chemistry Compulsory Problems English (AshwinImanuel50% (4)
- MT - 6 PAPER - I (QUESTION PAPER) NewDocument7 pagesMT - 6 PAPER - I (QUESTION PAPER) Newmaster aexpeckNo ratings yet
- Mock Test 6 P 2 Bks DDocument22 pagesMock Test 6 P 2 Bks DRare RootNo ratings yet
- XII Chemistry - Frequently Asked Question Bank PDFDocument175 pagesXII Chemistry - Frequently Asked Question Bank PDFYASH PATELNo ratings yet
- Carbonyl Compounds 13thDocument21 pagesCarbonyl Compounds 13thRaju SinghNo ratings yet
- Aep - CPP - 1Document9 pagesAep - CPP - 1ayesha sheikhNo ratings yet
- C-4.2 (Hydrocarbons & Aromatic Hydrocarbons) ADV - 1157477 - 2023 - 02 - 16 - 14 - 42 PDFDocument21 pagesC-4.2 (Hydrocarbons & Aromatic Hydrocarbons) ADV - 1157477 - 2023 - 02 - 16 - 14 - 42 PDFM2K AAYANo ratings yet
- Quiz-Alcohols, Phenols and Ethers-CLVK-finalDocument10 pagesQuiz-Alcohols, Phenols and Ethers-CLVK-finalayesha sheikhNo ratings yet
- Chemistry Paper - 1 (Question Paper) - 6Document6 pagesChemistry Paper - 1 (Question Paper) - 6Saumya MundraNo ratings yet
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- Ques Aldehydes and Ketones PDFDocument47 pagesQues Aldehydes and Ketones PDFChaitanyaPeshin100% (1)
- Alcohols, Phenols & Ethers QPDocument3 pagesAlcohols, Phenols & Ethers QPIniya RajasekharNo ratings yet
- Mock Test 5 Paper 1 Q. PaperDocument16 pagesMock Test 5 Paper 1 Q. PaperRare RootNo ratings yet
- Questions Chapter 1-10 PDFDocument107 pagesQuestions Chapter 1-10 PDFrashidNo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 3Document3 pagesAlkanes - Alkenes - Alkynes - DPP 3Vishal_93100% (1)
- C - Ch-26 - Aldehydes Ketones and Carboxylic AcidsDocument10 pagesC - Ch-26 - Aldehydes Ketones and Carboxylic AcidsRishi KeshNo ratings yet
- 07 Addition and Condensation of Enols and Enolate Ions (1) .PDF - 1Document15 pages07 Addition and Condensation of Enols and Enolate Ions (1) .PDF - 1JeetNo ratings yet
- Class XII Aldehydes, Ketones and Carboxylic AcidsDocument5 pagesClass XII Aldehydes, Ketones and Carboxylic AcidsvartikasinghNo ratings yet
- AEP QuestionsDocument8 pagesAEP QuestionsArihant BansalNo ratings yet
- Jms-3 Paper - 1 SolDocument15 pagesJms-3 Paper - 1 SoljanmanchiNo ratings yet
- Nsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020Document13 pagesNsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020KritikaNo ratings yet
- Single Correct: Class: Adv - CC Time: 45 Min Class Test-3: OzonolysisDocument4 pagesSingle Correct: Class: Adv - CC Time: 45 Min Class Test-3: Ozonolysisbruh pogNo ratings yet
- Iit 2011 FST1 QNS P1Document25 pagesIit 2011 FST1 QNS P1grdgerNo ratings yet
- Chemistry - Mains2 (Entire 11th)Document7 pagesChemistry - Mains2 (Entire 11th)Ravi Kiran KoduriNo ratings yet
- Aldehydes and Ketones - 3Document6 pagesAldehydes and Ketones - 3iitlectureNo ratings yet
- Paper 2 1923 SATDocument1 pagePaper 2 1923 SATAhana PoddarNo ratings yet
- Quiz Organic 1Document6 pagesQuiz Organic 1ronakgupta332005No ratings yet
- Mock Test 8 Paper 2 Question PDFDocument26 pagesMock Test 8 Paper 2 Question PDFSidNo ratings yet
- Fiitjee Class X Practice Worksheet Organic Chemistry-4Document11 pagesFiitjee Class X Practice Worksheet Organic Chemistry-4T3X1CNo ratings yet
- AIEEE Sample Paper-2Document21 pagesAIEEE Sample Paper-2aditya_kumar_meNo ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- JEE Advanced Aldehyde and Ketones Important QuestionsDocument23 pagesJEE Advanced Aldehyde and Ketones Important QuestionsthisissubhaNo ratings yet
- 10 - Phenol (Level) Module-4Document14 pages10 - Phenol (Level) Module-4Raju SinghNo ratings yet
- Chemistry: Section - IDocument8 pagesChemistry: Section - ISailendra Narayan SahuNo ratings yet
- Chemistry HOLIDAYS Assignment Questions (Class 12th)Document9 pagesChemistry HOLIDAYS Assignment Questions (Class 12th)Aayush SahuNo ratings yet
- Most Important PaperDocument11 pagesMost Important PaperVILLAIN EX.No ratings yet
- Aldehyde, Ketone and Carboxylic acidPYQsJEEMainsDocument45 pagesAldehyde, Ketone and Carboxylic acidPYQsJEEMainsmjonfire3023No ratings yet
- Class 10 Science CBSEDocument8 pagesClass 10 Science CBSEschoolhelpmentorNo ratings yet
- Chemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Document7 pagesChemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Surya Charan Reddy100% (1)
- Jee 2014 Booklet7 HWT Oxygen Containing Organic Compounds IIDocument10 pagesJee 2014 Booklet7 HWT Oxygen Containing Organic Compounds IIvarunkohliinNo ratings yet
- JMS-5 Paper - 2Document7 pagesJMS-5 Paper - 2janmanchiNo ratings yet
- Class XII MOCK TEST TERMI 2021 CHEMISTRYDocument10 pagesClass XII MOCK TEST TERMI 2021 CHEMISTRYSumit KumarNo ratings yet
- Aromatic HydrocarbonDocument7 pagesAromatic HydrocarbonUtkarsh YadavNo ratings yet
- ORGANIC CHEMISTRY ExamDocument13 pagesORGANIC CHEMISTRY ExamIkramNo ratings yet
- Graphene Oxide: Fundamentals and ApplicationsFrom EverandGraphene Oxide: Fundamentals and ApplicationsAyrat M. DimievNo ratings yet
- Chemistry Paper Pattern Vasai 26.05.19: Section A Q.1 1M Q.2 1M Q.3 1M Section BDocument1 pageChemistry Paper Pattern Vasai 26.05.19: Section A Q.1 1M Q.2 1M Q.3 1M Section BSachin DedhiaNo ratings yet
- Board Question Paper: March 2014 Physics - Ii: Section - Ii Q. 5. Attempt Any SIXDocument3 pagesBoard Question Paper: March 2014 Physics - Ii: Section - Ii Q. 5. Attempt Any SIXSachin DedhiaNo ratings yet
- HSC Chemistry 2014 Part 1Document2 pagesHSC Chemistry 2014 Part 1Sachin DedhiaNo ratings yet
- Chemistry in Everyday LifeDocument3 pagesChemistry in Everyday LifeSachin DedhiaNo ratings yet
- Aldehyde 1 To 8 + Acid 1 To 2 JEE & NEET Roboassess Question CodeDocument4 pagesAldehyde 1 To 8 + Acid 1 To 2 JEE & NEET Roboassess Question CodeSachin DedhiaNo ratings yet
- Aldehyde 1 To 5 JEE & NEET Roboassess Question CodeDocument4 pagesAldehyde 1 To 5 JEE & NEET Roboassess Question CodeSachin DedhiaNo ratings yet
- HSC Maths 2014 Part 1Document2 pagesHSC Maths 2014 Part 1Sachin DedhiaNo ratings yet
- STD 12 Maths 2 Board Question Paper Maharashtra Board PDFDocument6 pagesSTD 12 Maths 2 Board Question Paper Maharashtra Board PDFSachin DedhiaNo ratings yet
- HSC Maths 2014 Part 2Document2 pagesHSC Maths 2014 Part 2Sachin DedhiaNo ratings yet
- HSC Maths 2014 Part 2Document2 pagesHSC Maths 2014 Part 2Sachin DedhiaNo ratings yet
- HSC Zoology Board Paper 2013Document2 pagesHSC Zoology Board Paper 2013Sachin DedhiaNo ratings yet
- Board Question Paper: March 2014 Biology - IiDocument2 pagesBoard Question Paper: March 2014 Biology - IiSachin DedhiaNo ratings yet
- HSC Biology Feb 2014 Part 1Document2 pagesHSC Biology Feb 2014 Part 1Sachin DedhiaNo ratings yet
- NEET - Haloalkanes & Haloarenes - (Q+S)Document18 pagesNEET - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNo ratings yet
- HSC Botany Board Paper 2013Document2 pagesHSC Botany Board Paper 2013Sachin DedhiaNo ratings yet
- No. Topics Total VS BO 13 General Organic Chemistry 21 CSD CSD 14 Isomerism 9 CSD CSDDocument1 pageNo. Topics Total VS BO 13 General Organic Chemistry 21 CSD CSD 14 Isomerism 9 CSD CSDSachin DedhiaNo ratings yet
- HSC Maths II Board Paper 2013Document2 pagesHSC Maths II Board Paper 2013Sachin DedhiaNo ratings yet
- HSC Maths I Board Paper 2013Document2 pagesHSC Maths I Board Paper 2013Sachin DedhiaNo ratings yet
- Prelim - I Chem - Section II - QDocument3 pagesPrelim - I Chem - Section II - QSachin DedhiaNo ratings yet
- JEE - Haloalkanes & Haloarenes - (Q+S)Document13 pagesJEE - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNo ratings yet
- Stokes Drag FormulaDocument4 pagesStokes Drag FormulaAjith KrishnanNo ratings yet
- Simplified Modified Compression Field Theory For Calculating Shear Strength of Reinforced Concrete ElementsDocument11 pagesSimplified Modified Compression Field Theory For Calculating Shear Strength of Reinforced Concrete ElementsGiuseppe TizzaniNo ratings yet
- Nature Chemistry Volume 02 No 06 Pp425-510Document84 pagesNature Chemistry Volume 02 No 06 Pp425-510Natalia DankovaNo ratings yet
- Hisopos ATP LiquidosDocument2 pagesHisopos ATP LiquidosNeidy FloresNo ratings yet
- Recip. Comp ECDPDocument51 pagesRecip. Comp ECDPSkydriver Paul100% (1)
- ABAQUS Tutorial - Elastic Perfectly Plastic Buckling Analysis of A Cone-Cylinder Transition Under Axial CompressionDocument7 pagesABAQUS Tutorial - Elastic Perfectly Plastic Buckling Analysis of A Cone-Cylinder Transition Under Axial CompressionDang Quang MinhNo ratings yet
- Dew Point Apparatus 2000 DDDDocument11 pagesDew Point Apparatus 2000 DDDParmeshwar Nath TripathiNo ratings yet
- Electricity Test AnswersDocument3 pagesElectricity Test AnswersAdelaide MonyethabengNo ratings yet
- Amoeba Seminar Presentation 2Document28 pagesAmoeba Seminar Presentation 2Abhishek Isaac Mathew100% (2)
- There'll Be Equal and Opposite ReactionDocument8 pagesThere'll Be Equal and Opposite Reactionamarkiran vinayakNo ratings yet
- Mark Scheme Maximum Mark: 25 Syllabus/Component: 8700/3 Biology (Practical)Document2 pagesMark Scheme Maximum Mark: 25 Syllabus/Component: 8700/3 Biology (Practical)Remon AdelNo ratings yet
- Unit1 Mod 1 3 AnsDocument29 pagesUnit1 Mod 1 3 AnsAhmed JomaaNo ratings yet
- Nust ChemistryDocument137 pagesNust Chemistryahmed ilyasNo ratings yet
- Saturn's Moon Titan: "A Unique World in The Solar System" For Life?Document2 pagesSaturn's Moon Titan: "A Unique World in The Solar System" For Life?Sıla Nas ÇilekNo ratings yet
- 8.ionic EquilibriumDocument64 pages8.ionic EquilibriumhosifaNo ratings yet
- CN4122 Module SynopsisDocument2 pagesCN4122 Module SynopsisGary LiangNo ratings yet
- Leds-C4 Retail 2015Document252 pagesLeds-C4 Retail 2015VEMATELNo ratings yet
- A New Concept For Tilted-Component Telescopes: by Erwin HerrigDocument4 pagesA New Concept For Tilted-Component Telescopes: by Erwin HerrigbirbiburbiNo ratings yet
- FRP Rods For Brittle Fracture ResistantDocument9 pagesFRP Rods For Brittle Fracture Resistantdmsoares1989No ratings yet
- BJP9 v.170Document71 pagesBJP9 v.170LourdesNo ratings yet
- Forensic Science QP New Syllabus (2010-2012)Document25 pagesForensic Science QP New Syllabus (2010-2012)Ae BanpongNo ratings yet
- EnzymeAssayUnits DeerlandDocument4 pagesEnzymeAssayUnits DeerlandPc type100% (1)
- Hair Dye NiggasDocument8 pagesHair Dye NiggasEduardo Javier Granados SanchezNo ratings yet
- Gas AnalyzerDocument5 pagesGas Analyzerengine5No ratings yet
- P103 Temperature, Heat, ExpansionDocument33 pagesP103 Temperature, Heat, ExpansionAnonymous 88kXqKNo ratings yet
- MSDS Loctite262Document5 pagesMSDS Loctite262Navin ChandarNo ratings yet
- 25 Civ Su 985 FDocument10 pages25 Civ Su 985 FBhattNo ratings yet
- 6 135Document6 pages6 135Ashok LenkaNo ratings yet
SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-15) Date: Topic:Ketones
SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-15) Date: Topic:Ketones
Uploaded by
Sachin DedhiaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-15) Date: Topic:Ketones
SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-15) Date: Topic:Ketones
Uploaded by
Sachin DedhiaCopyright:
Available Formats
ANDHERI / BORIVALI / DADAR / CHEMBUR / THANE / MULUND/ NERUL / POWAI
IIT JEE: 2015 CRASH COURSE (C-15) DATE:
TOPIC:KETONES
SECTIONI (Multiple Choice Questions)
This section contains 05 multiple choice questions. Each question has 4 choices (A),
(B), (C) and (D) for its answer, out which ONLY ONE is correct.
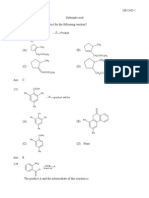
1. Which of the following carbonyl compounds reacts most rapidly with nucleophilic reagents?
(a) Benzaldehyde (b) 3,3 dimethylbutanal
(c) Acetophenone (d) 2,2 dimethylcyclohexanone
2. Which of the following amines would be best chosen for preparing an enamine derivative from
cyclohexanone?
(a) Dimethylmine (b) Ethylamine (c) Trimethylamine (d) Hydroxylamine
3. Which reaction or sequence of reactions would be best used to convert cyclohexanone to cis -1,2
cyclohexanediol?
(a) PCC in CH 2 Cl2 and base
(b) (i) NaBH 4 (ii) H 3 PO 4 &heat(iii) OsO 4 in pyridine
(c) (i) NaBH 4 ; (ii) H 3 PO 4 & heat;(iii) C6 H 5CO3 H
(d) (i) NaBH 4 ; (ii) OsO 4 in pyridine
4. Isolable hydrates are formed by
(a) CF3COCH 3 (b) PhCOPh (c) ( Me3C )2 CO (d) CH 3COCH 3
5. The product of the following reaction is
OH
Pb(OAc)4
OH
O O
O
O O
(a) O (b) (c) O (d) O
CENTERS: MUMBAI / DELHI / AKOLA / KOLKATA / LUCKNOW / NASHIK / GOA / PUNE # 1
SECTION-II (Multiple Choice Questions)
This section contains 3 multiple choice questions. Each question has 4 choices (A),
(B), (C) and (D) for its answer, out which ONE OR MORE is/are correct.
6. Following method(s) can not be used for the preparation of a ketone
(a) Passing of alcohol vapour over red hot copper
(b) Hydration of alkynes in presence of H 2SO 4 and HgSO 4
(c) Cleavage of 1, 3- diols with HIO 4
(d) Rosenmunds reduction
7. Dimethyl ketone can be prepared by
(a) Reaction of acetyl chloride with methyl magnesium chloride
(b) Reaction of acetyl chloride with dimethyl lithiumcuprate
(c) Reaction of acetyl chloride with dimethyl cadmium
(d) Reaction of methyl cyanide with methyl magnesium chloride followed by hydrolysis
8. The product(s) of the reaction of Ph ( CH 3 ) C ( OH ) C ( OH )( CH 3 ) Ph with BF3 is/are
(a) PhC ( CH 3 )2 COPh (b) Ph 2 C ( CH 3 ) COCH 3
(c) Ph 2 CCOC ( CH 3 )2 (d) CH 3OC6 H 4 COC6 H 4 OCH 3
SECTION III (Integer Answer Type)
This section contains 5 questions. The answer to each of the questions is a single
digit integer, ranging from 0 to 9. The correct digit below the question number in
the ORS is be bubbled.
9.
How many total chiral + geometrical centres will be there in the product?
10. A ( C4 H10 )
Br2
B
KOH
C
O3 H 2 O
D Product D does not give Tollens test but gives iodoform
test. How many carbon atoms are there in product D?
11. In the reaction
O
HO CH2 C CH3
How many distinct products (saturated) are possible?
12.
CENTERS: MUMBAI / DELHI / AKOLA / KOLKATA / LUCKNOW / NASHIK / GOA / PUNE # 2
O
+
Ph CH3
The no. of stereoisomers shown by the product of the following reaction would be
13.
OH OH
CH3
Ph
How many final products are possible in the above sequence of reaction (including stereo)?
SECTION IV (Paragraph Type)
This section contains 2 multiple choice questions relating to 1 paragraph. Each
question has four choices (A), (B), (C) and (D) out of which ONLY ONE is correct.
Passage for (Que. No. 14 to 15)
Oxidation of saturated C-H groups
Methylene group adjacent to carbonyl groups can by oxidized with SeO 2 to give dicarbonyl
compounds. In case of unsymmetrical ketones, oxidation gemerally occurs at that CH 2 group which
is most readily enolized. Two mechanisms have suggested for this oxidation. In first, the selenate
ester of the enol is involved. In the second proposal the principal intermediate is a ketoselenic
acid. SeO 2 can also be used to oxidize allylic or benzylic C H bonds. However in this case the
product is alcohols. If the oxidation is carried out in acetic acid as the solvent and then acetate esters
are formed
14. Predict the product of the following reaction:
O
SeO2
O O
O OH
O O
(a) O (b) (c) O (d)
15. Oxidation of toluene by SeO 2 gives
(a) Benzyl alcohol (b) Benzaldehyde (c) Acetophenone (d) Benzophenone
CENTERS: MUMBAI / DELHI / AKOLA / KOLKATA / LUCKNOW / NASHIK / GOA / PUNE # 3
SECTION - V (Matrix Match Type)
This section 1 Question. Each question has four statements Given in Column - I and
four statements in Column II. Any given statement in Column I can have correct
matching with one or more statement (s) given in column II.
16.
Column I Column II
(a) Benzilic Acid Rearrangement p. PhCOPh + NaOH
(b) CH 3CH ( CH 3 ) COCl CH 3CH ( CH 3 ) CHO q. Pd C (S or Quinoline)
(c) Rosenmund Reduction r. Lithium tri-t-butoxyaluminium
hydride
(d) Claisen Schmidt s. PhCHCH 3 + CH 3 COCH 3
t. PhCOCH 3 + ethanolic CN
CENTERS: MUMBAI / DELHI / AKOLA / KOLKATA / LUCKNOW / NASHIK / GOA / PUNE # 4
ANDHERI / BORIVALI / DADAR / CHEMBUR / THANE / MULUND/ NERUL / POWAI
TOPIC: KETONES
(ANSWER KEY) (C-15)
1 [b] 2 [a] 3 [b] 4 [a] 5 [d] 6 [cd] 7 [abcd]
8 [c] 9 [4] 10 [3] 11 [6] 12 [3] 13 [8]
14 [b] 15 [a] 16 [ a p; b q, r,;c q;d r ]
CENTERS: MUMBAI / DELHI / AKOLA / KOLKATA / LUCKNOW / NASHIK / GOA / PUNE # 5
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Christa Sommerer, Laurent Mignonneau (Eds.) - The Art and Science of Interface and Interaction DesignDocument199 pagesChrista Sommerer, Laurent Mignonneau (Eds.) - The Art and Science of Interface and Interaction DesignJuan Jose TirigallNo ratings yet
- Stargate CIA-RDP96-00787R000100200002-9Document19 pagesStargate CIA-RDP96-00787R000100200002-9taabenaNo ratings yet
- Aldehydes & KetonesDocument9 pagesAldehydes & Ketoneskrishna janamNo ratings yet
- Alcohol Phenol Ether and Carbonyl Compounds. Assignment Q. (Adv) .Document8 pagesAlcohol Phenol Ether and Carbonyl Compounds. Assignment Q. (Adv) .Anurag RamachandranNo ratings yet
- Test - A: BR (1) CH BR (2) (4) BRH C - H CDocument5 pagesTest - A: BR (1) CH BR (2) (4) BRH C - H CVansh ChauhanNo ratings yet
- IIT JAM Chemistry Test PaperDocument15 pagesIIT JAM Chemistry Test PaperAnil Kumar100% (1)
- Structure Identification & POCDocument8 pagesStructure Identification & POCHarshil rawal100% (1)
- Unit-12-Aldehydes, Ketones-MCQDocument5 pagesUnit-12-Aldehydes, Ketones-MCQArsenal Exploiter RepotsNo ratings yet
- SECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-12) Date: Topic: Halogen DerivativesDocument7 pagesSECTION-I (Multiple Choice Questions) : IIT - JEE: 2015 Crash Course (C-12) Date: Topic: Halogen DerivativesSachin DedhiaNo ratings yet
- Me Me CL BR CH-CH CH Oh: O PCLDocument3 pagesMe Me CL BR CH-CH CH Oh: O PCLAkhil JamwalNo ratings yet
- ALD and AMineDocument3 pagesALD and AMineAnubrata SarkarNo ratings yet
- Carbonyl Compund Subjective QuestionsDocument11 pagesCarbonyl Compund Subjective QuestionsVinod AgrawalNo ratings yet
- Guided Plan-6 (E)Document7 pagesGuided Plan-6 (E)abhiraw30062005No ratings yet
- GujCET - D26 Mar 2023Document34 pagesGujCET - D26 Mar 2023aadityabhagchandaniNo ratings yet
- 12th Chemistry Compulsory Problems English (Document34 pages12th Chemistry Compulsory Problems English (AshwinImanuel50% (4)
- MT - 6 PAPER - I (QUESTION PAPER) NewDocument7 pagesMT - 6 PAPER - I (QUESTION PAPER) Newmaster aexpeckNo ratings yet
- Mock Test 6 P 2 Bks DDocument22 pagesMock Test 6 P 2 Bks DRare RootNo ratings yet
- XII Chemistry - Frequently Asked Question Bank PDFDocument175 pagesXII Chemistry - Frequently Asked Question Bank PDFYASH PATELNo ratings yet
- Carbonyl Compounds 13thDocument21 pagesCarbonyl Compounds 13thRaju SinghNo ratings yet
- Aep - CPP - 1Document9 pagesAep - CPP - 1ayesha sheikhNo ratings yet
- C-4.2 (Hydrocarbons & Aromatic Hydrocarbons) ADV - 1157477 - 2023 - 02 - 16 - 14 - 42 PDFDocument21 pagesC-4.2 (Hydrocarbons & Aromatic Hydrocarbons) ADV - 1157477 - 2023 - 02 - 16 - 14 - 42 PDFM2K AAYANo ratings yet
- Quiz-Alcohols, Phenols and Ethers-CLVK-finalDocument10 pagesQuiz-Alcohols, Phenols and Ethers-CLVK-finalayesha sheikhNo ratings yet
- Chemistry Paper - 1 (Question Paper) - 6Document6 pagesChemistry Paper - 1 (Question Paper) - 6Saumya MundraNo ratings yet
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- Ques Aldehydes and Ketones PDFDocument47 pagesQues Aldehydes and Ketones PDFChaitanyaPeshin100% (1)
- Alcohols, Phenols & Ethers QPDocument3 pagesAlcohols, Phenols & Ethers QPIniya RajasekharNo ratings yet
- Mock Test 5 Paper 1 Q. PaperDocument16 pagesMock Test 5 Paper 1 Q. PaperRare RootNo ratings yet
- Questions Chapter 1-10 PDFDocument107 pagesQuestions Chapter 1-10 PDFrashidNo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 3Document3 pagesAlkanes - Alkenes - Alkynes - DPP 3Vishal_93100% (1)
- C - Ch-26 - Aldehydes Ketones and Carboxylic AcidsDocument10 pagesC - Ch-26 - Aldehydes Ketones and Carboxylic AcidsRishi KeshNo ratings yet
- 07 Addition and Condensation of Enols and Enolate Ions (1) .PDF - 1Document15 pages07 Addition and Condensation of Enols and Enolate Ions (1) .PDF - 1JeetNo ratings yet
- Class XII Aldehydes, Ketones and Carboxylic AcidsDocument5 pagesClass XII Aldehydes, Ketones and Carboxylic AcidsvartikasinghNo ratings yet
- AEP QuestionsDocument8 pagesAEP QuestionsArihant BansalNo ratings yet
- Jms-3 Paper - 1 SolDocument15 pagesJms-3 Paper - 1 SoljanmanchiNo ratings yet
- Nsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020Document13 pagesNsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020KritikaNo ratings yet
- Single Correct: Class: Adv - CC Time: 45 Min Class Test-3: OzonolysisDocument4 pagesSingle Correct: Class: Adv - CC Time: 45 Min Class Test-3: Ozonolysisbruh pogNo ratings yet
- Iit 2011 FST1 QNS P1Document25 pagesIit 2011 FST1 QNS P1grdgerNo ratings yet
- Chemistry - Mains2 (Entire 11th)Document7 pagesChemistry - Mains2 (Entire 11th)Ravi Kiran KoduriNo ratings yet
- Aldehydes and Ketones - 3Document6 pagesAldehydes and Ketones - 3iitlectureNo ratings yet
- Paper 2 1923 SATDocument1 pagePaper 2 1923 SATAhana PoddarNo ratings yet
- Quiz Organic 1Document6 pagesQuiz Organic 1ronakgupta332005No ratings yet
- Mock Test 8 Paper 2 Question PDFDocument26 pagesMock Test 8 Paper 2 Question PDFSidNo ratings yet
- Fiitjee Class X Practice Worksheet Organic Chemistry-4Document11 pagesFiitjee Class X Practice Worksheet Organic Chemistry-4T3X1CNo ratings yet
- AIEEE Sample Paper-2Document21 pagesAIEEE Sample Paper-2aditya_kumar_meNo ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- JEE Advanced Aldehyde and Ketones Important QuestionsDocument23 pagesJEE Advanced Aldehyde and Ketones Important QuestionsthisissubhaNo ratings yet
- 10 - Phenol (Level) Module-4Document14 pages10 - Phenol (Level) Module-4Raju SinghNo ratings yet
- Chemistry: Section - IDocument8 pagesChemistry: Section - ISailendra Narayan SahuNo ratings yet
- Chemistry HOLIDAYS Assignment Questions (Class 12th)Document9 pagesChemistry HOLIDAYS Assignment Questions (Class 12th)Aayush SahuNo ratings yet
- Most Important PaperDocument11 pagesMost Important PaperVILLAIN EX.No ratings yet
- Aldehyde, Ketone and Carboxylic acidPYQsJEEMainsDocument45 pagesAldehyde, Ketone and Carboxylic acidPYQsJEEMainsmjonfire3023No ratings yet
- Class 10 Science CBSEDocument8 pagesClass 10 Science CBSEschoolhelpmentorNo ratings yet
- Chemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Document7 pagesChemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Surya Charan Reddy100% (1)
- Jee 2014 Booklet7 HWT Oxygen Containing Organic Compounds IIDocument10 pagesJee 2014 Booklet7 HWT Oxygen Containing Organic Compounds IIvarunkohliinNo ratings yet
- JMS-5 Paper - 2Document7 pagesJMS-5 Paper - 2janmanchiNo ratings yet
- Class XII MOCK TEST TERMI 2021 CHEMISTRYDocument10 pagesClass XII MOCK TEST TERMI 2021 CHEMISTRYSumit KumarNo ratings yet
- Aromatic HydrocarbonDocument7 pagesAromatic HydrocarbonUtkarsh YadavNo ratings yet
- ORGANIC CHEMISTRY ExamDocument13 pagesORGANIC CHEMISTRY ExamIkramNo ratings yet
- Graphene Oxide: Fundamentals and ApplicationsFrom EverandGraphene Oxide: Fundamentals and ApplicationsAyrat M. DimievNo ratings yet
- Chemistry Paper Pattern Vasai 26.05.19: Section A Q.1 1M Q.2 1M Q.3 1M Section BDocument1 pageChemistry Paper Pattern Vasai 26.05.19: Section A Q.1 1M Q.2 1M Q.3 1M Section BSachin DedhiaNo ratings yet
- Board Question Paper: March 2014 Physics - Ii: Section - Ii Q. 5. Attempt Any SIXDocument3 pagesBoard Question Paper: March 2014 Physics - Ii: Section - Ii Q. 5. Attempt Any SIXSachin DedhiaNo ratings yet
- HSC Chemistry 2014 Part 1Document2 pagesHSC Chemistry 2014 Part 1Sachin DedhiaNo ratings yet
- Chemistry in Everyday LifeDocument3 pagesChemistry in Everyday LifeSachin DedhiaNo ratings yet
- Aldehyde 1 To 8 + Acid 1 To 2 JEE & NEET Roboassess Question CodeDocument4 pagesAldehyde 1 To 8 + Acid 1 To 2 JEE & NEET Roboassess Question CodeSachin DedhiaNo ratings yet
- Aldehyde 1 To 5 JEE & NEET Roboassess Question CodeDocument4 pagesAldehyde 1 To 5 JEE & NEET Roboassess Question CodeSachin DedhiaNo ratings yet
- HSC Maths 2014 Part 1Document2 pagesHSC Maths 2014 Part 1Sachin DedhiaNo ratings yet
- STD 12 Maths 2 Board Question Paper Maharashtra Board PDFDocument6 pagesSTD 12 Maths 2 Board Question Paper Maharashtra Board PDFSachin DedhiaNo ratings yet
- HSC Maths 2014 Part 2Document2 pagesHSC Maths 2014 Part 2Sachin DedhiaNo ratings yet
- HSC Maths 2014 Part 2Document2 pagesHSC Maths 2014 Part 2Sachin DedhiaNo ratings yet
- HSC Zoology Board Paper 2013Document2 pagesHSC Zoology Board Paper 2013Sachin DedhiaNo ratings yet
- Board Question Paper: March 2014 Biology - IiDocument2 pagesBoard Question Paper: March 2014 Biology - IiSachin DedhiaNo ratings yet
- HSC Biology Feb 2014 Part 1Document2 pagesHSC Biology Feb 2014 Part 1Sachin DedhiaNo ratings yet
- NEET - Haloalkanes & Haloarenes - (Q+S)Document18 pagesNEET - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNo ratings yet
- HSC Botany Board Paper 2013Document2 pagesHSC Botany Board Paper 2013Sachin DedhiaNo ratings yet
- No. Topics Total VS BO 13 General Organic Chemistry 21 CSD CSD 14 Isomerism 9 CSD CSDDocument1 pageNo. Topics Total VS BO 13 General Organic Chemistry 21 CSD CSD 14 Isomerism 9 CSD CSDSachin DedhiaNo ratings yet
- HSC Maths II Board Paper 2013Document2 pagesHSC Maths II Board Paper 2013Sachin DedhiaNo ratings yet
- HSC Maths I Board Paper 2013Document2 pagesHSC Maths I Board Paper 2013Sachin DedhiaNo ratings yet
- Prelim - I Chem - Section II - QDocument3 pagesPrelim - I Chem - Section II - QSachin DedhiaNo ratings yet
- JEE - Haloalkanes & Haloarenes - (Q+S)Document13 pagesJEE - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNo ratings yet
- Stokes Drag FormulaDocument4 pagesStokes Drag FormulaAjith KrishnanNo ratings yet
- Simplified Modified Compression Field Theory For Calculating Shear Strength of Reinforced Concrete ElementsDocument11 pagesSimplified Modified Compression Field Theory For Calculating Shear Strength of Reinforced Concrete ElementsGiuseppe TizzaniNo ratings yet
- Nature Chemistry Volume 02 No 06 Pp425-510Document84 pagesNature Chemistry Volume 02 No 06 Pp425-510Natalia DankovaNo ratings yet
- Hisopos ATP LiquidosDocument2 pagesHisopos ATP LiquidosNeidy FloresNo ratings yet
- Recip. Comp ECDPDocument51 pagesRecip. Comp ECDPSkydriver Paul100% (1)
- ABAQUS Tutorial - Elastic Perfectly Plastic Buckling Analysis of A Cone-Cylinder Transition Under Axial CompressionDocument7 pagesABAQUS Tutorial - Elastic Perfectly Plastic Buckling Analysis of A Cone-Cylinder Transition Under Axial CompressionDang Quang MinhNo ratings yet
- Dew Point Apparatus 2000 DDDDocument11 pagesDew Point Apparatus 2000 DDDParmeshwar Nath TripathiNo ratings yet
- Electricity Test AnswersDocument3 pagesElectricity Test AnswersAdelaide MonyethabengNo ratings yet
- Amoeba Seminar Presentation 2Document28 pagesAmoeba Seminar Presentation 2Abhishek Isaac Mathew100% (2)
- There'll Be Equal and Opposite ReactionDocument8 pagesThere'll Be Equal and Opposite Reactionamarkiran vinayakNo ratings yet
- Mark Scheme Maximum Mark: 25 Syllabus/Component: 8700/3 Biology (Practical)Document2 pagesMark Scheme Maximum Mark: 25 Syllabus/Component: 8700/3 Biology (Practical)Remon AdelNo ratings yet
- Unit1 Mod 1 3 AnsDocument29 pagesUnit1 Mod 1 3 AnsAhmed JomaaNo ratings yet
- Nust ChemistryDocument137 pagesNust Chemistryahmed ilyasNo ratings yet
- Saturn's Moon Titan: "A Unique World in The Solar System" For Life?Document2 pagesSaturn's Moon Titan: "A Unique World in The Solar System" For Life?Sıla Nas ÇilekNo ratings yet
- 8.ionic EquilibriumDocument64 pages8.ionic EquilibriumhosifaNo ratings yet
- CN4122 Module SynopsisDocument2 pagesCN4122 Module SynopsisGary LiangNo ratings yet
- Leds-C4 Retail 2015Document252 pagesLeds-C4 Retail 2015VEMATELNo ratings yet
- A New Concept For Tilted-Component Telescopes: by Erwin HerrigDocument4 pagesA New Concept For Tilted-Component Telescopes: by Erwin HerrigbirbiburbiNo ratings yet
- FRP Rods For Brittle Fracture ResistantDocument9 pagesFRP Rods For Brittle Fracture Resistantdmsoares1989No ratings yet
- BJP9 v.170Document71 pagesBJP9 v.170LourdesNo ratings yet
- Forensic Science QP New Syllabus (2010-2012)Document25 pagesForensic Science QP New Syllabus (2010-2012)Ae BanpongNo ratings yet
- EnzymeAssayUnits DeerlandDocument4 pagesEnzymeAssayUnits DeerlandPc type100% (1)
- Hair Dye NiggasDocument8 pagesHair Dye NiggasEduardo Javier Granados SanchezNo ratings yet
- Gas AnalyzerDocument5 pagesGas Analyzerengine5No ratings yet
- P103 Temperature, Heat, ExpansionDocument33 pagesP103 Temperature, Heat, ExpansionAnonymous 88kXqKNo ratings yet
- MSDS Loctite262Document5 pagesMSDS Loctite262Navin ChandarNo ratings yet
- 25 Civ Su 985 FDocument10 pages25 Civ Su 985 FBhattNo ratings yet
- 6 135Document6 pages6 135Ashok LenkaNo ratings yet