Professional Documents
Culture Documents
OCH:: C H CH Ohch:+ O C H OCH - CH OH
OCH:: C H CH Ohch:+ O C H OCH - CH OH
Uploaded by
Yash ShindeOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
OCH:: C H CH Ohch:+ O C H OCH - CH OH
OCH:: C H CH Ohch:+ O C H OCH - CH OH
Uploaded by
Yash ShindeCopyright:
Available Formats
597706-19-36E AID: 987 | 23/11/2015
RID : 102 | 22/12/2015
AID: 1112 |05/07/2017
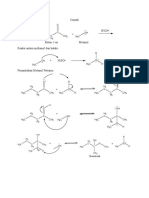
Acetals are formed reacting aldehydes with excess of alcohol, to replace the =O with two
alkoxy groups. A common method for the preparation of acetals is transacetalization. In a
symmetric acetal, both alkoxy groups are same, while in mixed acetals, the groups are
different.
The reaction starts with the attack of alcoholic oxygen on the acetal carbon, followed by
loss of alcohol and attack of another alcoholic group, to yield a cyclic acetal.
(a)
The mechanistic course of the reaction under consideration is shown in the following
diagram:
OHCH 3:+
OCH 3::
H3C O O CH3
H3C CH3
H3C CH3 -CH 3 OH H3C CH3
H O O H O O H
+
HO OH
-CH 3 OH
O O
H3C CH3
Therefore, the final product formed is 2,2-dimethyl-2H-1,3-benzodioxole.
(b)
The mechanistic course of the reaction under consideration is shown in the following
diagram:
OHCH 3:+
H3C O O CH3 OCH 3::
H3C
H3C CH3 H3C CH3
-CH 3OH
CH3
HO OH O OH
+
HO OH
-CH 3OH
O O
H3C CH3
Therefore, the final product formed is 2,2-diethyl-1,3-dioxolane.
You might also like
- AZ-500 Book PDFDocument253 pagesAZ-500 Book PDFAldo SENo ratings yet
- Midterm 2 - Key PDFDocument8 pagesMidterm 2 - Key PDFMichael NguyenNo ratings yet
- Esterification: Experiment 3. Ester Formation: Preparation of BenzocaineDocument3 pagesEsterification: Experiment 3. Ester Formation: Preparation of BenzocaineSurya kumar AhirwarNo ratings yet
- Esterification: Experiment 3. Ester Formation: Preparation of BenzocaineDocument3 pagesEsterification: Experiment 3. Ester Formation: Preparation of BenzocaineJasreen ArieshaNo ratings yet
- (2R) 2 Chloro 2 Methylbutan 1 OlDocument2 pages(2R) 2 Chloro 2 Methylbutan 1 OlYash ShindeNo ratings yet
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDocument13 pagesAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNo ratings yet
- 0331 S 05 BioenergyDocument44 pages0331 S 05 BioenergyDaisyNo ratings yet
- Experiment No. 7 Manufacturing of Butyl Acetate by Esterification ReactionDocument3 pagesExperiment No. 7 Manufacturing of Butyl Acetate by Esterification ReactionsumitNo ratings yet
- 100S120 CS19L01Document38 pages100S120 CS19L01b101112154No ratings yet
- Exp 7 Preparation of AlkenesDocument14 pagesExp 7 Preparation of AlkenesGeorge PiliposyanNo ratings yet
- Aldehydes Ketones: Subjective ProblemsDocument16 pagesAldehydes Ketones: Subjective ProblemsBhaskar AnandNo ratings yet
- OzonolysisDocument2 pagesOzonolysisAnubhav RajNo ratings yet
- Haloalcanos 3Document1 pageHaloalcanos 3MIRIAM SAN FRUTOS GARCÍANo ratings yet
- Worked Solutions To The Problems: Important General RemarkDocument15 pagesWorked Solutions To The Problems: Important General RemarkLê Hoàng MinhNo ratings yet
- 19 Prac Diel Alder AnsDocument2 pages19 Prac Diel Alder AnsNguyễn ThiệnNo ratings yet
- 19 Prac Diel Alder AnsDocument2 pages19 Prac Diel Alder Ansaminah hameedNo ratings yet
- Xercise: Socl Pyridine, O CH CL MG (CH) O O CH MGCLDocument5 pagesXercise: Socl Pyridine, O CH CL MG (CH) O O CH MGCLPriyanshu RajNo ratings yet
- Exp't 81: Synthesis of N-Butyl Acetate Via EsterificationDocument8 pagesExp't 81: Synthesis of N-Butyl Acetate Via EsterificationMuhammad Arif TaufiqNo ratings yet
- ALCOHOL : AlcoholsDocument11 pagesALCOHOL : AlcoholsSamirNo ratings yet
- Answers To AssignmentDocument1 pageAnswers To AssignmentIgbereyivwe TejiriNo ratings yet
- Reductions PPT 29-08-2020Document12 pagesReductions PPT 29-08-2020jkc collegeNo ratings yet
- Contoh: + Butan-2-On Metanol H SODocument2 pagesContoh: + Butan-2-On Metanol H SOElis TianiNo ratings yet
- Contoh: + Butan-2-On Metanol H SODocument2 pagesContoh: + Butan-2-On Metanol H SOElis TianiNo ratings yet
- GlysocideDocument70 pagesGlysocideAlan K MhamadNo ratings yet
- Trabajo de Química OrgánicaDocument1 pageTrabajo de Química OrgánicaPaulina Mota MacipNo ratings yet
- UNSTABLE FUNCTIONAL GROUPS IN ORGANIC by S.K.sinha See Chemistry Animations atDocument1 pageUNSTABLE FUNCTIONAL GROUPS IN ORGANIC by S.K.sinha See Chemistry Animations atmyiitchemistry100% (2)
- Esterfication MechanismDocument1 pageEsterfication MechanismrasikamuhandiramgeNo ratings yet
- Acetal Formation: O OCH H CO CH OH HDocument1 pageAcetal Formation: O OCH H CO CH OH Hkalli987No ratings yet
- Vollhardt Chapter 18 OChem PracticeDocument23 pagesVollhardt Chapter 18 OChem PracticeDanNo ratings yet
- Pyrolytic Elimination - Syn EliminationsDocument3 pagesPyrolytic Elimination - Syn EliminationssaheedvkNo ratings yet
- JEE Main 2019 Chemistry April Attempt Shift - 1 (08th April, 2019)Document16 pagesJEE Main 2019 Chemistry April Attempt Shift - 1 (08th April, 2019)Resonance Eduventures83% (24)
- CY2102Document3 pagesCY2102Prarabdha SharmaNo ratings yet
- Reaction and Preparation of Ethers and EpoxidesDocument2 pagesReaction and Preparation of Ethers and EpoxidesJAN JERICHO MENTOYNo ratings yet
- Steroids - IIDocument38 pagesSteroids - IIDhanaswamy Ilangeswaran100% (1)
- Aldol Reaction - ChemistryDocument7 pagesAldol Reaction - ChemistryGamer HelperNo ratings yet
- OH A, A Is: : CHN 1 EqvDocument4 pagesOH A, A Is: : CHN 1 EqvAtharva GanjuNo ratings yet
- Tugas Klmpok FitokimDocument3 pagesTugas Klmpok FitokimMARVA PERTIWINo ratings yet
- PS2 Carboxylic Acids and DerivativesDocument2 pagesPS2 Carboxylic Acids and Derivativesscarllee rogerNo ratings yet
- ReactiiDocument8 pagesReactiiEddyNo ratings yet
- Solution of Chemistry HSSC-II (3rd Set)Document11 pagesSolution of Chemistry HSSC-II (3rd Set)Ujala ShahidNo ratings yet
- Name Reactions Perkin Reaction DPPDocument1 pageName Reactions Perkin Reaction DPPSDMNo ratings yet
- Common MS Fragment IonsDocument2 pagesCommon MS Fragment IonsMarianaMuNo ratings yet
- Common MS Fragment Ions PDFDocument2 pagesCommon MS Fragment Ions PDFSeliaDestianingrumNo ratings yet
- Problem Set 4 - Key: 1. Suggest A Plausible Arrow-Pushing Mechanism For The Following Reactions. ADocument6 pagesProblem Set 4 - Key: 1. Suggest A Plausible Arrow-Pushing Mechanism For The Following Reactions. ATrần Nguyễn Quỳnh NhưNo ratings yet
- DM pp21-40Document20 pagesDM pp21-40MLUNGISI MkhwanaziNo ratings yet
- Alcohols, Phenols and Ethers - Short Notes - Prayas JEE 2024Document6 pagesAlcohols, Phenols and Ethers - Short Notes - Prayas JEE 2024yash vardhanNo ratings yet
- Cannizzaro Reaction: Reaction of The Day - 06Document4 pagesCannizzaro Reaction: Reaction of The Day - 06Raufa HussainNo ratings yet
- AlcoholsDocument7 pagesAlcoholsSuhaan GargNo ratings yet
- 11 - Alcohol Ethers Thiols Wks KeyDocument5 pages11 - Alcohol Ethers Thiols Wks KeyMaria Aira Mendoza100% (1)
- Alcohols, Phenols & Ether - AnswersDocument6 pagesAlcohols, Phenols & Ether - AnswersK. RupaNo ratings yet
- Problem 26 Chemical Structure and Absolute Stereochemistry of ConiineDocument6 pagesProblem 26 Chemical Structure and Absolute Stereochemistry of ConiineLê Hoàng MinhNo ratings yet
- DM pp61-80Document20 pagesDM pp61-80MLUNGISI MkhwanaziNo ratings yet
- Rapport Ester HT21Document8 pagesRapport Ester HT21Elizabeth queenNo ratings yet
- An Aldol Condensation To Synthesize ChalconesDocument6 pagesAn Aldol Condensation To Synthesize ChalconesAssyakurNo ratings yet
- COMPLETODocument13 pagesCOMPLETOMARITZA QUISPE VILLARREALNo ratings yet
- Chemistry 108M Final Exam Practice 1Document8 pagesChemistry 108M Final Exam Practice 1Norma Leticia RamosNo ratings yet
- AnswersDocument5 pagesAnswersSwastik DasNo ratings yet
- Chemistry Test-Ii: Part-I Section-I Single Correct Choice Type 1. (D)Document19 pagesChemistry Test-Ii: Part-I Section-I Single Correct Choice Type 1. (D)aayushNo ratings yet
- SpectrumDocument1 pageSpectrumYash ShindeNo ratings yet
- 15 39eDocument1 page15 39eYash ShindeNo ratings yet
- Methylidenecyclohexane.: CL CH Ona CH Oh CH CH + +Document2 pagesMethylidenecyclohexane.: CL CH Ona CH Oh CH CH + +Yash ShindeNo ratings yet
- CH CLDocument2 pagesCH CLYash ShindeNo ratings yet
- (2R) 2 Chloro 2 Methylbutan 1 OlDocument2 pages(2R) 2 Chloro 2 Methylbutan 1 OlYash ShindeNo ratings yet
- CH CH CH: Cyclononatetraenyl Radical, Cyclononatetraenyl Cation and Cyclononatetraenyl AnionDocument1 pageCH CH CH: Cyclononatetraenyl Radical, Cyclononatetraenyl Cation and Cyclononatetraenyl AnionYash ShindeNo ratings yet
- NARA - T733 - R3 - 48 (Records of German Field Commands Armies (Part VI) )Document102 pagesNARA - T733 - R3 - 48 (Records of German Field Commands Armies (Part VI) )V ManNo ratings yet
- Is 5 8Document218 pagesIs 5 8andispotifyaccNo ratings yet
- Egurukul OrbitDocument8 pagesEgurukul OrbitbetsyNo ratings yet
- Maria Montessori and Her Educational Philosophy: Breaking Barriers in EducationDocument5 pagesMaria Montessori and Her Educational Philosophy: Breaking Barriers in EducationfreddyNo ratings yet
- 6 Settlement Response of A Multi-Story BuildingDocument4 pages6 Settlement Response of A Multi-Story BuildingmazNo ratings yet
- Aluminium FormworkDocument14 pagesAluminium FormworkebinVettuchirayil100% (6)
- Characteristics of A ProfessionDocument1 pageCharacteristics of A ProfessionHenry BuemioNo ratings yet
- 42 Items Q and ADocument2 pages42 Items Q and AJoy NavalesNo ratings yet
- TDS BLR 896 EN ScreenDocument1 pageTDS BLR 896 EN ScreenLuis Angel MendozaNo ratings yet
- System and Surroundings: - SystemDocument19 pagesSystem and Surroundings: - SystemVighnesh ManojNo ratings yet
- Here Is The MoonDocument2 pagesHere Is The MoonDeepakNo ratings yet
- Water-Treatment (Rhen)Document13 pagesWater-Treatment (Rhen)Mark Anthony ReyesNo ratings yet
- A540E Automatic Transaxle: DescriptionDocument3 pagesA540E Automatic Transaxle: DescriptionDustNo ratings yet
- BS ConsolidationDocument25 pagesBS ConsolidationKamukwema johnNo ratings yet
- Crawljammer - #02Document36 pagesCrawljammer - #02JosephLouisNadeau100% (1)
- Unit Planner 2021 4b enDocument15 pagesUnit Planner 2021 4b enAineeNo ratings yet
- CGR1 Text ModeDocument11 pagesCGR1 Text ModeRaj SuraseNo ratings yet
- Maybee, Julie E. - Hegel, Georg W. - Picturing Hegel - An Illustrated Guide To Hegel's Encyclopaedia Logic-Lexington Books (2009) PDFDocument668 pagesMaybee, Julie E. - Hegel, Georg W. - Picturing Hegel - An Illustrated Guide To Hegel's Encyclopaedia Logic-Lexington Books (2009) PDFArroyo de FuegoNo ratings yet
- Designer Babies, The Key To A Better Developed World Student JournalismDocument4 pagesDesigner Babies, The Key To A Better Developed World Student Journalism宁丹No ratings yet
- GenmotDocument48 pagesGenmotBorislav AtanasovNo ratings yet
- Jurisprudence Research Paper TopicsDocument5 pagesJurisprudence Research Paper TopicsAmandeep MalikNo ratings yet
- Literature Review Ending Violence Against Women and GirlsDocument93 pagesLiterature Review Ending Violence Against Women and GirlsKyteNo ratings yet
- Drainage NOTESDocument9 pagesDrainage NOTESAuraNo ratings yet
- ToyotaDocument5 pagesToyotachristinaNo ratings yet
- Pulse Amplitude ModulationDocument19 pagesPulse Amplitude ModulationShubhangBaghelNo ratings yet
- MCGB - Data Sheet For Suppliers Old MAT Nos.: 142, - , - : Bright Steel, Unalloyed, Cold DrawnDocument3 pagesMCGB - Data Sheet For Suppliers Old MAT Nos.: 142, - , - : Bright Steel, Unalloyed, Cold Drawnbaskaran ayyapparajNo ratings yet
- Notes in Criminal SociologyDocument8 pagesNotes in Criminal SociologyWevinneNo ratings yet
- Series: LTJ: Toe Capacity: 2.5 - 25 Ton - Head Capacity: 5 - 50 Ton - Stroke Length For Toe: 50 MMDocument1 pageSeries: LTJ: Toe Capacity: 2.5 - 25 Ton - Head Capacity: 5 - 50 Ton - Stroke Length For Toe: 50 MM220479No ratings yet
- Famous Filipino AthleteDocument5 pagesFamous Filipino AthleteSandara SarciaNo ratings yet