Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
30 viewsFormation Constants of Complex Ions
Formation Constants of Complex Ions
Uploaded by
IuliaHortThis document lists the formation constants (Kf) of various complex ions at 25°C, organized into categories of halide complexes, ammonia complexes, cyanide complexes, complexes with other monodentate ligands, and complexes with bidentate ligands. The complex ions range from simple halide complexes like [AlF6]3- to more complex ligands binding multiple atoms like [Co(en)3]3+. The values provided are equilibrium constants that represent the strength of binding between metal cations and ligands in complex ion formations.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Page 2 Examples: FULL WORKED SOLUTIONS Are Available To Subscribers ofDocument13 pagesPage 2 Examples: FULL WORKED SOLUTIONS Are Available To Subscribers ofАЙЗАТ ЖАРИМБЕТОВА100% (5)
- Chang 1997Document17 pagesChang 1997Satyam Bhuyan100% (1)
- Solubility Product Constant (K) Values at 25 C: Salt K Salt K Salt K Salt K Bromides Carbonates Oxalates SulfidesDocument3 pagesSolubility Product Constant (K) Values at 25 C: Salt K Salt K Salt K Salt K Bromides Carbonates Oxalates SulfidesHerlina PanggabeanNo ratings yet
- Volumetric Analysis - Class Assignment Part 01 PDFDocument2 pagesVolumetric Analysis - Class Assignment Part 01 PDFyug agarwalNo ratings yet
- Nathaniel Herod - BalancingpracticeDocument10 pagesNathaniel Herod - BalancingpracticeNathaniel HerodNo ratings yet
- Coordination CoDocument19 pagesCoordination CoHandugan Quinlog NoelNo ratings yet
- Inorganic Chemistry by PMS: OF Co-Ordination CompoundsDocument15 pagesInorganic Chemistry by PMS: OF Co-Ordination CompoundsKumar AzadNo ratings yet
- 5.3.2 Transition Metals PDFDocument11 pages5.3.2 Transition Metals PDFkrishnaviNo ratings yet
- Lesson 1 Transition Metals IntroductionDocument48 pagesLesson 1 Transition Metals Introductiontiahayes2801No ratings yet
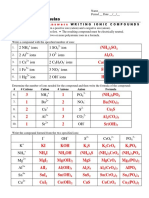
- 7 - Chemical Formulas: AnswersDocument1 page7 - Chemical Formulas: AnswersAlyssa Mae MayonadoNo ratings yet
- The D-Block Elements. General Properties: Mr. Kale Vinod NDocument23 pagesThe D-Block Elements. General Properties: Mr. Kale Vinod NLavinia DonaldNo ratings yet
- First Row Transition Metals - Complexes - Ligand ExchangeDocument4 pagesFirst Row Transition Metals - Complexes - Ligand ExchangeDanielle BelconNo ratings yet
- The Number of Antibonding Electron Pairs in O Ion On The Basis of Molecular Orbital Theory Is: (At. No. of O-Atom Is 8)Document23 pagesThe Number of Antibonding Electron Pairs in O Ion On The Basis of Molecular Orbital Theory Is: (At. No. of O-Atom Is 8)ronald.nazareth5469No ratings yet
- Utilization Os Nickel Slag PDFDocument7 pagesUtilization Os Nickel Slag PDFGaetanoD'AloiaNo ratings yet
- (Career Endeavour) MOLECULAR COMPOUNDSDocument15 pages(Career Endeavour) MOLECULAR COMPOUNDSVaibhav NikharNo ratings yet
- Unit 10. Complexometric Titration: I. Complex Ion FormationDocument5 pagesUnit 10. Complexometric Titration: I. Complex Ion FormationRaymond R. SantosNo ratings yet
- Balancing Equations: Practice ProblemsDocument10 pagesBalancing Equations: Practice ProblemsAyesha TauseefNo ratings yet
- Coordination Compound - Theory (Page 1-30)Document30 pagesCoordination Compound - Theory (Page 1-30)chemistrylectureihl2No ratings yet
- VBT Coordination CompoundsDocument4 pagesVBT Coordination CompoundsShashwat NiranjanNo ratings yet
- VbtcoorDocument4 pagesVbtcoorBishwadeep RoyNo ratings yet
- Semester Test 2 MemoDocument9 pagesSemester Test 2 MemoMac'Ann Ditshego MashaoNo ratings yet
- Pauling's Table of Electrode PotentialsDocument16 pagesPauling's Table of Electrode PotentialsDean GermetenNo ratings yet
- Heating Effect of Carbonate & Bicarbonate SaltsDocument3 pagesHeating Effect of Carbonate & Bicarbonate Saltsvishwajit patilNo ratings yet
- Environmental Mineralogy: Dr. Doni P E PutraDocument47 pagesEnvironmental Mineralogy: Dr. Doni P E Putramuh apriawan noorNo ratings yet
- Chapter 5 Coordination CompoundDocument36 pagesChapter 5 Coordination Compoundammar zakariaNo ratings yet
- Dissociation Constants For Some Complex IonsDocument1 pageDissociation Constants For Some Complex IonsabasakNo ratings yet
- Heating Effects (12th&13th)Document4 pagesHeating Effects (12th&13th)Raju SinghNo ratings yet
- Chem D and F BlockDocument5 pagesChem D and F BlockVIKAS SHARMANo ratings yet
- Cambridge IGCSE: CHEMISTRY 0620/31Document20 pagesCambridge IGCSE: CHEMISTRY 0620/31Tshegofatso SaliNo ratings yet
- CH2 Transition Metals Unit V A2 LevelDocument9 pagesCH2 Transition Metals Unit V A2 LevelbillaljavedNo ratings yet
- Coordination Compounds: DPP 01 (Of Lecture 3) - Yakeen 3.0 2023Document3 pagesCoordination Compounds: DPP 01 (Of Lecture 3) - Yakeen 3.0 2023PalakNo ratings yet
- Wa0005Document26 pagesWa0005Dixon ECNo ratings yet
- No Kation Nama No Anion NamaDocument2 pagesNo Kation Nama No Anion NamaAjeng Candra Arum PuspasariNo ratings yet
- Chemsheets-A2-Transition-Metals WorkbookDocument32 pagesChemsheets-A2-Transition-Metals WorkbookmlbgurpreetttNo ratings yet
- XII JEE Chemistry Practice Sheet 01Document406 pagesXII JEE Chemistry Practice Sheet 01Akshat MakwanaNo ratings yet
- JEE ChemistryDocument406 pagesJEE Chemistryak1740120No ratings yet
- Coordination Compounds (Exercise+Answers)Document32 pagesCoordination Compounds (Exercise+Answers)Hanukkah100% (1)
- COORDINATION COMPOUNDS - Class Notes - JEE MindmapDocument22 pagesCOORDINATION COMPOUNDS - Class Notes - JEE Mindmapadsaditya24No ratings yet
- Z, A, Valency, ConfigurationDocument2 pagesZ, A, Valency, ConfigurationnavyanonaNo ratings yet
- Coordination Compound Level-0 Part IDocument3 pagesCoordination Compound Level-0 Part IAnumuskan KashyapNo ratings yet
- Solubility ProductsDocument2 pagesSolubility Productsgracemizzi6No ratings yet
- Chemistry-Bridging The Gap AnswerDocument11 pagesChemistry-Bridging The Gap AnswerMuhammad IzzuanNo ratings yet
- RT3D - BTEX Degradation With Multiple Electron Acceptors: Gms 7.0 TutorialsDocument12 pagesRT3D - BTEX Degradation With Multiple Electron Acceptors: Gms 7.0 TutorialsAbdelhay ElomariNo ratings yet
- Standard Electrode PotentialDocument11 pagesStandard Electrode PotentialRSLNo ratings yet
- Co-Ordination and Organometallic CompDocument85 pagesCo-Ordination and Organometallic CompDr. Dhondiba Vishwanath100% (1)
- Redox Reactions Mini QuestionsDocument5 pagesRedox Reactions Mini QuestionsKL KNo ratings yet
- Coordination ChemistryDocument30 pagesCoordination ChemistryRizwanbhatNo ratings yet
- Chemical Bonding: Why Bond Anyway?Document45 pagesChemical Bonding: Why Bond Anyway?PutRi Charolin GintingNo ratings yet
- 3-Carbon Oxidation 1Document21 pages3-Carbon Oxidation 1Aditya GajbhiyeNo ratings yet
- (L1) - Coordinate Compounds - 28th NovDocument32 pages(L1) - Coordinate Compounds - 28th NovKhushi RathoreNo ratings yet
- B Masia c315 Exp 3Document6 pagesB Masia c315 Exp 3MphoNo ratings yet
- Coordination Chemistry - Diwali Assignment II (Pathshala 12th JEE 2023)Document6 pagesCoordination Chemistry - Diwali Assignment II (Pathshala 12th JEE 2023)Gayatri GuptaNo ratings yet
- Chelate Effect: Coordination ChemistryDocument13 pagesChelate Effect: Coordination ChemistryNikitha AkulaNo ratings yet
- Ferrioxalate SystemDocument6 pagesFerrioxalate SystemRohit ChauhanNo ratings yet
- Chemistry Periodic TableDocument2 pagesChemistry Periodic TableAbhinav KumarNo ratings yet
- Solution 2Document12 pagesSolution 2Varad DNo ratings yet
- C - Ch-18 - The D - F-Block ElementsDocument9 pagesC - Ch-18 - The D - F-Block Elementspanchaldalshukh46No ratings yet
- Tranisition Elements-03 - Assignments (New)Document13 pagesTranisition Elements-03 - Assignments (New)Raju SinghNo ratings yet
- Heating Effect - QBDocument12 pagesHeating Effect - QBRashi JalanNo ratings yet
- Chemistry Assignment - (JEE MainsDocument21 pagesChemistry Assignment - (JEE Mainsnikhil sridharaNo ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Assessment Specification 2017Document43 pagesAssessment Specification 2017IuliaHortNo ratings yet
- Accepted Manuscript: RSC - Li/pccpDocument10 pagesAccepted Manuscript: RSC - Li/pccpIuliaHortNo ratings yet
- Interviews in Natural Science and Computer Science: (Turn Over To See A Sample Paper)Document3 pagesInterviews in Natural Science and Computer Science: (Turn Over To See A Sample Paper)IuliaHortNo ratings yet
- Anode Polarization of Pb-Bi Alloys in The KCL-PBCL MeltDocument5 pagesAnode Polarization of Pb-Bi Alloys in The KCL-PBCL MeltIuliaHortNo ratings yet
- Assessment Specification 2017Document43 pagesAssessment Specification 2017IuliaHortNo ratings yet
- Productflyer - 978 3 540 00352 6Document1 pageProductflyer - 978 3 540 00352 6IuliaHortNo ratings yet
- Marmox Branz Appraisal 895 - 2018 A1Document8 pagesMarmox Branz Appraisal 895 - 2018 A1Dougie WNo ratings yet
- Brass Alloys Metalurgija Alloys enDocument19 pagesBrass Alloys Metalurgija Alloys enebrahimikntu100% (1)
- Global Marketing For Tube & Pipe: JIS G 4903 Seamless Nickel-Chromium-Iron Alloy PipesDocument9 pagesGlobal Marketing For Tube & Pipe: JIS G 4903 Seamless Nickel-Chromium-Iron Alloy PipesGerardo Emmanuel Romana BrianoNo ratings yet
- E3wood MysoreDocument2 pagesE3wood Mysorerame3No ratings yet
- Homework - Weak Acid Strong Base TitrationsDocument11 pagesHomework - Weak Acid Strong Base Titrationssamchen984No ratings yet
- International Standard: Coated Abrasives - Grain Size AnalysisDocument6 pagesInternational Standard: Coated Abrasives - Grain Size AnalysisAfzal ImamNo ratings yet
- Anel de Trava BI e BEDocument120 pagesAnel de Trava BI e BERonildo DuarteNo ratings yet
- 2015 Jan Chem 1 MsDocument26 pages2015 Jan Chem 1 Mskosala naveen wijekulasuriyaNo ratings yet
- Salud - Lab Activity 4 - Dialysis - Biochem - BSN 1-Coc A. PDFDocument1 pageSalud - Lab Activity 4 - Dialysis - Biochem - BSN 1-Coc A. PDFFaye SaludNo ratings yet
- Specification Points Covered: Ionic SubstancesDocument11 pagesSpecification Points Covered: Ionic SubstancesIsabella ThomasNo ratings yet
- Tutorial Getting Started With Code Aster PDFDocument12 pagesTutorial Getting Started With Code Aster PDFEnriqueNo ratings yet
- Ammonia Mass BalanceDocument24 pagesAmmonia Mass BalanceNurulFatimahalzahra100% (1)
- Reinforcement at Pipe Penetrations Through Steel Column TS-COL-01Document4 pagesReinforcement at Pipe Penetrations Through Steel Column TS-COL-01hahaerNo ratings yet
- Rusting of Metal - Home ExperimentDocument5 pagesRusting of Metal - Home ExperimentJustine Ryan L. MalgapoNo ratings yet
- Physicochemical Problems of Mineral Processing: ISSN 1643-1049 Index No. 32213XDocument329 pagesPhysicochemical Problems of Mineral Processing: ISSN 1643-1049 Index No. 32213Xravibelavadi100% (1)
- Pa6 GF20 - RTP Company RTP Pa6 20 GFDocument1 pagePa6 GF20 - RTP Company RTP Pa6 20 GFarmandoNo ratings yet
- Stainless Steel DefectsDocument30 pagesStainless Steel Defects0502ravi100% (1)
- Grooved Wooden Acoustic Panel Data SheetDocument30 pagesGrooved Wooden Acoustic Panel Data SheetzbignevtvNo ratings yet
- Charpy Test Determination of Impact Energy Using The Charpy TestDocument3 pagesCharpy Test Determination of Impact Energy Using The Charpy Testseelan10No ratings yet
- Petroleum Generation MigrationDocument63 pagesPetroleum Generation Migrationrobin2806100% (2)
- Ferro Molybdenum Powder - Ferro Alloy Powders - Kamman GroupDocument3 pagesFerro Molybdenum Powder - Ferro Alloy Powders - Kamman GroupHossein Hosseini RadNo ratings yet
- Shell Minimum Thickness Calculations: D (FT) G E Fill Height (FT)Document2 pagesShell Minimum Thickness Calculations: D (FT) G E Fill Height (FT)trijaya landscapeNo ratings yet
- Advanced Treatment of Shale Gas Fracturing Water To Produce Re-Use or Discharge Quality Water ... (PDFDrive)Document98 pagesAdvanced Treatment of Shale Gas Fracturing Water To Produce Re-Use or Discharge Quality Water ... (PDFDrive)سعيد الهاديNo ratings yet
- Chapter-1 Properties of SF6Document26 pagesChapter-1 Properties of SF6Aly AshrafNo ratings yet
- Chemical EqiulibriumDocument41 pagesChemical EqiulibriumZunaira Noreen100% (1)
- Opto Semi Koth0001eDocument17 pagesOpto Semi Koth0001eAnurag ChadhaNo ratings yet
- Fabrication of Nitrogen-Doped Porous Electrically Conductive Carbon Aerogel From Waste Cabbage For Supercapacitors and Oil Water SeparationDocument11 pagesFabrication of Nitrogen-Doped Porous Electrically Conductive Carbon Aerogel From Waste Cabbage For Supercapacitors and Oil Water SeparationAnh DuyNo ratings yet
- S E C Spe 1506 Experime Carbon D 634 Ental Stud Dioxide FL Dy For Op Lood Ptimizing Injected Surfacta NT Volum Meinami IscibleDocument9 pagesS E C Spe 1506 Experime Carbon D 634 Ental Stud Dioxide FL Dy For Op Lood Ptimizing Injected Surfacta NT Volum Meinami IscibleMariaNo ratings yet
- Tabel Besi PT Rangka RayaDocument3 pagesTabel Besi PT Rangka RayaMuhammad SumaidiNo ratings yet
Formation Constants of Complex Ions
Formation Constants of Complex Ions
Uploaded by
IuliaHort0 ratings0% found this document useful (0 votes)
30 views1 pageThis document lists the formation constants (Kf) of various complex ions at 25°C, organized into categories of halide complexes, ammonia complexes, cyanide complexes, complexes with other monodentate ligands, and complexes with bidentate ligands. The complex ions range from simple halide complexes like [AlF6]3- to more complex ligands binding multiple atoms like [Co(en)3]3+. The values provided are equilibrium constants that represent the strength of binding between metal cations and ligands in complex ion formations.
Original Description:
complex ions
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document lists the formation constants (Kf) of various complex ions at 25°C, organized into categories of halide complexes, ammonia complexes, cyanide complexes, complexes with other monodentate ligands, and complexes with bidentate ligands. The complex ions range from simple halide complexes like [AlF6]3- to more complex ligands binding multiple atoms like [Co(en)3]3+. The values provided are equilibrium constants that represent the strength of binding between metal cations and ligands in complex ion formations.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
30 views1 pageFormation Constants of Complex Ions
Formation Constants of Complex Ions
Uploaded by
IuliaHortThis document lists the formation constants (Kf) of various complex ions at 25°C, organized into categories of halide complexes, ammonia complexes, cyanide complexes, complexes with other monodentate ligands, and complexes with bidentate ligands. The complex ions range from simple halide complexes like [AlF6]3- to more complex ligands binding multiple atoms like [Co(en)3]3+. The values provided are equilibrium constants that represent the strength of binding between metal cations and ligands in complex ion formations.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
Formation Constants of Complex Ions at 25 C
Complex Ion Equilibrium Kf
Halide complexes
[AlF 6 ]3- 2.5104
[AlF 4 ] 1-
2.0108
[BeF 4 ] 2-
1.31013
[SnF 6 ]2- 11025
[CuCl 2 ] 1-
3105
[AgCl 2 ] 1-
1.8105
[PbCl 4 ] 2-
2.51015
[HgCl 4 ]2- 5.01015
[CuBr 2 ] 1-
8.0105
[AgBr 2 ] 1-
11011
[HgBr 4 ] 2-
3104
[CuI 2 ]1- 8108
[AgI 2 ] 1-
11011
[PbI 4 ] 2-
3104
[HgI 4 ] 2-
1.91030
Ammonia complexes
[Ag(NH 3 ) 2 ]1+ 1.6107
[Zn(NH 3 ) 4 ] 2+
7.8108
[Cu(NH 3 ) 4 ] 2+
1.11013
[Hg(NH 3 ) 4 ] 2+
1.81019
[Co(NH 3 ) 6 ]2+ 5.0104
[Co(NH 3 ) 6 ] 3+
4.61033
[Cd(NH 3 ) 6 ] 2+
2.6105
[Cd(NH 3 ) 4 ] 2+
1.0107
[Ni(NH 3 ) 6 ]2+ 2108
Cyanide complexes
[Fe(CN) 6 ]4- 1.01035
[Fe(CN) 6 ] 3-
9.11041
[Ag(CN) 2 ] 1-
5.31018
[Cu(CN) 2 ]1- 1.01016
[Cd(CN) 4 ] 2-
7.71016
[Au(CN) 2 ] 1-
21038
[Ni(CN) 4 ] 2-
11031
Complexes with other monodentate ligands
[Ag(CH 3 NH 2 ) 2 ]1+ 7.8106
[Ag(S 2 O 3 ) 2 ] 3-
2.91013
[Cd(SCN) 4 ] 2-
1103
[Cu(SCN) 2 ] 5.6103
[Fe(SCN) 3 ] 2106
[Hg(SCN) 4 ]2- 5.01021
[Cu(OH) 4 ] 2-
1.31016
[Zn(OH) 4 ] 2-
2.81015
Complexes with bidentate ligands (en = ethylenediamine )
[Mn(en) 3 ]2+ 6.5105
[Fe(en) 3 ] 2+
5.2109
[Co(en) 3 ] 2+
1.31014
[Co(en) 3 ] 3+
4.81048
[Ni(en) 3 ]2+ 4.11017
[Cu(en) 2 ] 2+
3.51019
[Co(C 2 O 4 ) 3 ] 4-
4.5106
[Fe(C 2 O 4 ) 3 ] 3-
3.31020
You might also like
- Page 2 Examples: FULL WORKED SOLUTIONS Are Available To Subscribers ofDocument13 pagesPage 2 Examples: FULL WORKED SOLUTIONS Are Available To Subscribers ofАЙЗАТ ЖАРИМБЕТОВА100% (5)
- Chang 1997Document17 pagesChang 1997Satyam Bhuyan100% (1)
- Solubility Product Constant (K) Values at 25 C: Salt K Salt K Salt K Salt K Bromides Carbonates Oxalates SulfidesDocument3 pagesSolubility Product Constant (K) Values at 25 C: Salt K Salt K Salt K Salt K Bromides Carbonates Oxalates SulfidesHerlina PanggabeanNo ratings yet
- Volumetric Analysis - Class Assignment Part 01 PDFDocument2 pagesVolumetric Analysis - Class Assignment Part 01 PDFyug agarwalNo ratings yet
- Nathaniel Herod - BalancingpracticeDocument10 pagesNathaniel Herod - BalancingpracticeNathaniel HerodNo ratings yet
- Coordination CoDocument19 pagesCoordination CoHandugan Quinlog NoelNo ratings yet
- Inorganic Chemistry by PMS: OF Co-Ordination CompoundsDocument15 pagesInorganic Chemistry by PMS: OF Co-Ordination CompoundsKumar AzadNo ratings yet
- 5.3.2 Transition Metals PDFDocument11 pages5.3.2 Transition Metals PDFkrishnaviNo ratings yet
- Lesson 1 Transition Metals IntroductionDocument48 pagesLesson 1 Transition Metals Introductiontiahayes2801No ratings yet
- 7 - Chemical Formulas: AnswersDocument1 page7 - Chemical Formulas: AnswersAlyssa Mae MayonadoNo ratings yet
- The D-Block Elements. General Properties: Mr. Kale Vinod NDocument23 pagesThe D-Block Elements. General Properties: Mr. Kale Vinod NLavinia DonaldNo ratings yet
- First Row Transition Metals - Complexes - Ligand ExchangeDocument4 pagesFirst Row Transition Metals - Complexes - Ligand ExchangeDanielle BelconNo ratings yet
- The Number of Antibonding Electron Pairs in O Ion On The Basis of Molecular Orbital Theory Is: (At. No. of O-Atom Is 8)Document23 pagesThe Number of Antibonding Electron Pairs in O Ion On The Basis of Molecular Orbital Theory Is: (At. No. of O-Atom Is 8)ronald.nazareth5469No ratings yet
- Utilization Os Nickel Slag PDFDocument7 pagesUtilization Os Nickel Slag PDFGaetanoD'AloiaNo ratings yet
- (Career Endeavour) MOLECULAR COMPOUNDSDocument15 pages(Career Endeavour) MOLECULAR COMPOUNDSVaibhav NikharNo ratings yet
- Unit 10. Complexometric Titration: I. Complex Ion FormationDocument5 pagesUnit 10. Complexometric Titration: I. Complex Ion FormationRaymond R. SantosNo ratings yet
- Balancing Equations: Practice ProblemsDocument10 pagesBalancing Equations: Practice ProblemsAyesha TauseefNo ratings yet
- Coordination Compound - Theory (Page 1-30)Document30 pagesCoordination Compound - Theory (Page 1-30)chemistrylectureihl2No ratings yet
- VBT Coordination CompoundsDocument4 pagesVBT Coordination CompoundsShashwat NiranjanNo ratings yet
- VbtcoorDocument4 pagesVbtcoorBishwadeep RoyNo ratings yet
- Semester Test 2 MemoDocument9 pagesSemester Test 2 MemoMac'Ann Ditshego MashaoNo ratings yet
- Pauling's Table of Electrode PotentialsDocument16 pagesPauling's Table of Electrode PotentialsDean GermetenNo ratings yet
- Heating Effect of Carbonate & Bicarbonate SaltsDocument3 pagesHeating Effect of Carbonate & Bicarbonate Saltsvishwajit patilNo ratings yet
- Environmental Mineralogy: Dr. Doni P E PutraDocument47 pagesEnvironmental Mineralogy: Dr. Doni P E Putramuh apriawan noorNo ratings yet
- Chapter 5 Coordination CompoundDocument36 pagesChapter 5 Coordination Compoundammar zakariaNo ratings yet
- Dissociation Constants For Some Complex IonsDocument1 pageDissociation Constants For Some Complex IonsabasakNo ratings yet
- Heating Effects (12th&13th)Document4 pagesHeating Effects (12th&13th)Raju SinghNo ratings yet
- Chem D and F BlockDocument5 pagesChem D and F BlockVIKAS SHARMANo ratings yet
- Cambridge IGCSE: CHEMISTRY 0620/31Document20 pagesCambridge IGCSE: CHEMISTRY 0620/31Tshegofatso SaliNo ratings yet
- CH2 Transition Metals Unit V A2 LevelDocument9 pagesCH2 Transition Metals Unit V A2 LevelbillaljavedNo ratings yet
- Coordination Compounds: DPP 01 (Of Lecture 3) - Yakeen 3.0 2023Document3 pagesCoordination Compounds: DPP 01 (Of Lecture 3) - Yakeen 3.0 2023PalakNo ratings yet
- Wa0005Document26 pagesWa0005Dixon ECNo ratings yet
- No Kation Nama No Anion NamaDocument2 pagesNo Kation Nama No Anion NamaAjeng Candra Arum PuspasariNo ratings yet
- Chemsheets-A2-Transition-Metals WorkbookDocument32 pagesChemsheets-A2-Transition-Metals WorkbookmlbgurpreetttNo ratings yet
- XII JEE Chemistry Practice Sheet 01Document406 pagesXII JEE Chemistry Practice Sheet 01Akshat MakwanaNo ratings yet
- JEE ChemistryDocument406 pagesJEE Chemistryak1740120No ratings yet
- Coordination Compounds (Exercise+Answers)Document32 pagesCoordination Compounds (Exercise+Answers)Hanukkah100% (1)
- COORDINATION COMPOUNDS - Class Notes - JEE MindmapDocument22 pagesCOORDINATION COMPOUNDS - Class Notes - JEE Mindmapadsaditya24No ratings yet
- Z, A, Valency, ConfigurationDocument2 pagesZ, A, Valency, ConfigurationnavyanonaNo ratings yet
- Coordination Compound Level-0 Part IDocument3 pagesCoordination Compound Level-0 Part IAnumuskan KashyapNo ratings yet
- Solubility ProductsDocument2 pagesSolubility Productsgracemizzi6No ratings yet
- Chemistry-Bridging The Gap AnswerDocument11 pagesChemistry-Bridging The Gap AnswerMuhammad IzzuanNo ratings yet
- RT3D - BTEX Degradation With Multiple Electron Acceptors: Gms 7.0 TutorialsDocument12 pagesRT3D - BTEX Degradation With Multiple Electron Acceptors: Gms 7.0 TutorialsAbdelhay ElomariNo ratings yet
- Standard Electrode PotentialDocument11 pagesStandard Electrode PotentialRSLNo ratings yet
- Co-Ordination and Organometallic CompDocument85 pagesCo-Ordination and Organometallic CompDr. Dhondiba Vishwanath100% (1)
- Redox Reactions Mini QuestionsDocument5 pagesRedox Reactions Mini QuestionsKL KNo ratings yet
- Coordination ChemistryDocument30 pagesCoordination ChemistryRizwanbhatNo ratings yet
- Chemical Bonding: Why Bond Anyway?Document45 pagesChemical Bonding: Why Bond Anyway?PutRi Charolin GintingNo ratings yet
- 3-Carbon Oxidation 1Document21 pages3-Carbon Oxidation 1Aditya GajbhiyeNo ratings yet
- (L1) - Coordinate Compounds - 28th NovDocument32 pages(L1) - Coordinate Compounds - 28th NovKhushi RathoreNo ratings yet
- B Masia c315 Exp 3Document6 pagesB Masia c315 Exp 3MphoNo ratings yet
- Coordination Chemistry - Diwali Assignment II (Pathshala 12th JEE 2023)Document6 pagesCoordination Chemistry - Diwali Assignment II (Pathshala 12th JEE 2023)Gayatri GuptaNo ratings yet
- Chelate Effect: Coordination ChemistryDocument13 pagesChelate Effect: Coordination ChemistryNikitha AkulaNo ratings yet
- Ferrioxalate SystemDocument6 pagesFerrioxalate SystemRohit ChauhanNo ratings yet
- Chemistry Periodic TableDocument2 pagesChemistry Periodic TableAbhinav KumarNo ratings yet
- Solution 2Document12 pagesSolution 2Varad DNo ratings yet
- C - Ch-18 - The D - F-Block ElementsDocument9 pagesC - Ch-18 - The D - F-Block Elementspanchaldalshukh46No ratings yet
- Tranisition Elements-03 - Assignments (New)Document13 pagesTranisition Elements-03 - Assignments (New)Raju SinghNo ratings yet
- Heating Effect - QBDocument12 pagesHeating Effect - QBRashi JalanNo ratings yet
- Chemistry Assignment - (JEE MainsDocument21 pagesChemistry Assignment - (JEE Mainsnikhil sridharaNo ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Assessment Specification 2017Document43 pagesAssessment Specification 2017IuliaHortNo ratings yet
- Accepted Manuscript: RSC - Li/pccpDocument10 pagesAccepted Manuscript: RSC - Li/pccpIuliaHortNo ratings yet
- Interviews in Natural Science and Computer Science: (Turn Over To See A Sample Paper)Document3 pagesInterviews in Natural Science and Computer Science: (Turn Over To See A Sample Paper)IuliaHortNo ratings yet
- Anode Polarization of Pb-Bi Alloys in The KCL-PBCL MeltDocument5 pagesAnode Polarization of Pb-Bi Alloys in The KCL-PBCL MeltIuliaHortNo ratings yet
- Assessment Specification 2017Document43 pagesAssessment Specification 2017IuliaHortNo ratings yet
- Productflyer - 978 3 540 00352 6Document1 pageProductflyer - 978 3 540 00352 6IuliaHortNo ratings yet
- Marmox Branz Appraisal 895 - 2018 A1Document8 pagesMarmox Branz Appraisal 895 - 2018 A1Dougie WNo ratings yet
- Brass Alloys Metalurgija Alloys enDocument19 pagesBrass Alloys Metalurgija Alloys enebrahimikntu100% (1)
- Global Marketing For Tube & Pipe: JIS G 4903 Seamless Nickel-Chromium-Iron Alloy PipesDocument9 pagesGlobal Marketing For Tube & Pipe: JIS G 4903 Seamless Nickel-Chromium-Iron Alloy PipesGerardo Emmanuel Romana BrianoNo ratings yet
- E3wood MysoreDocument2 pagesE3wood Mysorerame3No ratings yet
- Homework - Weak Acid Strong Base TitrationsDocument11 pagesHomework - Weak Acid Strong Base Titrationssamchen984No ratings yet
- International Standard: Coated Abrasives - Grain Size AnalysisDocument6 pagesInternational Standard: Coated Abrasives - Grain Size AnalysisAfzal ImamNo ratings yet
- Anel de Trava BI e BEDocument120 pagesAnel de Trava BI e BERonildo DuarteNo ratings yet
- 2015 Jan Chem 1 MsDocument26 pages2015 Jan Chem 1 Mskosala naveen wijekulasuriyaNo ratings yet
- Salud - Lab Activity 4 - Dialysis - Biochem - BSN 1-Coc A. PDFDocument1 pageSalud - Lab Activity 4 - Dialysis - Biochem - BSN 1-Coc A. PDFFaye SaludNo ratings yet
- Specification Points Covered: Ionic SubstancesDocument11 pagesSpecification Points Covered: Ionic SubstancesIsabella ThomasNo ratings yet
- Tutorial Getting Started With Code Aster PDFDocument12 pagesTutorial Getting Started With Code Aster PDFEnriqueNo ratings yet
- Ammonia Mass BalanceDocument24 pagesAmmonia Mass BalanceNurulFatimahalzahra100% (1)
- Reinforcement at Pipe Penetrations Through Steel Column TS-COL-01Document4 pagesReinforcement at Pipe Penetrations Through Steel Column TS-COL-01hahaerNo ratings yet
- Rusting of Metal - Home ExperimentDocument5 pagesRusting of Metal - Home ExperimentJustine Ryan L. MalgapoNo ratings yet
- Physicochemical Problems of Mineral Processing: ISSN 1643-1049 Index No. 32213XDocument329 pagesPhysicochemical Problems of Mineral Processing: ISSN 1643-1049 Index No. 32213Xravibelavadi100% (1)
- Pa6 GF20 - RTP Company RTP Pa6 20 GFDocument1 pagePa6 GF20 - RTP Company RTP Pa6 20 GFarmandoNo ratings yet
- Stainless Steel DefectsDocument30 pagesStainless Steel Defects0502ravi100% (1)
- Grooved Wooden Acoustic Panel Data SheetDocument30 pagesGrooved Wooden Acoustic Panel Data SheetzbignevtvNo ratings yet
- Charpy Test Determination of Impact Energy Using The Charpy TestDocument3 pagesCharpy Test Determination of Impact Energy Using The Charpy Testseelan10No ratings yet
- Petroleum Generation MigrationDocument63 pagesPetroleum Generation Migrationrobin2806100% (2)
- Ferro Molybdenum Powder - Ferro Alloy Powders - Kamman GroupDocument3 pagesFerro Molybdenum Powder - Ferro Alloy Powders - Kamman GroupHossein Hosseini RadNo ratings yet
- Shell Minimum Thickness Calculations: D (FT) G E Fill Height (FT)Document2 pagesShell Minimum Thickness Calculations: D (FT) G E Fill Height (FT)trijaya landscapeNo ratings yet
- Advanced Treatment of Shale Gas Fracturing Water To Produce Re-Use or Discharge Quality Water ... (PDFDrive)Document98 pagesAdvanced Treatment of Shale Gas Fracturing Water To Produce Re-Use or Discharge Quality Water ... (PDFDrive)سعيد الهاديNo ratings yet
- Chapter-1 Properties of SF6Document26 pagesChapter-1 Properties of SF6Aly AshrafNo ratings yet
- Chemical EqiulibriumDocument41 pagesChemical EqiulibriumZunaira Noreen100% (1)
- Opto Semi Koth0001eDocument17 pagesOpto Semi Koth0001eAnurag ChadhaNo ratings yet
- Fabrication of Nitrogen-Doped Porous Electrically Conductive Carbon Aerogel From Waste Cabbage For Supercapacitors and Oil Water SeparationDocument11 pagesFabrication of Nitrogen-Doped Porous Electrically Conductive Carbon Aerogel From Waste Cabbage For Supercapacitors and Oil Water SeparationAnh DuyNo ratings yet
- S E C Spe 1506 Experime Carbon D 634 Ental Stud Dioxide FL Dy For Op Lood Ptimizing Injected Surfacta NT Volum Meinami IscibleDocument9 pagesS E C Spe 1506 Experime Carbon D 634 Ental Stud Dioxide FL Dy For Op Lood Ptimizing Injected Surfacta NT Volum Meinami IscibleMariaNo ratings yet
- Tabel Besi PT Rangka RayaDocument3 pagesTabel Besi PT Rangka RayaMuhammad SumaidiNo ratings yet