Professional Documents
Culture Documents
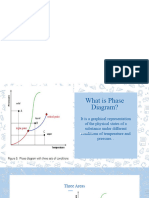
Figure 1: Temperature-Pressure Phase Diagram of Pure Water in A Closed System
Figure 1: Temperature-Pressure Phase Diagram of Pure Water in A Closed System
Uploaded by
JYOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Figure 1: Temperature-Pressure Phase Diagram of Pure Water in A Closed System
Figure 1: Temperature-Pressure Phase Diagram of Pure Water in A Closed System
Uploaded by
JYCopyright:
Available Formats
Figure 1 below shows the phase diagram of pure water which contains inside a closed
cylinder in terms of its temperature and absolute pressure. There are three main areas (solid, liquid
and vapour) separated from each other by three lines (melting, vaporization and sublimation) in
this temperature-pressure phase diagram. Both the solid and liquid states are separated by melting
line whereas the vaporization line separated the liquid and vapour regions. Meanwhile, sublimation
line lies between the solid and vapour regions. These three lines are the conditions where the two
separated states are existed at the same time. Then, the three lines meet at triple point, where all
the three states can be presented in equilibrium.
Figure 1: Temperature-Pressure Phase Diagram of Pure Water in a Closed System
You might also like
- MksowmdoxwDocument11 pagesMksowmdoxwMikhela Janielle MartinNo ratings yet
- Phase and One Component SystemDocument5 pagesPhase and One Component SystemMumtaz AhmadNo ratings yet
- Chem 2 LecDocument4 pagesChem 2 LecHeidi BardillonNo ratings yet
- 5) Phase RuleDocument17 pages5) Phase RuleSHANJIDA ALI RIA100% (1)
- Phase Diagram - WikipediaDocument14 pagesPhase Diagram - Wikipediasterling goinNo ratings yet
- Unit 3-Cy19241Document28 pagesUnit 3-Cy19241Suresh Kumar A PNo ratings yet
- Critical So TemperatureDocument49 pagesCritical So TemperatureThakur Aditya PratapNo ratings yet
- Phase Diagram: From Wikipedia, The Free EncyclopediaDocument7 pagesPhase Diagram: From Wikipedia, The Free EncyclopediaPawan PatelNo ratings yet
- Phase Diagrams: Along AB LineDocument4 pagesPhase Diagrams: Along AB LinebudhladaNo ratings yet
- Lecture No. 5 Phase DiagramDocument32 pagesLecture No. 5 Phase DiagramarslanNo ratings yet
- Thermodynamics /: Chpter 4Document7 pagesThermodynamics /: Chpter 4yashwantmoganaradjouNo ratings yet
- A Phase Diagram in Physical ChemistryDocument12 pagesA Phase Diagram in Physical Chemistryreeta1981No ratings yet
- The Basic Phase Diagram What Is A Phase?Document7 pagesThe Basic Phase Diagram What Is A Phase?SAMANTHA GABRIELA MESA REYESNo ratings yet
- ThermodynamicsDocument16 pagesThermodynamicsaneeda shabirNo ratings yet
- Binary Phase DiagramsDocument20 pagesBinary Phase DiagramsRaja AKNo ratings yet
- Kimfis Grafik Iodin Dan SulfurDocument7 pagesKimfis Grafik Iodin Dan SulfurAldyMaulanaNo ratings yet
- Phase Diagrams NotesDocument8 pagesPhase Diagrams NotesRamesh PotnuriNo ratings yet
- NAME: Ridah Khushnud: BATCH: 2019-2023 Topic: Phase DiagramDocument6 pagesNAME: Ridah Khushnud: BATCH: 2019-2023 Topic: Phase DiagramRidah KhanNo ratings yet
- Phase DiagramDocument5 pagesPhase DiagramErvz MissionNo ratings yet
- Phase EqualibriumDocument16 pagesPhase EqualibriumRatna BairagiNo ratings yet
- NOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxideDocument8 pagesNOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxidestephniedayaoNo ratings yet
- 3 - Phase Diagram of Naphthalene-BiphenylDocument7 pages3 - Phase Diagram of Naphthalene-Biphenyldidikkris100% (3)
- Capili Jefferson 9Document13 pagesCapili Jefferson 9Christian Al EncarnacionNo ratings yet
- Distillation Lecture Note-2Document20 pagesDistillation Lecture Note-2BasseyNo ratings yet
- Phase Rule Water & CO2systemsDocument9 pagesPhase Rule Water & CO2systemsAtul GautamNo ratings yet
- Lesson 4 Intermolecular Forces of Liquids and Solids - Phase DiagramsDocument33 pagesLesson 4 Intermolecular Forces of Liquids and Solids - Phase DiagramsLyndy PantaoNo ratings yet
- Phase Behavior OilDocument6 pagesPhase Behavior OilBashir KizitoNo ratings yet
- Phase Diagram: For The Use of This Term in Mathematics and Physics, See Phase SpaceDocument5 pagesPhase Diagram: For The Use of This Term in Mathematics and Physics, See Phase SpaceMurali Krishna.HARINo ratings yet
- NOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxideDocument8 pagesNOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxidestephniedayaoNo ratings yet
- Phase Diagrams: Figure 1. General Phase DiagramDocument3 pagesPhase Diagrams: Figure 1. General Phase DiagramRONo ratings yet
- Physical Equilibrium: Holistic Approach To Physical Chemistry by JBDocument31 pagesPhysical Equilibrium: Holistic Approach To Physical Chemistry by JBLavenz EltonNo ratings yet
- New Microsoft Office Word DocumentDocument17 pagesNew Microsoft Office Word DocumentmanojNo ratings yet
- Phase Diagram - WikipediaDocument7 pagesPhase Diagram - WikipediaYn FoanNo ratings yet
- The Phase Behavior of Water and Hydrocarbon SystemsDocument5 pagesThe Phase Behavior of Water and Hydrocarbon Systemsأصلان أصلانNo ratings yet
- Phase RuleDocument14 pagesPhase Ruleapi-26041653100% (1)
- Concentration: BinaryDocument2 pagesConcentration: BinarySaiful AzrieNo ratings yet
- Unit 7 PhaseruleDocument11 pagesUnit 7 Phaseruleengineeringchemistry100% (2)
- Volumetric Properties of Pure Fluids: ThermodynamicDocument9 pagesVolumetric Properties of Pure Fluids: ThermodynamicLulav BarwaryNo ratings yet
- Phase BehaviorDocument10 pagesPhase Behaviorfri_13thNo ratings yet
- Phase EquilibriaDocument11 pagesPhase EquilibriaNandhanNo ratings yet
- AlifDocument6 pagesAlifKiran YaqoobNo ratings yet
- CONTINUOUS DistillationDocument5 pagesCONTINUOUS DistillationNaseer SattarNo ratings yet
- One Component System Examples of One Component System: VSG & Ky 11/21/2014Document7 pagesOne Component System Examples of One Component System: VSG & Ky 11/21/2014MadhavanIceNo ratings yet
- Phase RuleDocument27 pagesPhase RulejaiminNo ratings yet
- Phase Diagrams: By: Cherides P. MarianoDocument25 pagesPhase Diagrams: By: Cherides P. MarianoWild RiftNo ratings yet
- Experiment 3Document14 pagesExperiment 3HafiniHambaliNo ratings yet
- Bahir Dar Universtity Department of Material Science and EngineeringDocument5 pagesBahir Dar Universtity Department of Material Science and EngineeringKetemaw ZemeneNo ratings yet
- Lab 2 - Phase Diagrams Hoai Nguyen 912292469 ENG 45Y Section 24Document4 pagesLab 2 - Phase Diagrams Hoai Nguyen 912292469 ENG 45Y Section 24Anonymous eJYLPeEo1No ratings yet
- Phase RuleDocument4 pagesPhase RuleKiran BarikNo ratings yet
- One Compenent Water SystemDocument7 pagesOne Compenent Water Systemslchem80% (5)
- Vapor-Liquid EquilibriumDocument8 pagesVapor-Liquid EquilibriumMagesh kumarNo ratings yet
- Quarter 2 - Module 6: Phase Diagram of Water and Carbon Dioxide ExplainDocument2 pagesQuarter 2 - Module 6: Phase Diagram of Water and Carbon Dioxide ExplainRic Anthony LayasanNo ratings yet
- Phase EquilibriumDocument32 pagesPhase EquilibriumSaif Khan100% (1)
- Wk2-GeneralChemistry2 Quarter1Document37 pagesWk2-GeneralChemistry2 Quarter1chelcieariendeleonNo ratings yet
- Extending The Diagrams To Include The Solid PhaseDocument4 pagesExtending The Diagrams To Include The Solid PhaseawaisahmedkhanNo ratings yet
- Phase Behavior of Petroleum Fluids: A. El-BanbiDocument6 pagesPhase Behavior of Petroleum Fluids: A. El-BanbimmatjNo ratings yet
- UNIT-5 Phase EquilibriaDocument13 pagesUNIT-5 Phase EquilibriaALOK KUMARNo ratings yet
- Phase RuleDocument20 pagesPhase RuleAshish KumarNo ratings yet
- Vapour Liquid Equilibria & Dew Point Calculations: Thermodynamics Ii Project CHE - 03Document12 pagesVapour Liquid Equilibria & Dew Point Calculations: Thermodynamics Ii Project CHE - 03Mallick Zeyshan TariqNo ratings yet
- Concept Question ThermoDocument1 pageConcept Question ThermoJYNo ratings yet
- Open - Ended - Assignment For EMDocument2 pagesOpen - Ended - Assignment For EMJYNo ratings yet
- Design of ExperimentsDocument44 pagesDesign of ExperimentsJYNo ratings yet
- Figure 1: RF Fixed Attenuator Figure 2: Variable AttenuatorDocument1 pageFigure 1: RF Fixed Attenuator Figure 2: Variable AttenuatorJYNo ratings yet