Professional Documents
Culture Documents
Polycystic Ovary Syndrome
Polycystic Ovary Syndrome
Uploaded by
ABC11Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Polycystic Ovary Syndrome
Polycystic Ovary Syndrome
Uploaded by
ABC11Copyright:
Available Formats
Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is a set of symptoms due to elevated androgens (male
hormones) in women.[4][14] Signs and symptoms of PCOS include irregular or no menstrual
periods, heavy periods, excess body and facial hair, acne, pelvic pain, difficulty getting
pregnant, and patches of thick, darker, velvety skin.[3] Associated conditions include type 2
diabetes, obesity, obstructive sleep apnea, heart disease, mood disorders, and endometrial
cancer.[4]
PCOS is due to a combination of genetic and environmental factors.[6][7][15] Risk factors
include obesity, not enough physical exercise, and a family history of someone with the
condition.[8] Diagnosis is based on two of the following three findings: no ovulation, high
androgen levels, and ovarian cysts.[4] Cysts may be detectable by ultrasound.[9] Other
conditions that produce similar symptoms include adrenal hyperplasia, hypothyroidism, and
hyperprolactinemia.[9]
PCOS has no cure.[5] Treatment may involve lifestyle changes such as weight loss and
exercise.[10][11] Birth control pills may help with improving the regularity of periods, excess
hair growth, and acne.[12] Metformin and anti-androgens may also help.[12] Other typical
acne treatments and hair removal techniques may be used.[12] Efforts to improve fertility
include weight loss, clomiphene, or metformin.[16] In vitro fertilization is used by some in
whom other measures are not effective.[16]
PCOS is the most common endocrine disorder among women between the ages of 18 and
44.[17] It affects approximately 2% to 20% of this age group depending on how it is
defined.[8][13] It is one of the leading causes of poor fertility.[4] The earliest known
description of what is now recognized as PCOS dates from 1721 in Italy.[18]
Signs and symptoms[edit]
Further information: Infertility in polycystic ovary syndrome
Common signs and symptoms of PCOS include the following:
Menstrual disorders: PCOS mostly produces oligomenorrhea (fewer than nine menstrual
periods in a year) or amenorrhea (no menstrual periods for three or more consecutive
months), but other types of menstrual disorders may also occur.[17][19]
Infertility:[19] This generally results directly from chronic anovulation (lack of ovulation).[17]
High levels of masculinizing hormones: Known as hyperandrogenism, the most common signs
are acne and hirsutism (male pattern of hair growth, such as on the chin or chest), but it may
produce hypermenorrhea (heavy and prolonged menstrual periods), androgenic alopecia
(increased hair thinning or diffuse hair loss), or other symptoms.[17][20] Approximately
three-quarters of women with PCOS (by the diagnostic criteria of NIH/NICHD 1990) have
evidence of hyperandrogenemia.[21]
Metabolic syndrome:[19] This appears as a tendency towards central obesity and other
symptoms associated with insulin resistance.[17] Serum insulin, insulin resistance, and
homocysteine levels are higher in women with PCOS.[22]
Asians affected by PCOS are less likely to develop hirsutism than those of other ethnic
backgrounds.[23]
Cause[edit]
PCOS is a heterogeneous disorder of uncertain cause.[19][24][25] There is some evidence that
it is a genetic disease. Such evidence includes the familial clustering of cases, greater
concordance in monozygotic compared with dizygotic twins and heritability of endocrine and
metabolic features of PCOS.[7][24][25]
The genetic component appears to be inherited in an autosomal dominant fashion with high
genetic penetrance but variable expressivity in females; this means that each child has a 50%
chance of inheriting the predisposing genetic variant(s) from a parent, and, if a daughter
receives the variant(s), the daughter will have the disease to some extent.[25][26][27][28]
The genetic variant(s) can be inherited from either the father or the mother, and can be
passed along to both sons (who may be asymptomatic carriers or may have symptoms such
as early baldness and/or excessive hair) and daughters, who will show signs of PCOS.[26][28]
The phenotype appears to manifest itself at least partially via heightened androgen levels
secreted by ovarian follicle theca cells from women with the allele.[27] The exact gene
affected has not yet been identified.[7][25][29] In rare instances, single-gene mutations can
give rise to the phenotype of the syndrome.[30] Current understanding of the pathogenesis
of the syndrome suggests, however, that it is a complex multigenic disorder.[31]
The severity of PCOS symptoms appears to be largely determined by factors such as
obesity.[7][17][32]
PCOS has some aspects of a metabolic disorder, since its symptoms are partly reversible. Even
though considered as a gynecological problem, PCOS consists of 28 clinical symptoms.
Even though the name suggests that the ovaries are central to disease pathology, cysts are a
symptom instead of the cause of the disease. Some symptoms of PCOS will persist even if
both ovaries are removed; the disease can appear even if cysts are absent. Since its first
description by Stein and Leventhal in 1935, the criteria of diagnosis, symptoms, and causative
factors are subject to debate. Gynecologists often see it as a gynecological problem, with the
ovaries being the primary organ affected. However, recent insights show a multisystem
disorder, with the primary problem lying in hormonal regulation in the hypothalamus, with
the involvement of many organs. The name PCOD is used when there is ultrasonographic
evidence. The term PCOS is used since there is a wide spectrum of symptoms possible, and
cysts in the ovaries are seen only in 15% of people.[33]
PCOS may be related to or worsened by exposures during the prenatal period, epigenetic
factors, environmental impacts (especially industrial endocrine disruptors[34] such as
bisphenol A and certain drugs) and the increasing rates of
obesity.[34][35][36][37][38][39][40]
Pathogenesis[edit]
Polycystic ovaries develop when the ovaries are stimulated to produce excessive amounts of
androgenic hormones, in particular testosterone, by either one or a combination of the
following (almost certainly combined with genetic susceptibility[27]):
the release of excessive luteinizing hormone (LH) by the anterior pituitary gland[citation
needed]
through high levels of insulin in the blood (hyperinsulinaemia) in women whose ovaries are
sensitive to this stimulus[19]
The syndrome acquired its most widely used name due to the common sign on ultrasound
examination of multiple (poly) ovarian cysts. These "cysts" are actually immature follicles not
cysts. The follicles have developed from primordial follicles, but the development has stopped
("arrested") at an early antral stage due to the disturbed ovarian function. The follicles may
be oriented along the ovarian periphery, appearing as a 'string of pearls' on ultrasound
examination.[citation needed]
Women with PCOS experience an increased frequency of hypothalamic GnRH pulses, which
in turn results in an increase in the LH/FSH ratio.[41]
A majority of women with PCOS have insulin resistance and/or are obese. Their elevated
insulin levels contribute to or cause the abnormalities seen in the hypothalamic-pituitary-
ovarian axis that lead to PCOS. Hyperinsulinemia increases GnRH pulse frequency, LH over
FSH dominance, increased ovarian androgen production,[19] decreased follicular maturation,
and decreased SHBG binding. Furthermore, excessive insulin, acting through its cognate
receptor in the presence of component cAMP signalling, upregulates 17-hydroxylase activity
via PI3K, 17-hydroxylase activity being responsible for synthesising androgen precursors.[42]
The combined effects of hyperinsulinemia contribute to an increased risk of PCOS.[43] Insulin
resistance is a common finding among women with a normal weight as well as overweight
women.[17][22][10]
Adipose tissue possesses aromatase, an enzyme that converts androstenedione to estrone
and testosterone to estradiol. The excess of adipose tissue in obese women creates the
paradox of having both excess androgens (which are responsible for hirsutism and virilization)
and estrogens (which inhibits FSH via negative feedback).[44]
PCOS may be associated with chronic inflammation,[19][45] with several investigators
correlating inflammatory mediators with anovulation and other PCOS symptoms.[46][47]
Similarly, there seems to be a relation between PCOS and increased level of oxidative
stress.[48]
It has previously been suggested that the excessive androgen production in PCOS could be
caused by a decreased serum level of IGFBP-1, in turn increasing the level of free IGF-I, which
stimulates ovarian androgen production, but recent data concludes this mechanism to be
unlikely.[49]
PCOS has also been associated with a specific FMR1 sub-genotype. The research suggests that
women with heterozygous-normal/low FMR1 have polycystic-like symptoms of excessive
follicle-activity and hyperactive ovarian function.[50]
Transgender men may experience a higher than expected rate of PCOS due to increased
testosterone, if they choose to take hormone therapy as part of their gender
presentation.[51][52]
Diagnosis[edit]
Not everyone with PCOS has polycystic ovaries (PCO), nor does everyone with ovarian cysts
have PCOS; although a pelvic ultrasound is a major diagnostic tool, it is not the only one.[53]
The diagnosis is straightforward using the Rotterdam criteria, even when the syndrome is
associated with a wide range of symptoms.
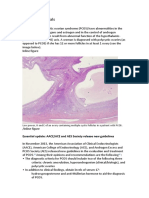
Polycystic Ovary as seen on Sonography
Transvaginal ultrasound scan of polycystic ovary
Polycystic Ovary as seen on Sonography
Definition[edit]
Two definitions are commonly used:
NIH[edit]
In 1990 a consensus workshop sponsored by the NIH/NICHD suggested that a person has
PCOS if they have all of the following:[54]
oligoovulation
signs of androgen excess (clinical or biochemical)
exclusion of other disorders that can result in menstrual irregularity and hyperandrogenism
Rotterdam[edit]
In 2003 a consensus workshop sponsored by ESHRE/ASRM in Rotterdam indicated PCOS to
be present if any 2 out of 3 criteria are met, in the absence of other entities that might cause
these findings[17][55][56]
oligoovulation and/or anovulation
excess androgen activity
polycystic ovaries (by gynecologic ultrasound)
The Rotterdam definition is wider, including many more women, the most notable ones being
women without androgen excess. Critics say that findings obtained from the study of women
with androgen excess cannot necessarily be extrapolated to women without androgen
excess.[57][58]
Androgen Excess PCOS Society
In 2006, the Androgen Excess PCOS Society suggested a tightening of the diagnostic criteria
to all of the following:[17]
excess androgen activity
oligoovulation/anovulation and/or polycystic ovaries
exclusion of other entities that would cause excess androgen activity
Standard assessment[edit]
History-taking, specifically for menstrual pattern, obesity, hirsutism and acne. A clinical
prediction rule found that these four questions can diagnose PCOS with a sensitivity of 77.1%
(95% confidence interval [CI] 62.7%88.0%) and a specificity of 93.8% (95% CI 82.8%
98.7%).[59]
Gynecologic ultrasonography, specifically looking for small ovarian follicles. These are
believed to be the result of disturbed ovarian function with failed ovulation, reflected by the
infrequent or absent menstruation that is typical of the condition. In a normal menstrual
cycle, one egg is released from a dominant follicle in essence, a cyst that bursts to release
the egg. After ovulation, the follicle remnant is transformed into a progesterone-producing
corpus luteum, which shrinks and disappears after approximately 1214 days. In PCOS, there
is a so-called "follicular arrest"; i.e., several follicles develop to a size of 57 mm, but not
further. No single follicle reaches the preovulatory size (16 mm or more). According to the
Rotterdam criteria, which are widely used for diagnosis,[10] 12 or more small follicles should
be seen in an ovary on ultrasound examination.[54] More recent research suggests that there
should be at least 25 follicles in an ovary to designate it as having polycystic ovarian
morphology (PCOM) in women aged 1835 years.[60] The follicles may be oriented in the
periphery, giving the appearance of a 'string of pearls'.[61] If a high resolution transvaginal
ultrasonography machine is not available, an ovarian volume of at least 10 ml is regarded as
an acceptable definition of having polycystic ovarian morphology instead of follicle count.[60]
Laparoscopic examination may reveal a thickened, smooth, pearl-white outer surface of the
ovary. (This would usually be an incidental finding if laparoscopy were performed for some
other reason, as it would not be routine to examine the ovaries in this way to confirm a
diagnosis of PCOS.)[citation needed]
Serum (blood) levels of androgens (hormones associated with male development), including
androstenedione and testosterone may be elevated.[17] Dehydroepiandrosterone sulfate
levels above 700800 g/dL are highly suggestive of adrenal dysfunction because DHEA-S is
made exclusively by the adrenal glands.[62][63] The free testosterone level is thought to be
the best measure,[63][64] with ~60% of PCOS patients demonstrating supranormal levels.[21]
The Free androgen index (FAI) of the ratio of testosterone to sex hormone-binding globulin
(SHBG) is high[17][63] and is meant to be a predictor of free testosterone, but is a poor
parameter for this and is no better than testosterone alone as a marker for PCOS,[65] possibly
because FAI is correlated with the degree of obesity.[66]
Some other blood tests are suggestive but not diagnostic. The ratio of LH (Luteinizing
hormone) to FSH (Follicle-stimulating hormone), when measured in international units, is
elevated in women with PCOS. Common cut-offs to designate abnormally high LH/FSH ratios
are 2:1[67] or 3:1[63] as tested on Day 3 of the menstrual cycle. The pattern is not very
sensitive; a ratio of 2:1 or higher was present in less than 50% of women with PCOS in one
study.[67] There are often low levels of sex hormone-binding globulin,[63] in particular
among obese or overweight women.[citation needed]
Anti-Mllerian hormone (AMH) is increased in PCOS, and may become part of its diagnostic
criteria.[68][69][70]
Associated conditions[edit]
Fasting biochemical screen and lipid profile[63]
2-Hour oral glucose tolerance test (GTT) in women with risk factors (obesity, family history,
history of gestational diabetes)[17] may indicate impaired glucose tolerance (insulin
resistance) in 1533% of women with PCOS.[63] Frank diabetes can be seen in 6568% of
women with this condition.[citation needed] Insulin resistance can be observed in both
normal weight and overweight people, although it is more common in the latter (and in those
matching the stricter NIH criteria for diagnosis); 5080% of people with PCOS may have insulin
resistance at some level.[17]
Fasting insulin level or GTT with insulin levels (also called IGTT). Elevated insulin levels have
been helpful to predict response to medication and may indicate women needing higher
dosages of metformin or the use of a second medication to significantly lower insulin levels.
Elevated blood sugar and insulin values do not predict who responds to an insulin-lowering
medication, low-glycemic diet, and exercise. Many women with normal levels may benefit
from combination therapy. A hypoglycemic response in which the two-hour insulin level is
higher and the blood sugar lower than fasting is consistent with insulin resistance. A
mathematical derivation known as the HOMAI, calculated from the fasting values in glucose
and insulin concentrations, allows a direct and moderately accurate measure of insulin
sensitivity (glucose-level x insulin-level/22.5).[citation needed]
Glucose tolerance testing (GTT) instead of fasting glucose can increase diagnosis of impaired
glucose tolerance and frank diabetes among people with PCOS according to a prospective
controlled trial.[71] While fasting glucose levels may remain within normal limits, oral glucose
tests revealed that up to 38% of asymptomatic women with PCOS (versus 8.5% in the general
population) actually had impaired glucose tolerance, 7.5% of those with frank diabetes
according to ADA guidelines.[71]
Differential diagnosis[edit]
Other causes of irregular or absent menstruation and hirsutism, such as hypothyroidism,
congenital adrenal hyperplasia (21-hydroxylase deficiency), Cushing's syndrome,
hyperprolactinemia, androgen secreting neoplasms, and other pituitary or adrenal disorders,
should be investigated.[17][56][63]
Management[edit]
The primary treatments for PCOS include: lifestyle changes and medications.[72]
Goals of treatment may be considered under four categories:
Lowering of insulin resistance levels
Restoration of fertility
Treatment of hirsutism or acne
Restoration of regular menstruation, and prevention of endometrial hyperplasia and
endometrial cancer
In each of these areas, there is considerable debate as to the optimal treatment. One of the
major reasons for this is the lack of large-scale clinical trials comparing different treatments.
Smaller trials tend to be less reliable and hence may produce conflicting results.
General interventions that help to reduce weight or insulin resistance can be beneficial for all
these aims, because they address what is believed to be the underlying cause.
As PCOS appears to cause significant emotional distress, appropriate support may be
useful.[73]
Diet[edit]
Where PCOS is associated with overweight or obesity, successful weight loss is the most
effective method of restoring normal ovulation/menstruation, but many women find it very
difficult to achieve and sustain significant weight loss. A scientific review in 2013 found similar
decreases in weight and body composition and improvements in pregnancy rate, menstrual
regularity, ovulation, hyperandrogenism, insulin resistance, lipids, and quality of life to occur
with weight loss independent of diet composition.[74] Still, a low GI diet, in which a significant
part of total carbohydrates are obtained from fruit, vegetables, and whole-grain sources, has
resulted in greater menstrual regularity than a macronutrient-matched healthy diet.[74]
Vitamin D deficiency may play some role in the development of the metabolic syndrome, so
treatment of any such deficiency is indicated.[75][76] However, a systematic review of 2015
found no evidence that vitamin D supplementation reduced or mitigated metabolic and
hormonal dysregulations in PCOS.[77] As of 2012, interventions using dietary supplements to
correct metabolic deficiencies in people with PCOS had been tested in small, uncontrolled
and nonrandomized clinical trials; the resulting data is insufficient to recommend their
use.[78]
Medications[edit]
Medications for PCOS include oral contraceptives and metformin. The oral contraceptives
increase sex hormone binding globulin production, which increases binding of free
testosterone. This reduces the symptoms of hirsutism caused by high testosterone and
regulates return to normal menstrual periods. Metformin is a drug commonly used in type 2
diabetes to reduce insulin resistance, and is used off label (in the UK, US, AU and EU) to treat
insulin resistance seen in PCOS. In many cases, metformin also supports ovarian function and
return to normal ovulation.[19][75][79] Spironolactone can be used for its antiandrogenic
effects, and the topical cream eflornithine can be used to reduce facial hair. A newer insulin
resistance drug class, the thiazolidinediones (glitazones), have shown equivalent efficacy to
metformin, but metformin has a more favorable side effect profile.[80][81] The United
Kingdom's National Institute for Health and Clinical Excellence recommended in 2004 that
women with PCOS and a body mass index above 25 be given metformin when other therapy
has failed to produce results.[82][83] Metformin may not be effective in every type of PCOS,
and therefore there is some disagreement about whether it should be used as a general first
line therapy.[84] The use of statins in the management of underlying metabolic syndrome
remains unclear.[85]
It can be difficult to become pregnant with PCOS because it causes irregular ovulation.
Medications to induce fertility when trying to conceive include the ovulation inducer
clomiphene or pulsatile leuprolide. Metformin improves the efficacy of fertility treatment
when used in combination with clomiphene.[86] Metformin is thought to be safe to use
during pregnancy (pregnancy category B in the US).[87] A review in 2014 concluded that the
use of metformin does not increase the risk of major birth defects in women treated with
metformin during the first trimester.[88]
Infertility[edit]
Main article: Infertility in polycystic ovary syndrome
Not all women with PCOS have difficulty becoming pregnant. For those that do, anovulation
or infrequent ovulation is a common cause. Other factors include changed levels of
gonadotropins, hyperandrogenemia and hyperinsulinemia.[89] Like women without PCOS,
women with PCOS that are ovulating may be infertile due to other causes, such as tubal
blockages due to a history of sexually transmitted diseases.
For overweight, anovulatory women with PCOS, weight loss and diet adjustments, especially
to reduce the intake of simple carbohydrates, are associated with resumption of natural
ovulation.
For those women that after weight loss still are anovulatory or for anovulatory lean women,
then the ovulation-inducing medications clomiphene citrate[75] and FSH are the principal
treatments used to promote ovulation.[19] Previously, the anti-diabetes medication
metformin was recommended treatment for anovulation,[19] but it appears less effective
than clomiphene.[90]
For women not responsive to clomiphene and diet and lifestyle modification, there are
options available including assisted reproductive technology procedures such as controlled
ovarian hyperstimulation with follicle-stimulating hormone (FSH) injections followed by in
vitro fertilisation (IVF).
Though surgery is not commonly performed, the polycystic ovaries can be treated with a
laparoscopic procedure called "ovarian drilling" (puncture of 410 small follicles with
electrocautery, laser, or biopsy needles), which often results in either resumption of
spontaneous ovulations[75] or ovulations after adjuvant treatment with clomiphene or
FSH.[citation needed] (Ovarian wedge resection is no longer used as much due to
complications such as adhesions and the presence of frequently effective medications.) There
are, however, concerns about the long-term effects of ovarian drilling on ovarian
function.[75]
Hirsutism and acne[edit]
For more details on this topic, see Hirsutism.
When appropriate (e.g., in women of child-bearing age who require contraception), a
standard contraceptive pill is frequently effective in reducing hirsutism.[19][75] Progestogens
such as norgestrel and levonorgestrel should be avoided due to their androgenic effects.[75]
Other drugs with anti-androgen effects include flutamide,[91] and spironolactone,[19][75]
which can give some improvement in hirsutism. Metformin can reduce hirsutism, perhaps by
reducing insulin resistance, and is often used if there are other features such as insulin
resistance, diabetes, or obesity that should also benefit from metformin. Eflornithine (Vaniqa)
is a drug that is applied to the skin in cream form, and acts directly on the hair follicles to
inhibit hair growth. It is usually applied to the face.[75] 5-alpha reductase inhibitors (such as
finasteride and dutasteride) may also be used;[92] they work by blocking the conversion of
testosterone to dihydrotestosterone (the latter of which responsible for most hair growth
alterations and androgenic acne).
Although these agents have shown significant efficacy in clinical trials (for oral contraceptives,
in 60100% of individuals[75]), the reduction in hair growth may not be enough to eliminate
the social embarrassment of hirsutism, or the inconvenience of plucking or shaving.
Individuals vary in their response to different therapies. It is usually worth trying other drug
treatments if one does not work, but drug treatments do not work well for all individuals.
Menstrual irregularity[edit]
If fertility is not the primary aim, then menstruation can usually be regulated with a
contraceptive pill.[19][75] The purpose of regulating menstruation, in essence, is for the
woman's convenience, and perhaps her sense of well-being; there is no medical requirement
for regular periods, as long as they occur sufficiently often.
If a regular menstrual cycle is not desired, then therapy for an irregular cycle is not necessarily
required. Most experts say that, if a menstrual bleed occurs at least every three months, then
the endometrium (womb lining) is being shed sufficiently often to prevent an increased risk
of endometrial abnormalities or cancer.[93] If menstruation occurs less often or not at all,
some form of progestogen replacement is recommended.[92] An alternative is oral
progestogen taken at intervals (e.g., every three months) to induce a predictable menstrual
bleeding.[19]
Alternative medicine[edit]
A 2017 review concluded that while both myo-inositol and D-chiro-inositols may regulate
menstrual cycles and improve ovulation, there is a lack of evidence regarding effects on the
probability of pregnancy.[94] A 2012 review, found myo-inositol supplementation appears to
be effective in improving several of the hormonal disturbances of PCOS.[95] A 2011 review
found not enough evidence to conclude any beneficial effect from D-chiro-inositol.[96] There
is insufficient evidence to support the use of acupuncture.[97][98]
Prognosis[edit]
A diagnosis of PCOS suggests an increased risk of the following:
Endometrial hyperplasia and endometrial cancer (cancer of the uterine lining) are possible,
due to overaccumulation of uterine lining, and also lack of progesterone resulting in
prolonged stimulation of uterine cells by estrogen.[19][54][99] It is not clear whether this risk
is directly due to the syndrome or from the associated obesity, hyperinsulinemia, and
hyperandrogenism.[100][101][102]
Insulin resistance/Type II diabetes.[19] A review published in 2010 concluded that women
with PCOS have an elevated prevalence of insulin resistance and type II diabetes, even when
controlling for body mass index (BMI).[54][103] PCOS also makes a woman, particularly if
obese, prone to gestational diabetes.[19]
High blood pressure, in particular if obese or during pregnancy[19]
Depression and anxiety[17][104]
Dyslipidemia[19] disorders of lipid metabolism cholesterol and triglycerides. Women with
PCOS show a decreased removal of atherosclerosis-inducing remnants, seemingly
independent of insulin resistance/Type II diabetes.[105]
Cardiovascular disease,[19][54] with a meta-analysis estimating a 2-fold risk of arterial
disease for women with PCOS relative to women without PCOS, independent of BMI.[106]
Strokes[54]
Weight gain[19]
Miscarriage[107][108]
Sleep apnea, particularly if obesity is present[19]
Non-alcoholic fatty liver disease, again particularly if obesity is present[19]
Acanthosis nigricans (patches of darkened skin under the arms, in the groin area, on the back
of the neck)[54]
Autoimmune thyroiditis[109]
Early diagnosis and treatment may reduce the risk of some of these, such as type 2 diabetes
and heart disease.[19]
The risk of ovarian cancer and breast cancer is not significantly increased overall.[99]
Epidemiology[edit]
The prevalence of PCOS depends on the choice of diagnostic criteria. The World Health
Organization estimates that it affects 116 million women worldwide as of 2010 (3.4% of
women).[110] One community-based prevalence study using the Rotterdam criteria found
that about 18% of women had PCOS, and that 70% of them were previously undiagnosed.[17]
Ultrasonographic findings of polycystic ovaries are found in 825% of normal
women.[111][112][113][114] 14% women on oral contraceptives are found to have polycystic
ovaries.[112] Ovarian cysts are also a common side effect of intrauterine devices (IUDs).[115]
History[edit]
The condition was first described in 1935 by American gynecologists Irving F. Stein, Sr. and
Michael L. Leventhal, from whom its original name of SteinLeventhal syndrome is
taken.[53][54]
The earliest published description of a person with what is now recognized as PCOS was in
1721 in Italy.[18] Cyst-related changes to the ovaries were described in 1844.[18]
Names[edit]
Other names for this syndrome include polycystic ovary disease, functional ovarian
hyperandrogenism, ovarian hyperthecosis, sclerocystic ovary syndrome, and SteinLeventhal
syndrome. The eponymous last option is the original name; it is now used, if at all, only for
the subset of women with all the symptoms of amenorrhea with infertility, hirsutism, and
enlarged polycystic ovaries.[53]
Most common names for this disease derive from a typical finding on medical images, called
a polycystic ovary.[19] A polycystic ovary has an abnormally large number of developing eggs
visible near its surface,[53] looking like many small cysts[116] or a string of pearls.
You might also like
- PcosDocument17 pagesPcosshrabon001No ratings yet
- Name Class Section Subject: Sohon Sen XI B BiologyDocument36 pagesName Class Section Subject: Sohon Sen XI B BiologySohon SenNo ratings yet
- Bab IDocument36 pagesBab IDiga AnaNo ratings yet
- Introduction PCOSDocument14 pagesIntroduction PCOSalex.avenko1030No ratings yet
- Polycystic Ovarian Syndrome (PCOS)Document57 pagesPolycystic Ovarian Syndrome (PCOS)Michelle FynesNo ratings yet
- Polycystic Ovarian SyndromeDocument18 pagesPolycystic Ovarian Syndromeshalika42598No ratings yet
- Bpj12 Polycystic Pages 7-13Document0 pagesBpj12 Polycystic Pages 7-13Kimsha ConcepcionNo ratings yet
- Shruti Review Article IjpsdrDocument17 pagesShruti Review Article IjpsdrPayal DoiphodeNo ratings yet
- Biology Project Vidhya-4Document16 pagesBiology Project Vidhya-4suriya251004No ratings yet
- V Polycystic Ovarysyndrome: EpidemiologyDocument13 pagesV Polycystic Ovarysyndrome: Epidemiologyrolla_hiraNo ratings yet
- Polycystic Ovary SyndromeDocument9 pagesPolycystic Ovary SyndromeMARCUS GREENENo ratings yet
- 014 Gowri Pcos BloodDocument15 pages014 Gowri Pcos BloodPriyanka GandhiNo ratings yet
- MetforminDocument14 pagesMetforminnidyaNo ratings yet
- Polycystic Ovary Syndrome: Under Supervision ofDocument7 pagesPolycystic Ovary Syndrome: Under Supervision ofHend HamedNo ratings yet
- Pcos - Clinical Case DiscussionDocument4 pagesPcos - Clinical Case Discussionreham macadatoNo ratings yet
- The Association of Thyroid-Stimulating Hormone With Testosterone in Sudanese Women With Polycystic Ovarian Syndrome in Khartoum StateDocument15 pagesThe Association of Thyroid-Stimulating Hormone With Testosterone in Sudanese Women With Polycystic Ovarian Syndrome in Khartoum StateMustafa KhandgawiNo ratings yet
- Polycystic Ovary Syndrome (PCOS) - Reproductive and Metabolic Aspects - Sundhed - DKDocument8 pagesPolycystic Ovary Syndrome (PCOS) - Reproductive and Metabolic Aspects - Sundhed - DKPavel BerlinschiNo ratings yet
- Polycystic Ovarian SyndromeDocument19 pagesPolycystic Ovarian SyndromeAndreeaBiancaPuiuNo ratings yet
- Polycystic Ovary Syndrome (PCOS) : Made by Hamza Shawal 60Document29 pagesPolycystic Ovary Syndrome (PCOS) : Made by Hamza Shawal 60Muhammad HamadNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument23 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsPranav Kumar PrabhakarNo ratings yet
- Epidemiology, Phenotype, and Genetics of The Polycystic Ovary Syndrome in AdultsDocument23 pagesEpidemiology, Phenotype, and Genetics of The Polycystic Ovary Syndrome in AdultsApotik ApotekNo ratings yet
- Polycystic Ovary Syndrome (PCOS)Document3 pagesPolycystic Ovary Syndrome (PCOS)Sava1988No ratings yet
- Herbal Drugs For The Treatment of Polycystic Ovary Syndrome (Pcos) and Its ComplicationsDocument9 pagesHerbal Drugs For The Treatment of Polycystic Ovary Syndrome (Pcos) and Its ComplicationsDoc MailNo ratings yet
- PcosDocument9 pagesPcosMonomay HalderNo ratings yet
- PCOS-Journal Article 2Document8 pagesPCOS-Journal Article 2dinarosman02No ratings yet
- Polycystic Ovary SyndromeDocument3 pagesPolycystic Ovary SyndromezeenahNo ratings yet
- 3.review of Literature PDFDocument38 pages3.review of Literature PDFJalajarani AridassNo ratings yet
- Polycystric Ovary Syndrome FilesDocument7 pagesPolycystric Ovary Syndrome FilesElla Jean B. LunzagaNo ratings yet
- Polycystic Ovary Syndrome: Adam BalenDocument14 pagesPolycystic Ovary Syndrome: Adam BalenRufino Guarniz CapristanNo ratings yet
- PCOS Forum: Research in Polycystic Ovary Syndrome Today and TomorrowDocument2 pagesPCOS Forum: Research in Polycystic Ovary Syndrome Today and TomorrowTrisha Marie JimenezNo ratings yet
- Jurnal 1Document8 pagesJurnal 1lomba Panah Dies UnsriNo ratings yet
- Acog 194Document15 pagesAcog 194Marco DiestraNo ratings yet
- Infertility Treatment in Patients With Polycystic Ovary Syndrome Pcos 2165 7491.1000e113Document3 pagesInfertility Treatment in Patients With Polycystic Ovary Syndrome Pcos 2165 7491.1000e113Yogi AnjasmaraNo ratings yet
- Faltu Muskan Pcos FinalDocument5 pagesFaltu Muskan Pcos Finalmbhatti00021No ratings yet
- Polycystic Ovarian Syndrome: Effect of Hormones, Associated Comorbidities and Recent Advances in TherapyDocument12 pagesPolycystic Ovarian Syndrome: Effect of Hormones, Associated Comorbidities and Recent Advances in TherapyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Pcos Fto 8Document11 pagesPcos Fto 8Rafli RizaldyNo ratings yet
- Infertility: SymptomsDocument9 pagesInfertility: SymptomsVyshak KrishnanNo ratings yet
- Plagrism Copy Last ModfiedDocument41 pagesPlagrism Copy Last ModfiedAfzal MuhammadNo ratings yet
- Do I Have PCOS? Understanding the Cause and Reversing Symptoms of Polycystic Ovary Syndrome (PCOS)From EverandDo I Have PCOS? Understanding the Cause and Reversing Symptoms of Polycystic Ovary Syndrome (PCOS)No ratings yet
- Clinical Medicine and ResearchDocument26 pagesClinical Medicine and ResearchDavid SuhendraNo ratings yet
- Polycystic Ovary Syndrome: Dr. Rizwana ShaheenDocument34 pagesPolycystic Ovary Syndrome: Dr. Rizwana ShaheenAmaan Hakim100% (1)
- About The Conference: Benefits of Attending PCOS Conference 2020Document8 pagesAbout The Conference: Benefits of Attending PCOS Conference 2020Roxana FrincuNo ratings yet
- Pcos May Be AutoimmunDocument9 pagesPcos May Be AutoimmunHAVIZ YUADNo ratings yet
- 4 1 36 151Document4 pages4 1 36 151Shelly GargNo ratings yet
- Group 3 - PCOS and SD Written OutputDocument25 pagesGroup 3 - PCOS and SD Written OutputJolly S. SendinNo ratings yet
- Hyperandrogenism: Arrest Occurs When The Granulosa Cells of The Ovaries Normally Begin To ProduceDocument7 pagesHyperandrogenism: Arrest Occurs When The Granulosa Cells of The Ovaries Normally Begin To ProduceNathan JeffreyNo ratings yet
- Jurnal Pcos InternationalDocument5 pagesJurnal Pcos InternationalPuput Anistiya Hariani100% (1)
- Final Presentation 3 MayDocument20 pagesFinal Presentation 3 MayBhavuk MittalNo ratings yet
- PCOSDocument15 pagesPCOSAndyan Adlu PrasetyajiNo ratings yet
- Diagnóstico Del SopDocument13 pagesDiagnóstico Del SopxcarlosfxNo ratings yet
- PCOS Case Report-3Document12 pagesPCOS Case Report-3Joshua Patrick MuljadiNo ratings yet
- H 7 D B6 Rehl WW XUW8Document5 pagesH 7 D B6 Rehl WW XUW8Abdul HaiNo ratings yet
- Chapter 27 The Polycystic Ovary SyndromeDocument4 pagesChapter 27 The Polycystic Ovary Syndromepmj050gpNo ratings yet
- Teede 2010 PDFDocument10 pagesTeede 2010 PDFKe XuNo ratings yet
- Polycystic Ovarian SyndromeDocument32 pagesPolycystic Ovarian SyndromeRaras MayangNo ratings yet
- (1479683X - European Journal of Endocrinology) An Adolescent With Polycystic Ovary SyndromeDocument4 pages(1479683X - European Journal of Endocrinology) An Adolescent With Polycystic Ovary SyndromeAllyssa Mae LaudeNo ratings yet
- JFMK 05 00035 v2Document24 pagesJFMK 05 00035 v2syakira00putriNo ratings yet
- Polycystic Ovary SyndromeDocument5 pagesPolycystic Ovary SyndromeAle W.S.No ratings yet
- The Mechanism of Androgen Actions in PCOS EtiologyDocument12 pagesThe Mechanism of Androgen Actions in PCOS EtiologyXime RdzNo ratings yet
- PCODDocument14 pagesPCODHardikNo ratings yet
- Stairway To HeavenDocument17 pagesStairway To HeavenFrank gNo ratings yet
- Genitive Case Evidence T3Document2 pagesGenitive Case Evidence T3Juan Jose Arcila AlvarezNo ratings yet
- Intersex Special Project (Briones, de Guzman, Balagtas Part)Document29 pagesIntersex Special Project (Briones, de Guzman, Balagtas Part)Eins BalagtasNo ratings yet
- Lab Instructions and Answer Key: Configuring and Troubleshooting A Windows Server® 2008 Network InfrastructureDocument297 pagesLab Instructions and Answer Key: Configuring and Troubleshooting A Windows Server® 2008 Network InfrastructureCarlos Ivan Chavez FuentesNo ratings yet
- Prevalent Moral Issues and Dubious Practices in The WorkplaceDocument1 pagePrevalent Moral Issues and Dubious Practices in The WorkplaceChristian Ü Fer Ibañez100% (1)
- Election Dispute ResolutionDocument3 pagesElection Dispute ResolutionOly VerNo ratings yet
- MATH 121 Assignment DDocument4 pagesMATH 121 Assignment DVladlightNo ratings yet
- Dick Detox - The Ultimate Guide To Beating PornDocument34 pagesDick Detox - The Ultimate Guide To Beating PornAlexander75% (4)
- Word by Word Surah 55 Ar RahmanDocument5 pagesWord by Word Surah 55 Ar RahmanBriliant Dzikrillah littaqwaNo ratings yet
- Sterling Scholar EssaysDocument5 pagesSterling Scholar Essaysapi-255099497No ratings yet
- (123doc - VN) - 41-De-Thi-Thu-Trac-Nhiem-Dai-Hoc-Tieng-Anh-Co-Dap-An PDFDocument248 pages(123doc - VN) - 41-De-Thi-Thu-Trac-Nhiem-Dai-Hoc-Tieng-Anh-Co-Dap-An PDFTrang NguyenNo ratings yet
- Ec8392 Digital Electronics SyllabusDocument2 pagesEc8392 Digital Electronics SyllabusNandha KumarNo ratings yet
- Implementation of Lean Six Sigma PDFDocument8 pagesImplementation of Lean Six Sigma PDFRenato MayorgaNo ratings yet
- Effects of Complementary Medicine On Nausea and VoDocument10 pagesEffects of Complementary Medicine On Nausea and VoDelviNo ratings yet
- Wound Care ThesisDocument4 pagesWound Care Thesiscandacedaiglelafayette100% (2)
- Speak Effortless English Learn Fluent English Grammatically-1Document556 pagesSpeak Effortless English Learn Fluent English Grammatically-1Petre Mihaela Georgiana100% (2)
- On Negative Theology - Hilary Putnam PDFDocument16 pagesOn Negative Theology - Hilary Putnam PDFjusrmyrNo ratings yet
- Kmpfe Sedlmeier RenkewitzDocument27 pagesKmpfe Sedlmeier RenkewitzAntonio Mauricio MorenoNo ratings yet
- Disco Inferno in CM en EbmDocument1 pageDisco Inferno in CM en EbmMichaelNo ratings yet
- Nils Persson 2018 Teacher ResumeDocument3 pagesNils Persson 2018 Teacher Resumeapi-379998608No ratings yet
- Sleep Hygiene: "Sleep Is The Key To Health, Performance, Safety, and Quality of Life"Document8 pagesSleep Hygiene: "Sleep Is The Key To Health, Performance, Safety, and Quality of Life"Child and Family InstituteNo ratings yet
- Strut Tie Model - STMDocument10 pagesStrut Tie Model - STMshish0iitrNo ratings yet
- No Mind-Map: Map / Pic Pedalogical Strategies Skills / HOT / Kbat Teaching Aids Noble Values Thinking SkillsDocument2 pagesNo Mind-Map: Map / Pic Pedalogical Strategies Skills / HOT / Kbat Teaching Aids Noble Values Thinking Skillscyberbat2008No ratings yet
- COBECON - Math ProblemsDocument16 pagesCOBECON - Math ProblemsdocumentsNo ratings yet
- Atpl Theory Formulas PDFDocument64 pagesAtpl Theory Formulas PDFKumar AirplanefreakNo ratings yet
- Letter of Justice Renato S PDFDocument2 pagesLetter of Justice Renato S PDFROSE CAMILLE O DE ASISNo ratings yet
- BIO 22 MODULE 1 - Chemical Basis of LifeDocument14 pagesBIO 22 MODULE 1 - Chemical Basis of LifeBryan DGNo ratings yet
- Schedule of Evangelization - Noel AbanDocument2 pagesSchedule of Evangelization - Noel AbanJerome DesameroNo ratings yet
- Guru Prasad DubeyDocument2 pagesGuru Prasad DubeyGowtham SivamNo ratings yet
- MP Clerks ChrisDocument33 pagesMP Clerks ChrisChrisstael KaranjaNo ratings yet