Professional Documents
Culture Documents
EMAT04C01/EAX - S - 210 2015-2016 S2: Q (1) (15 Marks, Divided Equally)
EMAT04C01/EAX - S - 210 2015-2016 S2: Q (1) (15 Marks, Divided Equally)
Uploaded by
sherif115040 Bue0 ratings0% found this document useful (0 votes)
12 views4 pagesThis document provides a class test with 4 questions for the module "Fundamentals of Materials Science and Engineering". Question 1 involves analyzing stress-strain curve data for a low carbon steel specimen to determine properties like yield stress and ultimate tensile strength. Question 2 calculates deformation of a steel rod under tensile load. Question 3 calculates the number of gold atoms in a wire of given dimensions and material properties. Question 4 requires using a phase diagram to predict the microstructure of an Al-Cu alloy at two different temperatures.
Original Description:
phase diagram
Original Title
q3 answer
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides a class test with 4 questions for the module "Fundamentals of Materials Science and Engineering". Question 1 involves analyzing stress-strain curve data for a low carbon steel specimen to determine properties like yield stress and ultimate tensile strength. Question 2 calculates deformation of a steel rod under tensile load. Question 3 calculates the number of gold atoms in a wire of given dimensions and material properties. Question 4 requires using a phase diagram to predict the microstructure of an Al-Cu alloy at two different temperatures.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
12 views4 pagesEMAT04C01/EAX - S - 210 2015-2016 S2: Q (1) (15 Marks, Divided Equally)
EMAT04C01/EAX - S - 210 2015-2016 S2: Q (1) (15 Marks, Divided Equally)
Uploaded by
sherif115040 BueThis document provides a class test with 4 questions for the module "Fundamentals of Materials Science and Engineering". Question 1 involves analyzing stress-strain curve data for a low carbon steel specimen to determine properties like yield stress and ultimate tensile strength. Question 2 calculates deformation of a steel rod under tensile load. Question 3 calculates the number of gold atoms in a wire of given dimensions and material properties. Question 4 requires using a phase diagram to predict the microstructure of an Al-Cu alloy at two different temperatures.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 4
Programme: Chemical Engineering
Module Title: Fundamentals of Materials Science and Engineering
Module Code: EMAT04C01/EAX_S_210 2015-2016 S2
CLASS TEST: Problems based Questions
Q (1) (15 marks, divided equally)
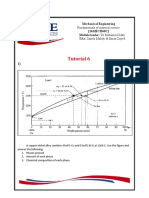
The following data were recorded during a test for a low carbon steel specimen:
Strain .001 .002 .005 .01 .02 .04 .06 .08 .10 .12 .14 .16 .18
mm/mm
Stress MPa 0 210 385 420 476 504 518 525 532 511 483 455 392
a) Describe the test that would have provided this data.
b) Sketch the resulting stress train curve.
c) Define what is meant by yield stress and hence find the 0.2% offset yield strength for this
steel in MPa.
d) What is meant by ultimate tensile strength? And hence find the ultimate tensile strength
UTS for this steel in MPa.
e) Use the appropriate strength to calculate the force in N that a 100 cm2 section made of
this steel can carry.
Q (2) (7.5 marks)
Calculate the type and amount of deformation that is expected for a steel cylindrical rod 380 mm
long and having a diameter of 10.0 mm if subjected to a tensile load of 25000 N. The material
properties are as follows: (Modulus of elasticity 207 GPa, yield strength 450 MPa and tensile
strength 550 MPa).
Stress= 25000N/(102/4) = 318 N/mm2 = 318 MPa
Strain = 318 MPa/ 207 GPa = 1.54 x 10-3
Since stress yield stress
Strain .002
This is elastic strain.
Q (3) (7.5 marks)
A gold wire is 0.70 mm in diameter and 8 cm in length. How many atoms does it contain? The
density of gold is 19.3 g/cm3.
Avogadros number = 6.023 x 1023 atoms/mol
Atomic mass of gold = 196.97 amu/ atom or g per mole
m = mass of Au = V where = 19.3 g/cm3
V = volume of wire = (area)(length)
Thus, m = (19.3 g/cm3 Au)(0.0308 cm3) = 0.5944 g Au
The number of atoms is then calculated as,
= 1.82x1021 atoms Au
Q (4) (10 marks)
Use the given Al-Cu phase diagram to predict the microstructure of an alloy containing 10% copper
at 548 and 300 0C.
At 548 oC
% = 10-4/53-4
%Al = 53-10/53-4
Or
%Al = 34-10/34-4
%eutectic=10-4/34-4
At 300 oC
% = 10-1/53-1
%Al = 53-10/53-1
Or
%Al = 34-10/34-1
%eutectic=10-1/34-1
You might also like
- Sloved QuestionDocument16 pagesSloved QuestionAtul KhodeNo ratings yet
- Problems Solutions On Fracture MechanicsDocument50 pagesProblems Solutions On Fracture MechanicsRajesh N Priya Gopinathan100% (5)
- QB Unit-1,2Document5 pagesQB Unit-1,2Agranshu BhardwajNo ratings yet
- ME 303 Study Set PDFDocument44 pagesME 303 Study Set PDFFajar RumantoNo ratings yet
- MCEN30017 Tutorial 5Document2 pagesMCEN30017 Tutorial 5vsanthanamNo ratings yet
- Assessment 15Document3 pagesAssessment 15ccharp123No ratings yet
- Homework 6-SolutionsDocument6 pagesHomework 6-Solutionsmirna sariNo ratings yet
- HW8 ch07Document7 pagesHW8 ch07Jeremy HernandezNo ratings yet
- MCEN30017 Tutorial 3Document2 pagesMCEN30017 Tutorial 3vsanthanamNo ratings yet
- Assignment 2 Fluctuating LoadDocument3 pagesAssignment 2 Fluctuating Loadabhishek chaurasiyaNo ratings yet
- Screenshot 2024-03-29 at 3.16.52 PMDocument22 pagesScreenshot 2024-03-29 at 3.16.52 PMabdoshetayaNo ratings yet
- Re Exam Paper Nov08, Smt211tDocument5 pagesRe Exam Paper Nov08, Smt211tPortia ShilengeNo ratings yet
- EMM7241-Advanced Machine Design Examination June 2016Document8 pagesEMM7241-Advanced Machine Design Examination June 2016Charles OndiekiNo ratings yet
- Applied Mechanics (ME 14.101) Tutorial Sheet-6: (Tension, Compression & Shear)Document1 pageApplied Mechanics (ME 14.101) Tutorial Sheet-6: (Tension, Compression & Shear)atulkumargaur26No ratings yet
- Bai Tap - Vat Lieu Hoc - Phan Tinh Chat - NTS - v1Document26 pagesBai Tap - Vat Lieu Hoc - Phan Tinh Chat - NTS - v1Hoàng BeeNo ratings yet
- Dme Question BankDocument4 pagesDme Question BankRavi Patil100% (1)
- HW Ch7 1Document12 pagesHW Ch7 1Chenhg ChengNo ratings yet
- M.D-I Final Tutorial and AssignmentDocument16 pagesM.D-I Final Tutorial and AssignmentnageshNo ratings yet
- Assignment 5 Solutions PDFDocument6 pagesAssignment 5 Solutions PDFMuhammad IdrisNo ratings yet
- MOM Assignment IDocument3 pagesMOM Assignment IAll_regNo ratings yet
- Sheet#8Document7 pagesSheet#8oreaby05No ratings yet
- Machine Design I - ME501 - Sup 011Document2 pagesMachine Design I - ME501 - Sup 011Saurav JainNo ratings yet
- All TutorialsDocument37 pagesAll TutorialsSimphiweNo ratings yet
- CH 07Document12 pagesCH 07Madeline NgoNo ratings yet
- 4363 111 Machine Design IDocument6 pages4363 111 Machine Design Iyogesh_b_k100% (2)
- 3094 HW ECBDocument2 pages3094 HW ECBradarskiNo ratings yet
- DMEDocument4 pagesDMEManivannanNo ratings yet
- 12ME5DCDM2Document3 pages12ME5DCDM2Abhyudaya SinghNo ratings yet
- Mse SW2BDocument25 pagesMse SW2BmarkkkkkNo ratings yet
- 09.material Lab Report (Lacx) IVDocument14 pages09.material Lab Report (Lacx) IVJack JongNo ratings yet
- Assign I MD IDocument3 pagesAssign I MD IAtharva KNo ratings yet
- Snist Dom Previous PaperDocument9 pagesSnist Dom Previous PaperKapil Siddhant DevulapalliNo ratings yet
- ME 352 - All Problem Class - 14-18 BatchDocument125 pagesME 352 - All Problem Class - 14-18 BatchEntertainment GamingNo ratings yet
- Mech Btech PapersDocument7 pagesMech Btech PapersThanatos XNo ratings yet
- Em MiDocument2 pagesEm MisathishskymechNo ratings yet
- Assignment 1 PDFDocument2 pagesAssignment 1 PDFvishal kabdwalNo ratings yet
- DRS1513 Assignment II2324Document4 pagesDRS1513 Assignment II2324Hazeeqah EzatyNo ratings yet
- Mem-402-Mos Final QB-2015Document9 pagesMem-402-Mos Final QB-2015Rohit DiwakarNo ratings yet
- Problems 4012 PDFDocument8 pagesProblems 4012 PDFjonthemesNo ratings yet
- Advanced Strength of Materials Paper ModelDocument3 pagesAdvanced Strength of Materials Paper ModeldurgaraokamireddyNo ratings yet
- Homework3 s450 1Document2 pagesHomework3 s450 1cassandra_wittenNo ratings yet
- Material ScienceDocument4 pagesMaterial Sciencediyana8894No ratings yet
- R7310305-Design of Machine Members-I2Document4 pagesR7310305-Design of Machine Members-I2slv_prasaad100% (1)
- Department of Mechanical Engineering MD - II: Tutorial Sheet For Fatigue Consideration in Design (Session 2020-21)Document2 pagesDepartment of Mechanical Engineering MD - II: Tutorial Sheet For Fatigue Consideration in Design (Session 2020-21)djadja nakamayaNo ratings yet
- Tutorial Chapter 6Document5 pagesTutorial Chapter 6SYAFIQAH ISMAILNo ratings yet
- Nadia Hamzawy Mohamed - Nadia PaperDocument7 pagesNadia Hamzawy Mohamed - Nadia PaperchatgptuseregyptNo ratings yet
- DMM 1 Assignment Questions FinalDocument2 pagesDMM 1 Assignment Questions FinalSrimanthula SrikanthNo ratings yet
- Design of Machine ElementDocument11 pagesDesign of Machine ElementVenkat RajaNo ratings yet
- T I TR NG Đ NGDocument40 pagesT I TR NG Đ NGVũ Hải HưngNo ratings yet
- Advanced Manufacturing ScienceDocument3 pagesAdvanced Manufacturing Sciencemukesh3021No ratings yet
- Suggested Problems (L12-15 Failure)Document2 pagesSuggested Problems (L12-15 Failure)Isaac MitchellNo ratings yet
- MOM2Tutorial 10-1Document3 pagesMOM2Tutorial 10-1Ishola mujeebNo ratings yet
- Dmm1 Mar2006Document8 pagesDmm1 Mar2006prk74No ratings yet
- Cyclic Plasticity of Engineering Materials: Experiments and ModelsFrom EverandCyclic Plasticity of Engineering Materials: Experiments and ModelsNo ratings yet
- Dynamic Damage and FragmentationFrom EverandDynamic Damage and FragmentationDavid Edward LambertNo ratings yet
- Non-Destructive Evaluation of Corrosion and Corrosion-assisted CrackingFrom EverandNon-Destructive Evaluation of Corrosion and Corrosion-assisted CrackingRaman SinghNo ratings yet
- Structural Steel Design to Eurocode 3 and AISC SpecificationsFrom EverandStructural Steel Design to Eurocode 3 and AISC SpecificationsNo ratings yet
- Lab AssignmentDocument11 pagesLab Assignmentsherif115040 BueNo ratings yet
- WS02 Welding Oxyfuel - MIGDocument2 pagesWS02 Welding Oxyfuel - MIGsherif115040 BueNo ratings yet
- Workshop 1 Welding : Arc Welding & Spot WeldingDocument2 pagesWorkshop 1 Welding : Arc Welding & Spot Weldingsherif115040 BueNo ratings yet
- WS03 Machining CNC - Lathe - Process SheetDocument2 pagesWS03 Machining CNC - Lathe - Process Sheetsherif115040 BueNo ratings yet
- Tutorial 6: Mechanical Engineering (16Mech60C) Module Leader: DR - Mohamed Zaky Tas: Sameh Mahdy & Eman ZayedDocument3 pagesTutorial 6: Mechanical Engineering (16Mech60C) Module Leader: DR - Mohamed Zaky Tas: Sameh Mahdy & Eman Zayedsherif115040 BueNo ratings yet