Professional Documents
Culture Documents
K. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de Spiegeleer
K. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de Spiegeleer
Uploaded by
Ina LabokCopyright:
Available Formats
You might also like
- A Amylase DirectDocument1 pageA Amylase DirectRisqon Anjahiranda Adiputra0% (1)
- Carbohydrate in Honey (AOAC-977.20)Document1 pageCarbohydrate in Honey (AOAC-977.20)Khalid100% (1)
- ThanatiaDocument670 pagesThanatiaJoão ClaudioNo ratings yet
- USP 42 Abacavir SulfateDocument4 pagesUSP 42 Abacavir SulfateAlejandro RestrepoNo ratings yet
- Si 02407Document4 pagesSi 02407AmineJaouedNo ratings yet
- PS01666 PDFDocument5 pagesPS01666 PDFEvelinaNo ratings yet
- USP-NF Abacavir SulfateDocument4 pagesUSP-NF Abacavir Sulfatemustafa bNo ratings yet
- Improved Sensitivity and Dynamic Range Using The Clarus SQ8 GC - MS System For EPA Method 8270DDocument6 pagesImproved Sensitivity and Dynamic Range Using The Clarus SQ8 GC - MS System For EPA Method 8270DIsaac AlvesNo ratings yet
- USP Monographs - Clavulanate PotassiumDocument6 pagesUSP Monographs - Clavulanate PotassiumMohamad IsmailNo ratings yet
- CVUA Karlsruhe Method Based On UHPLC-APCI-MS - MSDocument7 pagesCVUA Karlsruhe Method Based On UHPLC-APCI-MS - MSDip DuttaNo ratings yet
- Structural Comparability Assessment of Innovator and Biosimilar Rituximab Using The Biopharmaceutical System Solution With UNIFIDocument11 pagesStructural Comparability Assessment of Innovator and Biosimilar Rituximab Using The Biopharmaceutical System Solution With UNIFIMiyyada AichaouiNo ratings yet
- Application Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerDocument4 pagesApplication Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerPrianurraufikachmadNo ratings yet
- AN 21092 LC CX 1 PH Gradient Buffer Kit Mab AN21092 ENDocument8 pagesAN 21092 LC CX 1 PH Gradient Buffer Kit Mab AN21092 ENberkahNo ratings yet
- Ab 190Document4 pagesAb 190swapon kumar shillNo ratings yet
- Si 02361Document3 pagesSi 02361AmineJaouedNo ratings yet
- AN256 LC VitaminB12 Beverages 01sep2010 LPN2574Document11 pagesAN256 LC VitaminB12 Beverages 01sep2010 LPN2574RispaAndriyaniNo ratings yet
- AN 360 Rapid Determination of Azo Dyes in Textiles Using Dionex ASE and UltiMate 3000 HPLC SystemsDocument6 pagesAN 360 Rapid Determination of Azo Dyes in Textiles Using Dionex ASE and UltiMate 3000 HPLC SystemsDewi WulandhariNo ratings yet
- 0011 Food and Beverage Campaign Vitamins MMDocument4 pages0011 Food and Beverage Campaign Vitamins MMAdrianaNo ratings yet
- Amlodipine, Valsartan, and Hydrochlorothiazide Tablets USPDocument6 pagesAmlodipine, Valsartan, and Hydrochlorothiazide Tablets USPAyoub MglNo ratings yet
- J Institute Brewing - July August 1968 - Morgan - DETERMINATION OF HOP ALPHA ACIDS BY SEMI AUTOMATIC CONDUCTOMETRICDocument4 pagesJ Institute Brewing - July August 1968 - Morgan - DETERMINATION OF HOP ALPHA ACIDS BY SEMI AUTOMATIC CONDUCTOMETRICNguyễn Ngọc NamNo ratings yet
- Abacavir SulfateDocument2 pagesAbacavir SulfateNguyen Van ThaoNo ratings yet
- AN52072 - Pesticide-Analysis - Fast-GC-MSMS - TSQ QuantumDocument8 pagesAN52072 - Pesticide-Analysis - Fast-GC-MSMS - TSQ QuantumАлександрNo ratings yet
- WatersolubleVitamins UPLC-TQDDocument6 pagesWatersolubleVitamins UPLC-TQDLuisNo ratings yet
- CVS - Cyclic Voltammetric Stripping With Metrohm: CVS in The Laboratory CVS Atline Systems CVS Online SystemsDocument16 pagesCVS - Cyclic Voltammetric Stripping With Metrohm: CVS in The Laboratory CVS Atline Systems CVS Online SystemsEduardo LimaNo ratings yet
- Validación Métodos AnaliticosDocument12 pagesValidación Métodos AnaliticosJuan Gabriel GualterosNo ratings yet
- APP Analysis of Quaternary Ammonium Compounds in Milk by LC TOF 012225 01Document6 pagesAPP Analysis of Quaternary Ammonium Compounds in Milk by LC TOF 012225 01skype2121No ratings yet
- Boldenone UndDocument2 pagesBoldenone UndAhmedNo ratings yet
- Abacavir SulfateDocument2 pagesAbacavir SulfateDược K45 Nguyễn Thanh NgânNo ratings yet
- An - 01 00335 enDocument2 pagesAn - 01 00335 enPushpendu GauravNo ratings yet
- FY20-106-DPA-S - LC-ESI-HRMS Method For Detection of Nitrosamine Impurities in Metformin - For Sharing FINAL 6-3-20Document11 pagesFY20-106-DPA-S - LC-ESI-HRMS Method For Detection of Nitrosamine Impurities in Metformin - For Sharing FINAL 6-3-20Erdem SavasNo ratings yet
- Methodsx: Jens Søndergaard, Gert Asmund, Martin M. LarsenDocument8 pagesMethodsx: Jens Søndergaard, Gert Asmund, Martin M. LarsenThan HauNo ratings yet
- Fast Analysis of Coffee Bean Extracts HPLC ColumnDocument3 pagesFast Analysis of Coffee Bean Extracts HPLC ColumndmaingalNo ratings yet
- Abacavir SulfateDocument2 pagesAbacavir Sulfatefatima.batoolNo ratings yet
- LowLevelVOCs - VersaHEAD SPACEDocument37 pagesLowLevelVOCs - VersaHEAD SPACEomar velasquezNo ratings yet
- USP-NF Cefotaxime SodiumDocument6 pagesUSP-NF Cefotaxime SodiumCongluanNo ratings yet
- 70 - RJPT - 12 - 4 - 2019 - Dacetabine PaperDocument10 pages70 - RJPT - 12 - 4 - 2019 - Dacetabine PaperSureshreddy YelampalliNo ratings yet
- An 65200 LC Ms Phenylephrine Hydrochloride Qa QC An65200 enDocument6 pagesAn 65200 LC Ms Phenylephrine Hydrochloride Qa QC An65200 enMôùhamed BnsNo ratings yet
- Paracetamol TabletsDocument5 pagesParacetamol Tabletslucychamber1990No ratings yet
- GC - Shimadzu GCMS Terpene ApplicationDocument9 pagesGC - Shimadzu GCMS Terpene ApplicationpilarNo ratings yet
- 0015 Pharmaceutical Impurity Profiling Esomeprazole Magnesium MKDocument1 page0015 Pharmaceutical Impurity Profiling Esomeprazole Magnesium MKCECILIA PNo ratings yet
- Absorbance Ratio Spectrophotometric Method For The Simultaneous Estimation of Dexamethasone Sodium Phosphate and Atropine Sulphate in Eye DropDocument5 pagesAbsorbance Ratio Spectrophotometric Method For The Simultaneous Estimation of Dexamethasone Sodium Phosphate and Atropine Sulphate in Eye DropPutri AgustinaNo ratings yet
- USP Betamethasone MMDocument5 pagesUSP Betamethasone MMThai HocNo ratings yet
- Resveratrol Dietary SupsupplementsDocument1 pageResveratrol Dietary SupsupplementsJuan Carlos BeristainNo ratings yet
- Development of A Reversed Phase - HPLC Method For Determination of Meloxicam in Tablet Formulation and Human SerumDocument11 pagesDevelopment of A Reversed Phase - HPLC Method For Determination of Meloxicam in Tablet Formulation and Human SerumfenypristianaNo ratings yet
- AU150 V27 releasedJC121305Document6 pagesAU150 V27 releasedJC121305juakfuenmayorNo ratings yet
- 217 - 036 - 02 - Streptomycin Sulfate USPDocument6 pages217 - 036 - 02 - Streptomycin Sulfate USPNataliaNo ratings yet
- An 175 Food Packaging Shs-Gc-TofDocument7 pagesAn 175 Food Packaging Shs-Gc-Tofabdlkdr3460No ratings yet
- Quantitative Amino AcidsDocument4 pagesQuantitative Amino Acidsait el hocine tarekNo ratings yet
- 10660-Article Text-36214-1-10-20160308Document6 pages10660-Article Text-36214-1-10-20160308Rifki SaufiNo ratings yet
- Phenoxyethanol-Cosmetics en PDFDocument2 pagesPhenoxyethanol-Cosmetics en PDFHiềnNo ratings yet
- Practical 11 - WPMDocument7 pagesPractical 11 - WPM门门No ratings yet
- Abiraterone Acetate Tablets - USPDocument3 pagesAbiraterone Acetate Tablets - USPДарія ОсадчаNo ratings yet
- 19-Design of An Amperometric Biosensor For The Determination ofDocument4 pages19-Design of An Amperometric Biosensor For The Determination ofwardaninurindahNo ratings yet
- Review Jurnal 1 - TrehaloseDocument3 pagesReview Jurnal 1 - TrehaloseMuhammad Saepul AnwarNo ratings yet
- Knauer - Food PreservativesDocument1 pageKnauer - Food PreservativesMaryanta HadisudiroNo ratings yet
- Rapid Analysis of Genotoxic Nitrosamines by HPLC MS MSDocument3 pagesRapid Analysis of Genotoxic Nitrosamines by HPLC MS MSMarcio SchiavelliNo ratings yet
- Exp 3 DNSDocument10 pagesExp 3 DNSEmelya NatraNo ratings yet
- GPC System OperationDocument49 pagesGPC System OperationFahmi Januar AnugrahNo ratings yet
- Hydrolytic Degradation Profiling of Ezetimibe by HPLC MethodDocument6 pagesHydrolytic Degradation Profiling of Ezetimibe by HPLC MethodTJPRC PublicationsNo ratings yet
- Analisis Dan Pemurnian Protein-FinalDocument44 pagesAnalisis Dan Pemurnian Protein-FinalEndah Sekar PalupiNo ratings yet
- Electrochemistry of Dihydroxybenzene Compounds: Carbon Based Electrodes and Their Uses in Synthesis and SensorsFrom EverandElectrochemistry of Dihydroxybenzene Compounds: Carbon Based Electrodes and Their Uses in Synthesis and SensorsNo ratings yet
- Rumah Sakit Ibu Dan Anak Dedari: Section: Farmasi Periode: Feb 01, 2017 S/D Feb 28, 2017 No Opname: 1702-OPN-000212Document12 pagesRumah Sakit Ibu Dan Anak Dedari: Section: Farmasi Periode: Feb 01, 2017 S/D Feb 28, 2017 No Opname: 1702-OPN-000212Ina LabokNo ratings yet
- Formularium Dedari DraftDocument663 pagesFormularium Dedari DraftIna LabokNo ratings yet
- Book 1Document16 pagesBook 1Ina LabokNo ratings yet
- Label InjDocument1 pageLabel InjIna LabokNo ratings yet
- GlobalCOD Method 2000 2015Document85 pagesGlobalCOD Method 2000 2015suma riadiNo ratings yet
- CHEMEXCILDocument28 pagesCHEMEXCILAmal JerryNo ratings yet
- D 12332Document108 pagesD 12332jesus_hfNo ratings yet
- Characteristics of g3 - An Alternative To SF6Document5 pagesCharacteristics of g3 - An Alternative To SF6Abdul MoizNo ratings yet
- KaizenDocument8 pagesKaizenGigih SetijawanNo ratings yet
- M1 - 02 Nature and Scope of BusinessDocument41 pagesM1 - 02 Nature and Scope of BusinessDiptendu RoyNo ratings yet
- DT81 Data Logger DatasheetDocument2 pagesDT81 Data Logger DatasheetDuška JarčevićNo ratings yet
- TCS AIP Take Home AssignmentDocument16 pagesTCS AIP Take Home AssignmentShruthiSAthreyaNo ratings yet
- ORTableAccessory Brochure INT en 05 NonUSDocument144 pagesORTableAccessory Brochure INT en 05 NonUSHarianto HarNo ratings yet
- Accounting All ChaptersDocument155 pagesAccounting All ChaptersHasnain Ahmad KhanNo ratings yet
- Bangladesh University of Professionals (Bup)Document17 pagesBangladesh University of Professionals (Bup)oldschoolNo ratings yet
- Distribution Channel of AMULDocument13 pagesDistribution Channel of AMULMeet JivaniNo ratings yet
- Scala AccesoriesDocument27 pagesScala AccesoriesZiggy BussyNo ratings yet
- Analytical Chem Chap11Document18 pagesAnalytical Chem Chap11Nicole Ann PedriñaNo ratings yet
- ClaytonDocument1 pageClaytonapi-3831340No ratings yet
- General Properties of Engineering MaterialsDocument9 pagesGeneral Properties of Engineering MaterialsRoderick VillanuevaNo ratings yet
- DK Pocket Genius - HorsesDocument158 pagesDK Pocket Genius - HorsesAnamaria RaduNo ratings yet
- Book Reviews: Supplemental Damping and Seismic Isolation by C. ChristopoulosDocument1 pageBook Reviews: Supplemental Damping and Seismic Isolation by C. ChristopoulosLuca AcetoNo ratings yet
- Final Project On Employee EngagementDocument97 pagesFinal Project On Employee EngagementSanju DurgapalNo ratings yet
- Friction Worksheet: Part A Questions - Multiple ChoiceDocument2 pagesFriction Worksheet: Part A Questions - Multiple ChoiceAmos JosephatNo ratings yet
- Raga Purna Pancama: Analysis ModesDocument1 pageRaga Purna Pancama: Analysis ModesKermitNo ratings yet
- RT00Document16 pagesRT00Jeganeswaran17% (6)
- Coast Artillery Journal - Oct 1946Document84 pagesCoast Artillery Journal - Oct 1946CAP History LibraryNo ratings yet
- Test Bank For Anatomy and Physiology 10th Edition by PattonDocument32 pagesTest Bank For Anatomy and Physiology 10th Edition by PattonCassandraDuncanmcytd100% (42)
- Submarine Diesel EngineDocument106 pagesSubmarine Diesel EngineDharma Gita Surya PrayogaNo ratings yet
- Extracting DnaDocument6 pagesExtracting DnaIya AlabastroNo ratings yet
- 1 IntroductionDocument28 pages1 Introductionkevnnicl921216No ratings yet
- Final DKSSKDocument79 pagesFinal DKSSKSarita Latthe100% (2)
- Economists' Corner: Weighing The Procompetitive and Anticompetitive Effects of RPM Under The Rule of ReasonDocument3 pagesEconomists' Corner: Weighing The Procompetitive and Anticompetitive Effects of RPM Under The Rule of ReasonHarsh GandhiNo ratings yet
K. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de Spiegeleer
K. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de Spiegeleer
Uploaded by
Ina LabokOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
K. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de Spiegeleer
K. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de Spiegeleer
Uploaded by
Ina LabokCopyright:
Available Formats
DruQuaR
Qbd analytical development of calcitonin bioadhesive formulation.
K. Geenens1, N. Clottens1, V. Vergote1, D. Coucke2, E. Mehuys2 and B. De Spiegeleer1*
1Drug Quality & Registration (DruQuaR) group, Faculty Pharmaceutical Sciences, and 2Pharmaceutical Technology, Ghent University, Belgium.
*Corresponding author: bart.despiegeleer@ugent.be (O.Ref.: 2009 – 244c)
INTRODUCTION
Calcitonin is a 32-amino acid polypeptide hormone ubiquitous in humans and animals. It acts i.a. to reduce blood calcium (Ca2+), opposing the effects of
parathyroid hormone (PTH). Both human (hu) and salmon (sa) calcitonins are clinically effective and currently approved as active pharmaceutical
ingredients (APIs).
A new bioadhesive nasal formulation is currently under development, which contains low dose sa-calcitonin in polymeric excipients (carbomer and starch).
The analytical development is confronted with several challenges: the low dose of the peptide in the formulation, its inherent instability, the polymeric matrix
interacting with the peptide influencing sample preparation and its undefined degradation impurity profile in this formulation.
The aim of this investigation was to develop a suitable method to determine the concentration of sa-calcitonin in this formulation and to establish its
degradation profile, using experimental designs which will also give us mechanistic information.
EXPERIMENTAL
Sample preparation development (spiked plcaebo) :
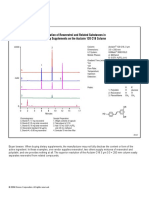
1. Placket Burman design (PBD): Figure 1: 3D visualisation of the Onion design
HPβCD (0-10 mg/ml), temperature (50-70°C), incubation time (1-2 h),
number of steps (1-2), mixing velocity (300-600 rpm), concentration FA (1-5 % V/V).
=> FA and temperature have a significant influence (p < 0.05), BUT: too low recoveries
2. Onion design (with change FA to TFA):
Temperature (20-70°C), incubation time (30-90 min), concentration TFA (0.1-0.75 % V/V)
=> Concentration TFA significant (p < 0.05), others regional (p < 0.10)
3. Verification robustness with PBD:
Temperature (50-60°C), incubation time (40-50 min),concentration TFA (0.45-0.65 % V/V)
=> Incubation temperature significant influence hence: range ± 2°C
HPLC-UV :
Variables optimised with PBD: acid (TFA vs FA), column temperature, ACN gradient slope
HPLC-MS analysis for determination of related substances :
Evaluation using ESI-iontrap MS (SIM), with selectivity optimised gradient FA
RESULTS AND DISCUSSION
The final HPLC conditions for the assay are listed in Table 1, while a typical chromatogram of the selecivity study for related compounds is given in Figure 2
Table 1: HPLC method for sa-calcitonin assay Figure 2: HPLC-MS for related substances
Parameter Specification
Column Everest C18 (300 Å), 250 × 4.6 mm, 5 µm (+ guard column)
1 2 3 5
Column temperature 40°C
- Solvent A: 0.1% V/V TFA in water.
Mobile phase
- Solvent B: 0.085% V/V TFA in acetonitrille.
Solvent constitution with TFA
Time (min)
%A %B
0 73 27
Gradiënt programme
20 63 37
21 73 27
40 73 27
Flow 1 ml/min
Injection volume 100 µl
Detection UV at 195 nm
Table 2: Peak identification corresponding to Figure 2
Peak identification RRT m/Z
1: cys-ser hydrolysis product 0.5 862.81-863.81
4
2: calcitonin trisulfide 0.9 866.30-876-30
3: sa-calcitonin 1.00 859.37-860-49
4: epimer 1.25 859.37-860-49
5: acetylated calcitonin 1.50 868.81-869.81
CONCLUSIONS
Optimised sample preparation obtained with Plackett-Burman and Onion designs: 0.45% V/V TFA at 60°C during 40 minutes
accuracy (recovery) = 97.37%, precision = 3.34%
HPLC-UV assay characterisation .
A selective method for specified related substances profiling for nasal powder was established, using HPLC-ESI/MS (SIM).
You might also like
- A Amylase DirectDocument1 pageA Amylase DirectRisqon Anjahiranda Adiputra0% (1)
- Carbohydrate in Honey (AOAC-977.20)Document1 pageCarbohydrate in Honey (AOAC-977.20)Khalid100% (1)
- ThanatiaDocument670 pagesThanatiaJoão ClaudioNo ratings yet
- USP 42 Abacavir SulfateDocument4 pagesUSP 42 Abacavir SulfateAlejandro RestrepoNo ratings yet
- Si 02407Document4 pagesSi 02407AmineJaouedNo ratings yet
- PS01666 PDFDocument5 pagesPS01666 PDFEvelinaNo ratings yet
- USP-NF Abacavir SulfateDocument4 pagesUSP-NF Abacavir Sulfatemustafa bNo ratings yet
- Improved Sensitivity and Dynamic Range Using The Clarus SQ8 GC - MS System For EPA Method 8270DDocument6 pagesImproved Sensitivity and Dynamic Range Using The Clarus SQ8 GC - MS System For EPA Method 8270DIsaac AlvesNo ratings yet
- USP Monographs - Clavulanate PotassiumDocument6 pagesUSP Monographs - Clavulanate PotassiumMohamad IsmailNo ratings yet
- CVUA Karlsruhe Method Based On UHPLC-APCI-MS - MSDocument7 pagesCVUA Karlsruhe Method Based On UHPLC-APCI-MS - MSDip DuttaNo ratings yet
- Structural Comparability Assessment of Innovator and Biosimilar Rituximab Using The Biopharmaceutical System Solution With UNIFIDocument11 pagesStructural Comparability Assessment of Innovator and Biosimilar Rituximab Using The Biopharmaceutical System Solution With UNIFIMiyyada AichaouiNo ratings yet
- Application Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerDocument4 pagesApplication Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerPrianurraufikachmadNo ratings yet
- AN 21092 LC CX 1 PH Gradient Buffer Kit Mab AN21092 ENDocument8 pagesAN 21092 LC CX 1 PH Gradient Buffer Kit Mab AN21092 ENberkahNo ratings yet
- Ab 190Document4 pagesAb 190swapon kumar shillNo ratings yet
- Si 02361Document3 pagesSi 02361AmineJaouedNo ratings yet
- AN256 LC VitaminB12 Beverages 01sep2010 LPN2574Document11 pagesAN256 LC VitaminB12 Beverages 01sep2010 LPN2574RispaAndriyaniNo ratings yet
- AN 360 Rapid Determination of Azo Dyes in Textiles Using Dionex ASE and UltiMate 3000 HPLC SystemsDocument6 pagesAN 360 Rapid Determination of Azo Dyes in Textiles Using Dionex ASE and UltiMate 3000 HPLC SystemsDewi WulandhariNo ratings yet
- 0011 Food and Beverage Campaign Vitamins MMDocument4 pages0011 Food and Beverage Campaign Vitamins MMAdrianaNo ratings yet
- Amlodipine, Valsartan, and Hydrochlorothiazide Tablets USPDocument6 pagesAmlodipine, Valsartan, and Hydrochlorothiazide Tablets USPAyoub MglNo ratings yet
- J Institute Brewing - July August 1968 - Morgan - DETERMINATION OF HOP ALPHA ACIDS BY SEMI AUTOMATIC CONDUCTOMETRICDocument4 pagesJ Institute Brewing - July August 1968 - Morgan - DETERMINATION OF HOP ALPHA ACIDS BY SEMI AUTOMATIC CONDUCTOMETRICNguyễn Ngọc NamNo ratings yet
- Abacavir SulfateDocument2 pagesAbacavir SulfateNguyen Van ThaoNo ratings yet
- AN52072 - Pesticide-Analysis - Fast-GC-MSMS - TSQ QuantumDocument8 pagesAN52072 - Pesticide-Analysis - Fast-GC-MSMS - TSQ QuantumАлександрNo ratings yet
- WatersolubleVitamins UPLC-TQDDocument6 pagesWatersolubleVitamins UPLC-TQDLuisNo ratings yet
- CVS - Cyclic Voltammetric Stripping With Metrohm: CVS in The Laboratory CVS Atline Systems CVS Online SystemsDocument16 pagesCVS - Cyclic Voltammetric Stripping With Metrohm: CVS in The Laboratory CVS Atline Systems CVS Online SystemsEduardo LimaNo ratings yet
- Validación Métodos AnaliticosDocument12 pagesValidación Métodos AnaliticosJuan Gabriel GualterosNo ratings yet
- APP Analysis of Quaternary Ammonium Compounds in Milk by LC TOF 012225 01Document6 pagesAPP Analysis of Quaternary Ammonium Compounds in Milk by LC TOF 012225 01skype2121No ratings yet
- Boldenone UndDocument2 pagesBoldenone UndAhmedNo ratings yet
- Abacavir SulfateDocument2 pagesAbacavir SulfateDược K45 Nguyễn Thanh NgânNo ratings yet
- An - 01 00335 enDocument2 pagesAn - 01 00335 enPushpendu GauravNo ratings yet
- FY20-106-DPA-S - LC-ESI-HRMS Method For Detection of Nitrosamine Impurities in Metformin - For Sharing FINAL 6-3-20Document11 pagesFY20-106-DPA-S - LC-ESI-HRMS Method For Detection of Nitrosamine Impurities in Metformin - For Sharing FINAL 6-3-20Erdem SavasNo ratings yet
- Methodsx: Jens Søndergaard, Gert Asmund, Martin M. LarsenDocument8 pagesMethodsx: Jens Søndergaard, Gert Asmund, Martin M. LarsenThan HauNo ratings yet
- Fast Analysis of Coffee Bean Extracts HPLC ColumnDocument3 pagesFast Analysis of Coffee Bean Extracts HPLC ColumndmaingalNo ratings yet
- Abacavir SulfateDocument2 pagesAbacavir Sulfatefatima.batoolNo ratings yet
- LowLevelVOCs - VersaHEAD SPACEDocument37 pagesLowLevelVOCs - VersaHEAD SPACEomar velasquezNo ratings yet
- USP-NF Cefotaxime SodiumDocument6 pagesUSP-NF Cefotaxime SodiumCongluanNo ratings yet
- 70 - RJPT - 12 - 4 - 2019 - Dacetabine PaperDocument10 pages70 - RJPT - 12 - 4 - 2019 - Dacetabine PaperSureshreddy YelampalliNo ratings yet
- An 65200 LC Ms Phenylephrine Hydrochloride Qa QC An65200 enDocument6 pagesAn 65200 LC Ms Phenylephrine Hydrochloride Qa QC An65200 enMôùhamed BnsNo ratings yet
- Paracetamol TabletsDocument5 pagesParacetamol Tabletslucychamber1990No ratings yet
- GC - Shimadzu GCMS Terpene ApplicationDocument9 pagesGC - Shimadzu GCMS Terpene ApplicationpilarNo ratings yet
- 0015 Pharmaceutical Impurity Profiling Esomeprazole Magnesium MKDocument1 page0015 Pharmaceutical Impurity Profiling Esomeprazole Magnesium MKCECILIA PNo ratings yet
- Absorbance Ratio Spectrophotometric Method For The Simultaneous Estimation of Dexamethasone Sodium Phosphate and Atropine Sulphate in Eye DropDocument5 pagesAbsorbance Ratio Spectrophotometric Method For The Simultaneous Estimation of Dexamethasone Sodium Phosphate and Atropine Sulphate in Eye DropPutri AgustinaNo ratings yet
- USP Betamethasone MMDocument5 pagesUSP Betamethasone MMThai HocNo ratings yet
- Resveratrol Dietary SupsupplementsDocument1 pageResveratrol Dietary SupsupplementsJuan Carlos BeristainNo ratings yet
- Development of A Reversed Phase - HPLC Method For Determination of Meloxicam in Tablet Formulation and Human SerumDocument11 pagesDevelopment of A Reversed Phase - HPLC Method For Determination of Meloxicam in Tablet Formulation and Human SerumfenypristianaNo ratings yet
- AU150 V27 releasedJC121305Document6 pagesAU150 V27 releasedJC121305juakfuenmayorNo ratings yet
- 217 - 036 - 02 - Streptomycin Sulfate USPDocument6 pages217 - 036 - 02 - Streptomycin Sulfate USPNataliaNo ratings yet
- An 175 Food Packaging Shs-Gc-TofDocument7 pagesAn 175 Food Packaging Shs-Gc-Tofabdlkdr3460No ratings yet
- Quantitative Amino AcidsDocument4 pagesQuantitative Amino Acidsait el hocine tarekNo ratings yet
- 10660-Article Text-36214-1-10-20160308Document6 pages10660-Article Text-36214-1-10-20160308Rifki SaufiNo ratings yet
- Phenoxyethanol-Cosmetics en PDFDocument2 pagesPhenoxyethanol-Cosmetics en PDFHiềnNo ratings yet
- Practical 11 - WPMDocument7 pagesPractical 11 - WPM门门No ratings yet
- Abiraterone Acetate Tablets - USPDocument3 pagesAbiraterone Acetate Tablets - USPДарія ОсадчаNo ratings yet
- 19-Design of An Amperometric Biosensor For The Determination ofDocument4 pages19-Design of An Amperometric Biosensor For The Determination ofwardaninurindahNo ratings yet
- Review Jurnal 1 - TrehaloseDocument3 pagesReview Jurnal 1 - TrehaloseMuhammad Saepul AnwarNo ratings yet
- Knauer - Food PreservativesDocument1 pageKnauer - Food PreservativesMaryanta HadisudiroNo ratings yet
- Rapid Analysis of Genotoxic Nitrosamines by HPLC MS MSDocument3 pagesRapid Analysis of Genotoxic Nitrosamines by HPLC MS MSMarcio SchiavelliNo ratings yet
- Exp 3 DNSDocument10 pagesExp 3 DNSEmelya NatraNo ratings yet
- GPC System OperationDocument49 pagesGPC System OperationFahmi Januar AnugrahNo ratings yet
- Hydrolytic Degradation Profiling of Ezetimibe by HPLC MethodDocument6 pagesHydrolytic Degradation Profiling of Ezetimibe by HPLC MethodTJPRC PublicationsNo ratings yet
- Analisis Dan Pemurnian Protein-FinalDocument44 pagesAnalisis Dan Pemurnian Protein-FinalEndah Sekar PalupiNo ratings yet
- Electrochemistry of Dihydroxybenzene Compounds: Carbon Based Electrodes and Their Uses in Synthesis and SensorsFrom EverandElectrochemistry of Dihydroxybenzene Compounds: Carbon Based Electrodes and Their Uses in Synthesis and SensorsNo ratings yet
- Rumah Sakit Ibu Dan Anak Dedari: Section: Farmasi Periode: Feb 01, 2017 S/D Feb 28, 2017 No Opname: 1702-OPN-000212Document12 pagesRumah Sakit Ibu Dan Anak Dedari: Section: Farmasi Periode: Feb 01, 2017 S/D Feb 28, 2017 No Opname: 1702-OPN-000212Ina LabokNo ratings yet
- Formularium Dedari DraftDocument663 pagesFormularium Dedari DraftIna LabokNo ratings yet
- Book 1Document16 pagesBook 1Ina LabokNo ratings yet
- Label InjDocument1 pageLabel InjIna LabokNo ratings yet
- GlobalCOD Method 2000 2015Document85 pagesGlobalCOD Method 2000 2015suma riadiNo ratings yet
- CHEMEXCILDocument28 pagesCHEMEXCILAmal JerryNo ratings yet
- D 12332Document108 pagesD 12332jesus_hfNo ratings yet
- Characteristics of g3 - An Alternative To SF6Document5 pagesCharacteristics of g3 - An Alternative To SF6Abdul MoizNo ratings yet
- KaizenDocument8 pagesKaizenGigih SetijawanNo ratings yet
- M1 - 02 Nature and Scope of BusinessDocument41 pagesM1 - 02 Nature and Scope of BusinessDiptendu RoyNo ratings yet
- DT81 Data Logger DatasheetDocument2 pagesDT81 Data Logger DatasheetDuška JarčevićNo ratings yet
- TCS AIP Take Home AssignmentDocument16 pagesTCS AIP Take Home AssignmentShruthiSAthreyaNo ratings yet
- ORTableAccessory Brochure INT en 05 NonUSDocument144 pagesORTableAccessory Brochure INT en 05 NonUSHarianto HarNo ratings yet
- Accounting All ChaptersDocument155 pagesAccounting All ChaptersHasnain Ahmad KhanNo ratings yet
- Bangladesh University of Professionals (Bup)Document17 pagesBangladesh University of Professionals (Bup)oldschoolNo ratings yet
- Distribution Channel of AMULDocument13 pagesDistribution Channel of AMULMeet JivaniNo ratings yet
- Scala AccesoriesDocument27 pagesScala AccesoriesZiggy BussyNo ratings yet
- Analytical Chem Chap11Document18 pagesAnalytical Chem Chap11Nicole Ann PedriñaNo ratings yet
- ClaytonDocument1 pageClaytonapi-3831340No ratings yet
- General Properties of Engineering MaterialsDocument9 pagesGeneral Properties of Engineering MaterialsRoderick VillanuevaNo ratings yet
- DK Pocket Genius - HorsesDocument158 pagesDK Pocket Genius - HorsesAnamaria RaduNo ratings yet
- Book Reviews: Supplemental Damping and Seismic Isolation by C. ChristopoulosDocument1 pageBook Reviews: Supplemental Damping and Seismic Isolation by C. ChristopoulosLuca AcetoNo ratings yet
- Final Project On Employee EngagementDocument97 pagesFinal Project On Employee EngagementSanju DurgapalNo ratings yet
- Friction Worksheet: Part A Questions - Multiple ChoiceDocument2 pagesFriction Worksheet: Part A Questions - Multiple ChoiceAmos JosephatNo ratings yet
- Raga Purna Pancama: Analysis ModesDocument1 pageRaga Purna Pancama: Analysis ModesKermitNo ratings yet
- RT00Document16 pagesRT00Jeganeswaran17% (6)
- Coast Artillery Journal - Oct 1946Document84 pagesCoast Artillery Journal - Oct 1946CAP History LibraryNo ratings yet
- Test Bank For Anatomy and Physiology 10th Edition by PattonDocument32 pagesTest Bank For Anatomy and Physiology 10th Edition by PattonCassandraDuncanmcytd100% (42)
- Submarine Diesel EngineDocument106 pagesSubmarine Diesel EngineDharma Gita Surya PrayogaNo ratings yet
- Extracting DnaDocument6 pagesExtracting DnaIya AlabastroNo ratings yet
- 1 IntroductionDocument28 pages1 Introductionkevnnicl921216No ratings yet
- Final DKSSKDocument79 pagesFinal DKSSKSarita Latthe100% (2)
- Economists' Corner: Weighing The Procompetitive and Anticompetitive Effects of RPM Under The Rule of ReasonDocument3 pagesEconomists' Corner: Weighing The Procompetitive and Anticompetitive Effects of RPM Under The Rule of ReasonHarsh GandhiNo ratings yet