Professional Documents
Culture Documents
Protons Neutrons Electrons: Elements
Protons Neutrons Electrons: Elements
Uploaded by
Thea Mari MagdasocOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Protons Neutrons Electrons: Elements
Protons Neutrons Electrons: Elements
Uploaded by
Thea Mari MagdasocCopyright:
Available Formats
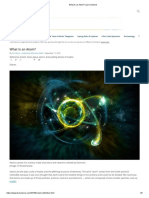
There are three parts to an atom.
Protons have a positive electrical charge and are
found together with neutrons (no electrical charge) in the nucleus of each atom.
Negatively charged electrons orbit the nucleus.
Atoms are the smallest particles that make up elements. Each element contains a
different number of protons. For example, all hydrogen atoms have 1 proton while all
carbon atoms have 6 protons. Some matter consists of one type of atom (e.g., gold),
while other matter is made of atoms bonded together to form compounds (e.g.,
sodium chloride).
Atoms are mostly empty space. The nucleus of an atom is extremely dense and
contains nearly all of the mass of each atom. Electrons contribute very little mass to
the atom (it takes 1836 electrons to equal the size of a proton) and orbit so far away
from the nucleus that each atom is 99.9% empty space. If the atom was the size of a
sports arena, the nucleus would be the size of a pea. Although the nucleus is much
more dense compared with the rest of the atom, it too consists mainly of empty
space.
There are over 100 different kinds of atoms. About 92 of them occur naturally, while
the remainder are made in labs. The first new atom made by man was technetium,
which has 43 protons. New atoms can be made by adding more protons to an
atomic nucleus. However, these new atoms (elements) are unstable and decay into
smaller atoms instantaneously. Usually, we only know a new atom was created by
identifying the smaller atoms from this decay.
The components of an atom are held together by three forces. Protons and neutrons
are held together by the strong and weak nuclear forces. Electrical attraction holds
electrons and protons. While electrical repulsion repels protons away from each
other, the attracting nuclear force is much stronger than electrical repulsion. The
strong force that binds together protons and neutrons is 1038 times more powerful
than gravity, but it acts over a very short range, so particles need to be very close to
each other to feel its effect.
The word "atom" comes from the Greek word for "uncuttable" or "undivided". The
Greek Democritus believed matter consists of particles that could not be cut into
smaller particles. For a long time, people believed atoms were the fundamental
"uncuttable" unit of matter. While atoms are the building blocks of elements, that can
be divided into still smaller particles. Also, nuclear fission and nuclear decay can
break atoms into smaller atoms.
Atoms are very small. The average atom is about one tenth of a billionth of a meter
across. The largest atom (cesium) is approximately nine times bigger than the
smallest atom (helium).
Although atoms are the smallest unit of an element, they consist of even tinier
particles called quarks and leptons. An electron is a lepton. Protons and neutrons
consist of three quarks each.
The most abundant type of atom in the universe is the hydrogen atom. Nearly 74%
of the atoms in the Milky Way galaxy are hydrogen atoms.
You have around 7 billion atoms in your body, yet you replace about 98% of them
every year!
You might also like
- AtomDocument22 pagesAtomVinayKumarNo ratings yet
- Atom FactsDocument3 pagesAtom Factsaaditya panwarNo ratings yet
- Lectura No3 - The AtomDocument2 pagesLectura No3 - The Atomjudasan9210No ratings yet
- Bai Doc 1 AtomsDocument2 pagesBai Doc 1 AtomsLê ÁnhNo ratings yet
- AtomDocument12 pagesAtomatgimale.comNo ratings yet
- Definition and Composition of An Atom.Document3 pagesDefinition and Composition of An Atom.Habibu AbdullahiNo ratings yet
- TheatomwhatareatomsPDFNotesHandout 1Document25 pagesTheatomwhatareatomsPDFNotesHandout 1shanettes2000No ratings yet
- Unit 3 AtomsDocument22 pagesUnit 3 AtomsmaryNo ratings yet
- Victoria Catholic School: Submitted By: Jessica V. MarceloDocument4 pagesVictoria Catholic School: Submitted By: Jessica V. MarceloEric CastilloNo ratings yet
- Atoms and ElementsDocument18 pagesAtoms and ElementsJACK CAMPBELLNo ratings yet
- Cience: Molecules ElementsDocument22 pagesCience: Molecules ElementsRudyliza CadiongNo ratings yet
- Mod 2 Book 1 PhysicsDocument41 pagesMod 2 Book 1 Physicsranjit prasadNo ratings yet
- Material Chapter OneDocument13 pagesMaterial Chapter OneTeshale AlemieNo ratings yet
- A Water Molecule Consists of An Oxygen Atom and Two Hydrogen Atoms, Which Are Attached at An Angle of 105°Document20 pagesA Water Molecule Consists of An Oxygen Atom and Two Hydrogen Atoms, Which Are Attached at An Angle of 105°Kunal LunawatNo ratings yet
- UntitledDocument1 pageUntitledJhomar B, Dela CruzNo ratings yet
- The Atom - Wikipedia, The Free EncyclopediaDocument20 pagesThe Atom - Wikipedia, The Free EncyclopediaBorn Magnetic AllahNo ratings yet
- Protons: Protons Are The Basis of Atoms. While An Atom CanDocument3 pagesProtons: Protons Are The Basis of Atoms. While An Atom CansaadNo ratings yet
- Atomic StructureDocument2 pagesAtomic StructureJessica BeasleyNo ratings yet
- What Is An Atom (English)Document2 pagesWhat Is An Atom (English)Andrew SilvaNo ratings yet
- Basic Atomic StructureDocument2 pagesBasic Atomic Structureanas subhanNo ratings yet
- Atomic Structure and Interatomic BondingDocument40 pagesAtomic Structure and Interatomic BondingJhomel EberoNo ratings yet
- Atomic StructureDocument28 pagesAtomic StructureJohn Vince Ramos PapNo ratings yet
- PeriodictableofelementsDocument6 pagesPeriodictableofelementswoodysseusNo ratings yet
- Nuclear Physics NotesDocument14 pagesNuclear Physics NotesJonathan ThomasNo ratings yet
- Structure and Characteristics of ProtonDocument2 pagesStructure and Characteristics of ProtonElok SorayaNo ratings yet
- What Is An Atom - Live ScienceDocument9 pagesWhat Is An Atom - Live ScienceSheena Shane CantelaNo ratings yet
- Atom and The Location of Its Major ComponentsDocument11 pagesAtom and The Location of Its Major ComponentsAldenramosNo ratings yet
- Atoms AND Elements: Chemistry Science Fair Project 7A Valen, Mathew, Davin NG, Catherine, SeanDocument7 pagesAtoms AND Elements: Chemistry Science Fair Project 7A Valen, Mathew, Davin NG, Catherine, Seansean gillmoreNo ratings yet
- Atoms AND Elements: Chemistry Science Fair Project 7A Valen, Mathew, Davin NG, Catherine, SeanDocument7 pagesAtoms AND Elements: Chemistry Science Fair Project 7A Valen, Mathew, Davin NG, Catherine, Seansean gillmoreNo ratings yet
- Definicion de AtomoDocument3 pagesDefinicion de AtomoJos eNo ratings yet
- Chapter 4-Student Reading: Parts of The AtomDocument12 pagesChapter 4-Student Reading: Parts of The AtomShimmy LimmyNo ratings yet
- Structure of Substance - Lesson - 1Document14 pagesStructure of Substance - Lesson - 1samsonNo ratings yet
- Safari - Dec 31, 2023 at 3:47 AM 2Document1 pageSafari - Dec 31, 2023 at 3:47 AM 2syansyncNo ratings yet
- Atoms: NucleusDocument6 pagesAtoms: Nucleusヒルデガルダ HILDEGARDENo ratings yet
- Chapter 4 Structure of The Atom Notes Class 9 ScienceDocument47 pagesChapter 4 Structure of The Atom Notes Class 9 ScienceChandan Kumar SinghNo ratings yet
- For The Internet Feed, See - For The Microprocessor, See .: Atom (Feed) Intel AtomDocument2 pagesFor The Internet Feed, See - For The Microprocessor, See .: Atom (Feed) Intel AtomDiego TrincadoNo ratings yet
- Atomic Structure: An Updated Version of This Lesson Is Available at Visionlearning: Atomic Theory & Ions & IsotopesDocument4 pagesAtomic Structure: An Updated Version of This Lesson Is Available at Visionlearning: Atomic Theory & Ions & IsotopesCarolina Angarita RodriguezNo ratings yet
- 01 Atomic Structure 2009Document8 pages01 Atomic Structure 2009api-27085921No ratings yet
- Nucleus: Carbon Hydrogen Oxygen Periodic Table of The ElementsDocument2 pagesNucleus: Carbon Hydrogen Oxygen Periodic Table of The ElementsThess Tecla Zerauc AzodnemNo ratings yet
- Today: - Chap 11, Atomic Nature of MatterDocument34 pagesToday: - Chap 11, Atomic Nature of Matterbaig79No ratings yet
- AtomDocument32 pagesAtommcmusbixNo ratings yet
- Helium Atom: Jump To Navigationjump To SearchDocument2 pagesHelium Atom: Jump To Navigationjump To SearchWYNNo ratings yet
- Dalton's Atomic Theory: Cations Are Ions With A Net Positive ChargeDocument2 pagesDalton's Atomic Theory: Cations Are Ions With A Net Positive ChargeJalaineLoisLatojaNo ratings yet
- 01 Chemistry 4 13Document40 pages01 Chemistry 4 13Alejandro AvalosNo ratings yet
- Unit - 4 Atomic Structure - 7th STDDocument29 pagesUnit - 4 Atomic Structure - 7th STDthangamuthu baskarNo ratings yet
- Prepared By: Group 6 Brent Pili Andrew Roque Bryan BelirDocument18 pagesPrepared By: Group 6 Brent Pili Andrew Roque Bryan BelirJoice Ann A. PolinarNo ratings yet
- Structure of The Atom:: Standard Model of Particle PhysicsDocument88 pagesStructure of The Atom:: Standard Model of Particle PhysicsAngel BenganNo ratings yet
- Chapter Three: The Atomic WorldDocument7 pagesChapter Three: The Atomic WorldEvonneNo ratings yet
- Project Standard 8Document6 pagesProject Standard 8Saqib MandhaiNo ratings yet
- Helium Atom: Electron CloudDocument14 pagesHelium Atom: Electron CloudSwati RoyNo ratings yet
- Atoms and Elements: Atomic StructureDocument5 pagesAtoms and Elements: Atomic StructureJohn Rey Siwala EduqueNo ratings yet
- Physical ScienceDocument27 pagesPhysical ScienceCarlos MasikaNo ratings yet
- Inorganic ChemistryDocument15 pagesInorganic Chemistryabdihakimhasssan344No ratings yet
- Welcome!: M H M Mubassir (MNS, Bracu)Document29 pagesWelcome!: M H M Mubassir (MNS, Bracu)raven ravenNo ratings yet
- What Is Atomic Structure?: ProtonsDocument22 pagesWhat Is Atomic Structure?: ProtonsCaleb MukaviNo ratings yet
- NucleusDocument7 pagesNucleusaditya2053No ratings yet
- What Is Electricity?: Prepared By: Engr. Renzo M. JognoDocument36 pagesWhat Is Electricity?: Prepared By: Engr. Renzo M. JognoRenzo M. JognoNo ratings yet
- AtomDocument1 pageAtomwingchun629No ratings yet
- Your Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2From EverandYour Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2Rating: 5 out of 5 stars5/5 (1)
- Revised Format of BU Syllabus SW With Groups UpdatedDocument12 pagesRevised Format of BU Syllabus SW With Groups UpdatedThea Mari MagdasocNo ratings yet
- Digital FootprintDocument2 pagesDigital FootprintThea Mari MagdasocNo ratings yet
- College of Social Sciences and PhilosophyDocument1 pageCollege of Social Sciences and PhilosophyThea Mari MagdasocNo ratings yet
- 3A SW115 Magdasoc at 3.2Document4 pages3A SW115 Magdasoc at 3.2Thea Mari MagdasocNo ratings yet
- How To Ride A BikeDocument1 pageHow To Ride A BikeThea Mari MagdasocNo ratings yet
- WorksheetDocument1 pageWorksheetThea Mari MagdasocNo ratings yet
- Combine All of The Like Terms and Move Them To One Side of The EquationDocument1 pageCombine All of The Like Terms and Move Them To One Side of The EquationThea Mari MagdasocNo ratings yet
- Understanding The Difference Between Negative and Positive FreedomDocument3 pagesUnderstanding The Difference Between Negative and Positive FreedomThea Mari MagdasocNo ratings yet
- Rhodnel I. Bejo Gen. Average - 91Document1 pageRhodnel I. Bejo Gen. Average - 91Thea Mari MagdasocNo ratings yet
- Ancient Rome: Did You Know? Four Decades After Constantine Made Christianity Rome's Official Religion, EmperorDocument6 pagesAncient Rome: Did You Know? Four Decades After Constantine Made Christianity Rome's Official Religion, EmperorThea Mari MagdasocNo ratings yet
- Vision: A World - Class University Producing Leaders and Change Agents For Social Transformation and DevelopmentDocument1 pageVision: A World - Class University Producing Leaders and Change Agents For Social Transformation and DevelopmentThea Mari MagdasocNo ratings yet
- Bata 2Document1 pageBata 2Thea Mari MagdasocNo ratings yet
- Jomina Remi M. MagdasocDocument1 pageJomina Remi M. MagdasocThea Mari MagdasocNo ratings yet
- Footnote To Youth by Jose Garcia VillaDocument3 pagesFootnote To Youth by Jose Garcia VillaThea Mari MagdasocNo ratings yet
- Final Exam in World ReligionDocument3 pagesFinal Exam in World ReligionThea Mari MagdasocNo ratings yet
- Sandy Hook Tragedy Is An Excellent ExampleDocument5 pagesSandy Hook Tragedy Is An Excellent ExampleThea Mari MagdasocNo ratings yet
- How To Write A Position PaperDocument3 pagesHow To Write A Position PaperThea Mari MagdasocNo ratings yet
- Section 1Document207 pagesSection 1gnanasekarNo ratings yet
- Physics II ProblemsDocument1 pagePhysics II ProblemsBOSS BOSSNo ratings yet
- Unit ChemistryDocument3 pagesUnit Chemistryapi-212901753No ratings yet
- A Primer On LogarithmDocument202 pagesA Primer On LogarithmVikas50% (2)
- Phy 124Document18 pagesPhy 124hardebolzem12No ratings yet
- Solution Manual For Chemistry An Atoms-Focused Approach, 3rd Edition, Thomas R Gilbert, Rein V Kirss, Stacey Lowery Bretz, Natalie FosterDocument10 pagesSolution Manual For Chemistry An Atoms-Focused Approach, 3rd Edition, Thomas R Gilbert, Rein V Kirss, Stacey Lowery Bretz, Natalie Fosterloudly.nereisnai6100% (21)
- (Undergraduate Texts in Mathematics) Stephanie Frank Singer-Linearity, Symmetry, and Prediction in The Hydrogen Atom-Springer (2005) PDFDocument404 pages(Undergraduate Texts in Mathematics) Stephanie Frank Singer-Linearity, Symmetry, and Prediction in The Hydrogen Atom-Springer (2005) PDFThiago Drummond100% (2)
- Atomic Structure 11Document303 pagesAtomic Structure 11aleena'No ratings yet
- Molar Mass SGDocument17 pagesMolar Mass SGMark Rouie FelicianoNo ratings yet
- 12 Strand DNA Morphogenetic EngineeringDocument14 pages12 Strand DNA Morphogenetic EngineeringTimothy Henriksen100% (1)
- G3 Module 5 - Intro To ElectricityDocument51 pagesG3 Module 5 - Intro To ElectricityEthan BlackNo ratings yet
- Multilayered Narration in Electroacoustic Music Composition Using Nuclear Magnetic Resonance Data Sonification and Acousmatic StorytellingDocument7 pagesMultilayered Narration in Electroacoustic Music Composition Using Nuclear Magnetic Resonance Data Sonification and Acousmatic StorytellingPatrizia MandolinoNo ratings yet
- Narayana Junior College. JR AIEEE & EAMCET IC. CDF Model Test.Document9 pagesNarayana Junior College. JR AIEEE & EAMCET IC. CDF Model Test.Vaishno Bharadwaj58% (19)
- Radioactivity Overview ArticleDocument4 pagesRadioactivity Overview Articleapi-267255264No ratings yet
- Division Budgeted Lesson and Tos Science 8 2Document24 pagesDivision Budgeted Lesson and Tos Science 8 2Marvin Obra100% (2)
- PHY4 January 2004Document3 pagesPHY4 January 2004api-3726022No ratings yet
- Atom and Ion Movements in Materials Group VDocument21 pagesAtom and Ion Movements in Materials Group VVictor Anthony CuaresmaNo ratings yet
- Radtech-113L ACTIVITY-113L.3 GROUP2Document3 pagesRadtech-113L ACTIVITY-113L.3 GROUP2Jnn FrzNo ratings yet
- G9 SLEM Q2 W2 Ionic Covalent PropertiesDocument17 pagesG9 SLEM Q2 W2 Ionic Covalent PropertiesStephanie Villanueva100% (1)
- Chapter 1 - Structure and BondingDocument12 pagesChapter 1 - Structure and BondingHerstine SistualNo ratings yet
- Dr. Homibhabha Competition Test Series.: Answer FileDocument25 pagesDr. Homibhabha Competition Test Series.: Answer FileSachin AgrawalNo ratings yet
- NCERT Exemplar Solution Class 9 Chapter 4Document14 pagesNCERT Exemplar Solution Class 9 Chapter 4Rahul N BNo ratings yet
- 13 NucleiDocument13 pages13 Nucleivallabh.moapNo ratings yet
- Understanding Zero Point Energy by Thomas Valone (1999)Document12 pagesUnderstanding Zero Point Energy by Thomas Valone (1999)Douglas BooyensNo ratings yet
- VV (Associated With: Plane Waves and Refractive IndexDocument18 pagesVV (Associated With: Plane Waves and Refractive IndexDaniel MejiaNo ratings yet
- Atomic Structure & Mole Concept (Question Paper)Document5 pagesAtomic Structure & Mole Concept (Question Paper)Param shahNo ratings yet
- MYC MYC: Equipp Review Center Inc. Radiologic PhysicsDocument37 pagesMYC MYC: Equipp Review Center Inc. Radiologic PhysicsZyrineNo ratings yet
- G. I. Epifanov - Solid State Physics (1979, Mir Publisher)Document345 pagesG. I. Epifanov - Solid State Physics (1979, Mir Publisher)puceiroaleNo ratings yet
- Lecture Notes 1 - Chemical HistoryDocument9 pagesLecture Notes 1 - Chemical HistoryannmarieNo ratings yet
- Lecture 1-8Document98 pagesLecture 1-8PrituuNo ratings yet