Professional Documents
Culture Documents
Experiment 1: Determination of Molecular Weight: 84.916 G/mol
Experiment 1: Determination of Molecular Weight: 84.916 G/mol
Uploaded by
camille.cruz0 ratings0% found this document useful (0 votes)
41 views1 pageThe group determined that unknown liquid sample #5 was dichloromethane (DCM) based on several experiments and comparisons to known properties of DCM. The unknown smelled familiar and had a molecular weight near that of DCM. Subsequent measurements of density, viscosity, surface tension, and heat of vaporization were also consistent with values for DCM within reported percent errors.
Original Description:
Unknown sample determination using molecular weight, heat of vaporization, etc.
Original Title
Determination of Unknown (Chem 156.1)
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe group determined that unknown liquid sample #5 was dichloromethane (DCM) based on several experiments and comparisons to known properties of DCM. The unknown smelled familiar and had a molecular weight near that of DCM. Subsequent measurements of density, viscosity, surface tension, and heat of vaporization were also consistent with values for DCM within reported percent errors.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
41 views1 pageExperiment 1: Determination of Molecular Weight: 84.916 G/mol
Experiment 1: Determination of Molecular Weight: 84.916 G/mol
Uploaded by
camille.cruzThe group determined that unknown liquid sample #5 was dichloromethane (DCM) based on several experiments and comparisons to known properties of DCM. The unknown smelled familiar and had a molecular weight near that of DCM. Subsequent measurements of density, viscosity, surface tension, and heat of vaporization were also consistent with values for DCM within reported percent errors.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
Determination of Identity of Unknown # 5
dela Cruz, Ma. Carmela and Mauricio, Theresa Marie
Group 9, Chem 156.1, HEJ
December 9, 2017
All liquid unknowns are organic compounds. The group’s unknown was sample number 5. When the
sample was given, the first thing we did was to smell it and we noted that the unknown smells familiar,
as if we have used it many times before.
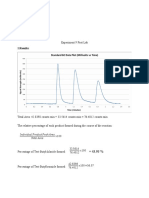
Experiment 1: Determination of Molecular Weight
Result Error True Value if (-) error True Value if (+) error
47.833 g/mol 43.67% 84.916 g/mol 33.294 g/mol
When we submitted our data for checking, the lab instructor remarked, “Ba’t ang layo?”. So, when we
calculated the true value based on the percent error given to us, we assumed that the true value was
that given by if the error is negative since 84.916 was far from 47.833 while 33.294 was near to it. Upon,
research, the only organic liquid with that molecular weight was dichloromethane.
MW DCM: 84.95 g/mol
Thus, from then on, we focused on checking if our results will correspond to the properties of DCM.
We also remarked that DCM being our unknown make sense because of the familiar smell since we
used that compound multiple times during Chem 35.1.
Experiment 2: Liquid Density
Result Error True Value if (-) error True Value if (+) error
1.2984 g/mL 2.13% 1.3267 g/mL 1.2713 g/mL
Before conducting expt.2, we noticed that the amount of unknown remaining in the bottle was
significantly lower than what we left from expt.1. This implies that the unknown is volatile which is one
of the characteristics of DCM that our instructor from Chem 35.1 told us. Thus, at this point we are pretty
confident that unknown 5 is indeed DCM which is further supported by the result of this experiment.
ρDCM: 1.3 g/mL
Since, the results support our hypothesis that the unknown is DCM, we decided to use the density of
DCM which is 1.3 g/mL for the subsequent experiments.
Experiment 3: Liquid Viscosity
Result Error True Value if (-) error True Value if (+) error
0.478 mPa.s 15.74% 0.567 mPa.s 0.413 mPa.s
ηDCM: 0.41 mPa.s
Experiment 4: Surface Tension
Result Error True Value if (-) error True Value if (+) error
37.5 mN/m 37.87% 60.36 mN/m 27.2 mN/m
γDCM: 28.2 mN/m
Experiment 9: Heat of Vaporization
Result Error True Value if (-) error True Value if (+) error
11.81 kJ/mol 59% 28.8 kJ/mol 7.42 kJ/mol
ΔHvap,DCM: 28.2 kJ/mol
Conclusion
Since true values obtained through the experimental results and given percent error, were more or less
equal to the values of DCM, we therefore conclude that unknown number 5 is dichloromethane.
Chem 156.1, Determination of Identity of Unknown # 5 Page 1 of 1
You might also like
- LAB REPORT 7 Aldol Reaction Synthesis 1 5 Diphenyl 1 4 Pentadien 3 OneDocument6 pagesLAB REPORT 7 Aldol Reaction Synthesis 1 5 Diphenyl 1 4 Pentadien 3 OnerodneyperuNo ratings yet
- Lab 21A and 21BDocument8 pagesLab 21A and 21BLateesha ThomasNo ratings yet
- ZINCALUME® G550 SteelDocument2 pagesZINCALUME® G550 SteelGeni SamudraNo ratings yet
- FYDP of DAP ProductionDocument124 pagesFYDP of DAP ProductionAhmed Mustafa100% (1)
- Lab 9 Post LabDocument2 pagesLab 9 Post LabAleksandra GlodNo ratings yet
- Experiment 14Document7 pagesExperiment 14Jc GohNo ratings yet
- Repeatability & Reproducibility of HFODocument5 pagesRepeatability & Reproducibility of HFOshishir4870No ratings yet
- A Laboratory Manual of Physical PharmaceuticsFrom EverandA Laboratory Manual of Physical PharmaceuticsRating: 2.5 out of 5 stars2.5/5 (2)
- Lab1 TeodoroDocument9 pagesLab1 TeodoroJherby TeodoroNo ratings yet
- Cadmium Astm-B766-86-2015Document8 pagesCadmium Astm-B766-86-2015Abbas RangoonwalaNo ratings yet
- Accuracy Precision Errors - SolutionDocument1 pageAccuracy Precision Errors - SolutionShaimaa SalamaNo ratings yet
- Laboratory Session - BiosensorsDocument5 pagesLaboratory Session - BiosensorsEtienne GattNo ratings yet
- Statistics Problem SetDocument2 pagesStatistics Problem SetDanielle Marie GevañaNo ratings yet
- Name-Surname: Şüheda Özek (Oxidation Reduction Titration) : Exp. 4 Report SheetDocument2 pagesName-Surname: Şüheda Özek (Oxidation Reduction Titration) : Exp. 4 Report SheetŞüheda ÖzekNo ratings yet
- Determination of K of IndicatorDocument2 pagesDetermination of K of IndicatorAmy WildesNo ratings yet
- 21_Triglycerides-_Test_KitDocument1 page21_Triglycerides-_Test_KitdeepakNo ratings yet
- PH TitrationDocument5 pagesPH TitrationOdaro OsayimwenNo ratings yet
- POVA TrainingDocument42 pagesPOVA TrainingJuly FojasNo ratings yet
- Materiales de Referencia CCRMP (CANMET)Document5 pagesMateriales de Referencia CCRMP (CANMET)Erick Leonardo Valle MendozaNo ratings yet
- Discussion PCR.Document2 pagesDiscussion PCR.diyanaNo ratings yet
- BetaDocument3 pagesBetacrg1234No ratings yet
- AppendixDocument2 pagesAppendixMiao MiaoNo ratings yet
- Understanding Experimental Error: Than The Population You Wish To Study. For Example, Say I Want To Study The AverageDocument13 pagesUnderstanding Experimental Error: Than The Population You Wish To Study. For Example, Say I Want To Study The AverageWajih M'likiNo ratings yet
- Final ExperimentDocument15 pagesFinal ExperimentqueenNo ratings yet
- Freezing Point DepressionDocument4 pagesFreezing Point DepressionJosh TingleyNo ratings yet
- Experiment 1 - DumasDocument5 pagesExperiment 1 - DumasVEnzi VeNjie Fontanilla EndicoNo ratings yet
- MKBS221. PRAC 5 REPORT Last OneDocument11 pagesMKBS221. PRAC 5 REPORT Last OneNOMKHULEKO ALICENo ratings yet
- Gravimetric Analysis Lab ReportDocument5 pagesGravimetric Analysis Lab Reportclaire_miller_16100% (1)
- Analysis and ConclusionDocument3 pagesAnalysis and ConclusionMichael SchoenfieldNo ratings yet
- Accuracy, Precision Error WSDocument3 pagesAccuracy, Precision Error WSRamón CamiloNo ratings yet
- Measure and ErrorDocument9 pagesMeasure and ErroralvesguidaNo ratings yet
- Determination of Copper by AASDocument18 pagesDetermination of Copper by AASWan ShamNo ratings yet
- Development and Validation of Related Substances Method With Gas Chromatography For Memantine Hydrochloride Drug SubstanceDocument5 pagesDevelopment and Validation of Related Substances Method With Gas Chromatography For Memantine Hydrochloride Drug SubstancevishnuvrgNo ratings yet
- Experiment 11Document12 pagesExperiment 11api-3702235No ratings yet
- Measurement Uncertainty: Case Study: Konas HKKI, Oct 31 2019, SoloDocument23 pagesMeasurement Uncertainty: Case Study: Konas HKKI, Oct 31 2019, SolohafizhNo ratings yet
- Experiment 1 Example Lab ReportDocument9 pagesExperiment 1 Example Lab ReportWinn FookNo ratings yet
- 2.0 Polymerase Chain Reaction (PCR)Document4 pages2.0 Polymerase Chain Reaction (PCR)Joan TooNo ratings yet
- Paracetamol SuppositiriesDocument6 pagesParacetamol SuppositiriesZainabNo ratings yet
- Experiment 2 OrganicDocument12 pagesExperiment 2 OrganicArif HilmiNo ratings yet
- Development of LC Method For Estimation of Diethyl Carbamazine Citrate and Chlorpheniramine Maleate in Combined Dosage FormDocument8 pagesDevelopment of LC Method For Estimation of Diethyl Carbamazine Citrate and Chlorpheniramine Maleate in Combined Dosage FormWhulan MudiaNo ratings yet
- ACTIVITY#1Document7 pagesACTIVITY#1Princess Krenzelle BañagaNo ratings yet
- Test Food AnalysisDocument12 pagesTest Food AnalysisFadhlin SakinahNo ratings yet
- Paper 3 - Analysis, Conclusions and EvaluationDocument5 pagesPaper 3 - Analysis, Conclusions and EvaluationKhadija KaziNo ratings yet
- Chapter 5 Lec 4Document3 pagesChapter 5 Lec 4IsaNo ratings yet
- Lab Report 2 AOTWDocument14 pagesLab Report 2 AOTWFatihah AlhataNo ratings yet
- PCR Labwork 2 ENGDocument4 pagesPCR Labwork 2 ENGmigas1996No ratings yet
- Comparative Analysis of The Quantitative and Qualitative Method For Determination of D - DimerDocument4 pagesComparative Analysis of The Quantitative and Qualitative Method For Determination of D - DimerDimitar KosturkovNo ratings yet
- Southern and Northern HybridizationDocument6 pagesSouthern and Northern Hybridizationyaqoob008No ratings yet
- Real Time PCR: Presented By: SUVODIP JANA 16/BT/06Document21 pagesReal Time PCR: Presented By: SUVODIP JANA 16/BT/06SUVODIP JANANo ratings yet
- Paper 3 - Analysis, Conclusions and EvaluationDocument5 pagesPaper 3 - Analysis, Conclusions and Evaluationbaryalkhan1236No ratings yet
- Paper 3 - Analysis, Conclusions and EvaluationDocument5 pagesPaper 3 - Analysis, Conclusions and EvaluationUrvah TauseefNo ratings yet
- Prepared By: KLEBACASEN University of The CordillerasDocument3 pagesPrepared By: KLEBACASEN University of The CordillerasEdison SisonNo ratings yet
- Kimia Fisik-Chemical Potential-Aska ZakiyaDocument2 pagesKimia Fisik-Chemical Potential-Aska ZakiyaAskaNo ratings yet
- PCR AmplificationDocument6 pagesPCR AmplificationDibyakNo ratings yet
- Ross Lab Report - Final DraftDocument6 pagesRoss Lab Report - Final DraftchronosplitzerNo ratings yet
- CHEM1Document1 pageCHEM1Cheena Francesca LucianoNo ratings yet
- Anachem NotesDocument10 pagesAnachem NotesAngelaNo ratings yet
- Density and Relative Density: Experiment NO.1Document13 pagesDensity and Relative Density: Experiment NO.1Anonymous a2CgV0gNo ratings yet
- Lab Waste WaterDocument8 pagesLab Waste WaterNur IzzatieNo ratings yet
- Exp 2 CHM 260Document8 pagesExp 2 CHM 2602023637002No ratings yet
- ArticleDocument3 pagesArticleSanggari AnamalaiNo ratings yet
- Limiting Reactant: Name: Abdulla Sadeq AL-Saegh ID: 20165113 Section: 30Document4 pagesLimiting Reactant: Name: Abdulla Sadeq AL-Saegh ID: 20165113 Section: 30Hasan RajabNo ratings yet
- Lecture Notes 19 - Accuracy and PrecisionDocument5 pagesLecture Notes 19 - Accuracy and PrecisionSurendra RamkissoonNo ratings yet
- Green Park International Senior Secondary School, Namakkal: Weekend Test - 8 (2 Year - Physical ScienceDocument8 pagesGreen Park International Senior Secondary School, Namakkal: Weekend Test - 8 (2 Year - Physical Sciences.hiruthivNo ratings yet
- Block Diagram New DM PlantDocument1 pageBlock Diagram New DM PlantDevraj RoyNo ratings yet
- Proper Handling and Storage of Chemicals: December 2014Document54 pagesProper Handling and Storage of Chemicals: December 2014Qais AlzamelNo ratings yet
- Development of Aluminium Scrap Melting Technology: © V. I. Gel, D. N. Rudakov UDC 669.054.8:669.71Document5 pagesDevelopment of Aluminium Scrap Melting Technology: © V. I. Gel, D. N. Rudakov UDC 669.054.8:669.71PrasanthanNo ratings yet
- Synthesis of AspirinDocument3 pagesSynthesis of AspirinShoomyla RashidNo ratings yet
- BASF Basement Waterproofing 602 - pdf5Document4 pagesBASF Basement Waterproofing 602 - pdf5Ciela RZNo ratings yet
- Oleochemical - WikipediaDocument3 pagesOleochemical - WikipediaNur Atiqah Ahmad100% (1)
- Review On Membranes For The Filtration of Aqueous Based Solution - Oil in Water Emulsion - 2018Document16 pagesReview On Membranes For The Filtration of Aqueous Based Solution - Oil in Water Emulsion - 2018Carmen StefanescuNo ratings yet
- Chemical Plant Consultancy ServicesDocument2 pagesChemical Plant Consultancy Servicesjaya46No ratings yet
- Wadi Cement Work Ordinary Portland Cement 53 Grade: Chemical CharacteristicsDocument1 pageWadi Cement Work Ordinary Portland Cement 53 Grade: Chemical CharacteristicsAshish SontakkeNo ratings yet
- Different Methods For Determination of Sodium Chloride in Cheese 1450-81091001065RDocument13 pagesDifferent Methods For Determination of Sodium Chloride in Cheese 1450-81091001065RgustavoesanchezNo ratings yet
- Cis-Bis (Imidazole) Bis (Picolinato) Copper (II) DihydrateDocument7 pagesCis-Bis (Imidazole) Bis (Picolinato) Copper (II) DihydrateAnonymous RkzuPewvydNo ratings yet
- Managing Sodic Soils: Quick FactsDocument3 pagesManaging Sodic Soils: Quick FactsArturo MunozNo ratings yet
- One Pot Synthesis, Characterization of ZN GO Nanocomposite For The Electrochemical Decoloration of A Textile DyeDocument7 pagesOne Pot Synthesis, Characterization of ZN GO Nanocomposite For The Electrochemical Decoloration of A Textile DyeEditor IJTSRDNo ratings yet
- 8.lectures Blend Dyeing 3-05-04-11Document2 pages8.lectures Blend Dyeing 3-05-04-11Shoaib WahabNo ratings yet
- Final Presentation Silica ExtractionDocument21 pagesFinal Presentation Silica Extractionramesh pokhrel33% (3)
- ZEISS Microscopy Solutions For Industrial Ceramics ResearchDocument19 pagesZEISS Microscopy Solutions For Industrial Ceramics Researchsamar209No ratings yet
- Detergenten Test ZeeppoedersDocument12 pagesDetergenten Test ZeeppoedersAdeel AshiqNo ratings yet
- The Law of Conservation of MassDocument14 pagesThe Law of Conservation of Masslevi0417No ratings yet
- Paal-Knorr Pyrrole Synthesis: A. General Description of The ReactionDocument4 pagesPaal-Knorr Pyrrole Synthesis: A. General Description of The Reactionjorge esteban guerrero poloNo ratings yet
- 09 - Chapter 2 PDFDocument34 pages09 - Chapter 2 PDFrajkumarNo ratings yet
- MithridatismDocument3 pagesMithridatismTodd WoosterNo ratings yet
- Coatings Resins On Bio Based Raw Materials DSMDocument22 pagesCoatings Resins On Bio Based Raw Materials DSMDharmendra B MistryNo ratings yet
- General Chemistry Topic 1 ReviewerDocument3 pagesGeneral Chemistry Topic 1 ReviewerNishka CarabeoNo ratings yet
- Lecture 1 - Fundamentals of Organic ChemistryDocument119 pagesLecture 1 - Fundamentals of Organic ChemistrymjmonforteNo ratings yet
- Dr. Umar Jan Pandit: Personal DetailsDocument6 pagesDr. Umar Jan Pandit: Personal Detailsumar panditNo ratings yet