Professional Documents
Culture Documents
Effects of Weight Loss On Mechanisms of Hyperglycemia in Obese Non-Insulin-Dependent Diabetes Mellitus
Effects of Weight Loss On Mechanisms of Hyperglycemia in Obese Non-Insulin-Dependent Diabetes Mellitus
Uploaded by
Christine Yohana SianturiCopyright:
Available Formats
You might also like
- A6 MF 1Document2 pagesA6 MF 1hidraulic100% (1)
- Itl 512 Learning Map Final DraftDocument5 pagesItl 512 Learning Map Final Draftapi-483586558No ratings yet
- Prophecies in Islam & Prophet Muhammad - A.aliDocument82 pagesProphecies in Islam & Prophet Muhammad - A.aliZaky MuzaffarNo ratings yet
- Naplex Complete Study Outline A Topic-Wise Approach DiabetesFrom EverandNaplex Complete Study Outline A Topic-Wise Approach DiabetesRating: 4 out of 5 stars4/5 (3)
- Cardiff Council Planning Committee Agenda For Wednesday February 13, 2013.Document222 pagesCardiff Council Planning Committee Agenda For Wednesday February 13, 2013.jessica_best2820No ratings yet
- Noninsulin-Dependent Diabetes: Effects of Insulin Peripheral Splanchnic Glucose Metabolism in 11)Document7 pagesNoninsulin-Dependent Diabetes: Effects of Insulin Peripheral Splanchnic Glucose Metabolism in 11)agiekNo ratings yet
- The Effect of Glimepiride On Glycemic Control and Fasting Insulin LevelsDocument3 pagesThe Effect of Glimepiride On Glycemic Control and Fasting Insulin Levelszahrosofi ahmadahNo ratings yet
- Effects of Glucose Ingestion On Postprandial LipemiaDocument6 pagesEffects of Glucose Ingestion On Postprandial LipemiaBram KNo ratings yet
- Глюкагон мен глюкоза, инсулинDocument5 pagesГлюкагон мен глюкоза, инсулинArsen IzbanovNo ratings yet
- Am J Clin Nutr 1979 Anderson 2312 21Document10 pagesAm J Clin Nutr 1979 Anderson 2312 21Riza Haida WardhaniNo ratings yet
- HFD MiceDocument5 pagesHFD Micechar462No ratings yet
- Obesity and Type 2 Diabetes Impair Insulin-Induced Suppression of Glycogenolysis As Well As GluconeogenesisDocument11 pagesObesity and Type 2 Diabetes Impair Insulin-Induced Suppression of Glycogenolysis As Well As GluconeogenesisFauzi RahmanNo ratings yet
- Effect of Insulin Analog Initiation Therapy On LDL/ HDL Subfraction Profile and HDL Associated Enzymes in Type 2 Diabetic PatientsDocument11 pagesEffect of Insulin Analog Initiation Therapy On LDL/ HDL Subfraction Profile and HDL Associated Enzymes in Type 2 Diabetic PatientsAan AlawiyahNo ratings yet
- Insulin Secretion and FunctionDocument8 pagesInsulin Secretion and FunctionWendy EscalanteNo ratings yet
- Beneficial Effects of Viscous Dietary Fiber From Konjac-Mannan in Subjects With The Insulin Resistance Syndro M eDocument6 pagesBeneficial Effects of Viscous Dietary Fiber From Konjac-Mannan in Subjects With The Insulin Resistance Syndro M eNaresh MaliNo ratings yet
- CHAPTER 7 - Inpatient Management of Diabetes and HyperglycemiaDocument6 pagesCHAPTER 7 - Inpatient Management of Diabetes and HyperglycemiaenesNo ratings yet
- Jurnal DMDocument5 pagesJurnal DMratihparmadiniNo ratings yet
- Glycemic and IRDocument10 pagesGlycemic and IRAyman AleemNo ratings yet
- Diabeties and CamelmilkDocument4 pagesDiabeties and Camelmilkarib syedNo ratings yet
- Gracia Ramos2016Document8 pagesGracia Ramos2016Rahmanu ReztaputraNo ratings yet
- Ratner 2011 Preprandial Vs Postprandial GlulisineDocument7 pagesRatner 2011 Preprandial Vs Postprandial GlulisineKusumaNo ratings yet
- Premixed Insulin Analogue Compared With Basal-Plus Regimen For Inpatient Glycemic ControlDocument9 pagesPremixed Insulin Analogue Compared With Basal-Plus Regimen For Inpatient Glycemic ControlAbraham RamonNo ratings yet
- Defining Insulin Resistance From HyperinsulinemicDocument19 pagesDefining Insulin Resistance From HyperinsulinemicWita Ferani KartikaNo ratings yet
- Glycemic Variability and Hypoglycemia Before and ADocument7 pagesGlycemic Variability and Hypoglycemia Before and ALeonorNo ratings yet
- Chronic Effect of PferospermuDocument1 pageChronic Effect of PferospermuamritaryaaligarghNo ratings yet
- Weight Reduction Improves Markers of Hepatic Function and InsulinDocument6 pagesWeight Reduction Improves Markers of Hepatic Function and Insulinmummy23572No ratings yet
- Medip, IJAM-3354 RDocument6 pagesMedip, IJAM-3354 RweasrandomnicolasNo ratings yet
- 216 orDocument1 page216 orBALTAZAR OTTONELLONo ratings yet
- The Effect of Postprandial Exercise On MealrelatedDocument7 pagesThe Effect of Postprandial Exercise On MealrelatedLeonardoValenzuelaNo ratings yet
- Home-Based Exercise Training Improves Capillary Glucose Profile in Women With Gestational DiabetesDocument4 pagesHome-Based Exercise Training Improves Capillary Glucose Profile in Women With Gestational DiabetesdiahadnyaNo ratings yet
- NIH Public Access: Author ManuscriptDocument13 pagesNIH Public Access: Author ManuscriptPaul SimononNo ratings yet
- Gestational DiabetesDocument51 pagesGestational Diabeteskhadzx100% (2)
- Manejo de La DMGDocument5 pagesManejo de La DMGsandymejiaNo ratings yet
- Accelerated Intestinal Glucose Absorption in Morbidly Obese Humans: Relationship To Glucose Transporters, Incretin Hormones, and GlycemiaDocument9 pagesAccelerated Intestinal Glucose Absorption in Morbidly Obese Humans: Relationship To Glucose Transporters, Incretin Hormones, and GlycemiaNissa SissariNo ratings yet
- Administration of Resveratrol For 5 WK Has No Effect On Glucagon-Like Peptide 1 Secretion, Gastric Emptying, or Glycemic Control in Type 2 Diabetes: A Randomized Controlled TrialDocument5 pagesAdministration of Resveratrol For 5 WK Has No Effect On Glucagon-Like Peptide 1 Secretion, Gastric Emptying, or Glycemic Control in Type 2 Diabetes: A Randomized Controlled TrialAmanda TeacaNo ratings yet
- Ada WhoDocument7 pagesAda WhoHironmoy RoyNo ratings yet
- Dia Care 1987 Feinglos 72 5Document4 pagesDia Care 1987 Feinglos 72 5Kang Opik TaufikNo ratings yet
- Diabetic EssayDocument6 pagesDiabetic Essayreet kaurNo ratings yet
- Track 3 Eating Patterns and Behaviour P63Document15 pagesTrack 3 Eating Patterns and Behaviour P63Yuriko AndreNo ratings yet
- Insulin in DMDocument46 pagesInsulin in DMask1400No ratings yet
- Nutrition in DMDocument33 pagesNutrition in DMkero R.habibNo ratings yet
- Diabetes 2Document9 pagesDiabetes 2Lakshmipriya GopinathNo ratings yet
- 2814 Full PDFDocument2 pages2814 Full PDFAnaNo ratings yet
- Insulin Sensitivity and Glucose Tolerance Are Altered by Maintenance On A Ketogenic DietDocument17 pagesInsulin Sensitivity and Glucose Tolerance Are Altered by Maintenance On A Ketogenic DietSebastiano ViganòNo ratings yet
- Associations of Glucose Control with Insulin Sensitivity and Pancreatic β-Cell Responsiveness in Newly Presenting Type 2 DiabetesDocument6 pagesAssociations of Glucose Control with Insulin Sensitivity and Pancreatic β-Cell Responsiveness in Newly Presenting Type 2 DiabetesJoseph PositivoNo ratings yet
- Effects of Olanzapine and Haloperidol On The Metabolic Status of Healthy MenDocument8 pagesEffects of Olanzapine and Haloperidol On The Metabolic Status of Healthy MenBenjamin paulNo ratings yet
- Insulin Resistance IcuDocument18 pagesInsulin Resistance IcuLucero GutierrezNo ratings yet
- DMJ 37 465Document10 pagesDMJ 37 465sn4s7nyxcbNo ratings yet
- Toward Improved Management of NIDDM: A Randomized, Controlled, Pilot Intervention Using A Lowfat, Vegetarian DietDocument5 pagesToward Improved Management of NIDDM: A Randomized, Controlled, Pilot Intervention Using A Lowfat, Vegetarian DietpepeNo ratings yet
- Continuous Glucose Profiles in Healthy Subjects Under Everyday Life Conditions and After Different MealsDocument9 pagesContinuous Glucose Profiles in Healthy Subjects Under Everyday Life Conditions and After Different MealsAsose TuNo ratings yet
- Insulin Analogs Versus Human Insulin in The Treatment of Patients With Diabetic KetoacidosisDocument6 pagesInsulin Analogs Versus Human Insulin in The Treatment of Patients With Diabetic KetoacidosisrosieveliaNo ratings yet
- Mitigating Effects of Vildagliptin in Experimental Diabetes With Metabolic SyndromeDocument9 pagesMitigating Effects of Vildagliptin in Experimental Diabetes With Metabolic Syndromerajesh sumanNo ratings yet
- DR - Rihab Pediatrics 02.pediatric DM Part TwoDocument7 pagesDR - Rihab Pediatrics 02.pediatric DM Part TwoMujtaba JawadNo ratings yet
- Original Article: A. Kashiwagi, H. Takahashi, H. Ishikawa, S. Yoshida, K. Kazuta, A. Utsuno & E. UeyamaDocument9 pagesOriginal Article: A. Kashiwagi, H. Takahashi, H. Ishikawa, S. Yoshida, K. Kazuta, A. Utsuno & E. UeyamadbcooperdkNo ratings yet
- Estudo CarboidratosDocument5 pagesEstudo CarboidratosSemi Chung AzekaNo ratings yet
- NONE BDocument11 pagesNONE BPincelito21No ratings yet
- Effect of A High-Protein, Low-Carbohydrate Diet On Blood Glucose Control in People With Type 2 DiabetesDocument8 pagesEffect of A High-Protein, Low-Carbohydrate Diet On Blood Glucose Control in People With Type 2 DiabetesFajar AgNo ratings yet
- Clinical: Influence of Pharmacokinetics Bioavailability Highly Purified Beef DependentDocument5 pagesClinical: Influence of Pharmacokinetics Bioavailability Highly Purified Beef Dependentsstrumello7395No ratings yet
- Pharmacological Management of Type 1 DiabetesDocument6 pagesPharmacological Management of Type 1 DiabetesMI RFNo ratings yet
- Carbohydrate-Last Meal Pattern Lowers Postprandial Glucose and Insulin Excursions in Type 2 DiabetesDocument5 pagesCarbohydrate-Last Meal Pattern Lowers Postprandial Glucose and Insulin Excursions in Type 2 DiabetesClaudio Soares da SilvaNo ratings yet
- Reduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic PatientsDocument5 pagesReduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic PatientsRoger CNo ratings yet
- Efficacy of Autologous Bone Marrow DerivDocument10 pagesEfficacy of Autologous Bone Marrow DerivsulaymanwaquarNo ratings yet
- 6992 Birkeland Diabetes Care 2011Document14 pages6992 Birkeland Diabetes Care 2011Pharmatopia CorpNo ratings yet
- Complementary and Alternative Medical Lab Testing Part 18: PsychiatryFrom EverandComplementary and Alternative Medical Lab Testing Part 18: PsychiatryRating: 5 out of 5 stars5/5 (1)
- Local Wound Care and Topical Management of Hidradenitis SupurativaDocument7 pagesLocal Wound Care and Topical Management of Hidradenitis SupurativaChristine Yohana SianturiNo ratings yet
- Flowchart PKTCRDDocument2 pagesFlowchart PKTCRDChristine Yohana SianturiNo ratings yet
- Jurnal Bahasa InggrisDocument8 pagesJurnal Bahasa InggrisChristine Yohana SianturiNo ratings yet
- Hasil StatistikDocument8 pagesHasil StatistikChristine Yohana SianturiNo ratings yet
- PDZVBB - 18 Jun 2018Document12 pagesPDZVBB - 18 Jun 2018Christine Yohana SianturiNo ratings yet
- Family Wellness Plan Keluarga Bapak GunawanDocument2 pagesFamily Wellness Plan Keluarga Bapak GunawanChristine Yohana SianturiNo ratings yet
- Comparative Widal and Blooc Culture 2014Document19 pagesComparative Widal and Blooc Culture 2014Christine Yohana SianturiNo ratings yet
- Hasil StatistikDocument8 pagesHasil StatistikChristine Yohana SianturiNo ratings yet
- Myopia Control: A Review: Jeffrey J. WallineDocument6 pagesMyopia Control: A Review: Jeffrey J. WallineChristine Yohana SianturiNo ratings yet
- V34n1a22 Which FactorsDocument13 pagesV34n1a22 Which FactorsChristine Yohana SianturiNo ratings yet
- Za2 PDFDocument7 pagesZa2 PDFChristine Yohana SianturiNo ratings yet
- PDZVBB - 18 Jun 2018 PDFDocument1 pagePDZVBB - 18 Jun 2018 PDFChristine Yohana SianturiNo ratings yet
- Materi Medical Emergency Response Plan (Kedok. Unila 2015)Document38 pagesMateri Medical Emergency Response Plan (Kedok. Unila 2015)Christine Yohana SianturiNo ratings yet
- Patient Safety Topic 11Document21 pagesPatient Safety Topic 11Christine Yohana SianturiNo ratings yet
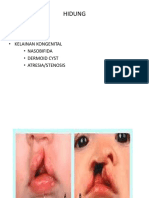
- Hidung: - Anatomi - Fisiologi - Kelainan Kongenital - Nasobifida - Dermoid Cyst - Atresia/StenosisDocument27 pagesHidung: - Anatomi - Fisiologi - Kelainan Kongenital - Nasobifida - Dermoid Cyst - Atresia/StenosisChristine Yohana SianturiNo ratings yet
- Flave5 PDFDocument12 pagesFlave5 PDFChristine Yohana SianturiNo ratings yet
- EBTax Tax Simulator Webcast 16 Jul 14pdfDocument49 pagesEBTax Tax Simulator Webcast 16 Jul 14pdfM.MedinaNo ratings yet
- The MoonDocument6 pagesThe MoonjaudreytuyNo ratings yet
- Hybrid Clutch Installation GuideDocument2 pagesHybrid Clutch Installation Guidebrucken1987No ratings yet
- DLP TLE Grade7 Carpentry 5S JH 012319Document1 pageDLP TLE Grade7 Carpentry 5S JH 012319Jonathan Magtibay HusainNo ratings yet
- 1-Module 1 Introduction 20181114 RCGDocument46 pages1-Module 1 Introduction 20181114 RCGRoger ChiuNo ratings yet
- Management Accounting Costing and BudgetingDocument31 pagesManagement Accounting Costing and BudgetingnileshdilushanNo ratings yet
- Unit-7: Iot SecurityDocument21 pagesUnit-7: Iot SecuritySAMPANo ratings yet
- Amol Sanap Teacher ResumeDocument3 pagesAmol Sanap Teacher ResumeAmol SanapNo ratings yet
- Distortion in Music ProductionDocument306 pagesDistortion in Music ProductionjansolostarNo ratings yet
- GC - Interview QuestionsDocument7 pagesGC - Interview QuestionsSasiNo ratings yet
- Strategic Intent Envisioning and MissionDocument23 pagesStrategic Intent Envisioning and Mission14122001riya2001No ratings yet
- Bonding QuizDocument7 pagesBonding Quiz卜一斐No ratings yet
- Controllogix FestoDocument43 pagesControllogix FestoEdwin Ramirez100% (1)
- IPC2022 87194 Enhancing MFL A Ultra UtilizingFEM JSpille FinalDocument5 pagesIPC2022 87194 Enhancing MFL A Ultra UtilizingFEM JSpille FinalOswaldo MontenegroNo ratings yet
- Curriculum Till DE-39 PDFDocument173 pagesCurriculum Till DE-39 PDFubaid umarNo ratings yet
- Datasheet: Delastic Preformed Pavement SealsDocument1 pageDatasheet: Delastic Preformed Pavement SealsUnited Construction Est. TechnicalNo ratings yet
- Contemporary World FinalDocument22 pagesContemporary World FinalApril Grace Genobatin BinayNo ratings yet
- Bagrut Module D Answers To The Treasure of Lemon BrownDocument8 pagesBagrut Module D Answers To The Treasure of Lemon BrownMohammed ArarNo ratings yet
- Major Fractures of The Pilon The Talus and The Calcaneus Current Concepts of TreatmentDocument241 pagesMajor Fractures of The Pilon The Talus and The Calcaneus Current Concepts of TreatmentPaul Radulescu - FizioterapeutNo ratings yet
- Schallert 2019Document7 pagesSchallert 2019Dian Putri NingsihNo ratings yet
- Experiment 1 Safety Measures: Use and Preparation of Chemical Substance 1.1 ObjectivesDocument8 pagesExperiment 1 Safety Measures: Use and Preparation of Chemical Substance 1.1 ObjectivesMaldini JosnonNo ratings yet
- Research ProposalDocument9 pagesResearch Proposalsyed arslan aliNo ratings yet
- Mwn-Wapr150n User GuideDocument116 pagesMwn-Wapr150n User GuideDan WrysinskiNo ratings yet
- All About Zookeeper and ClickHouse KeeperDocument45 pagesAll About Zookeeper and ClickHouse KeeperomyeudaihiepNo ratings yet
- Ashok Mali ReportDocument1 pageAshok Mali ReportAshok MaliNo ratings yet
- C16ac3 - 60T LoadcellDocument2 pagesC16ac3 - 60T LoadcellAmit AGRAWALNo ratings yet
Effects of Weight Loss On Mechanisms of Hyperglycemia in Obese Non-Insulin-Dependent Diabetes Mellitus
Effects of Weight Loss On Mechanisms of Hyperglycemia in Obese Non-Insulin-Dependent Diabetes Mellitus
Uploaded by
Christine Yohana SianturiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Effects of Weight Loss On Mechanisms of Hyperglycemia in Obese Non-Insulin-Dependent Diabetes Mellitus
Effects of Weight Loss On Mechanisms of Hyperglycemia in Obese Non-Insulin-Dependent Diabetes Mellitus
Uploaded by
Christine Yohana SianturiCopyright:
Available Formats
Effects of Weight Loss on Mechanisms
of Hyperglycemia in Obese
Non-Insulin-Dependent Diabetes Mellitus
R. R. HENRY, P. WALLACE, AND J. M. OLEFSKY
SUMMARY rates of 3-O-methylglucose transport increased from
To quantitate the effects of weight loss on the mecha- 0.21 ± 0.13 to 0.53 ± 0.24 pmol/(2 x 109 »tm2) x (10
nisms responsible for hyperglycemia in non-insulin-de- s~1) and maximal glucose transport rates increased
pendent diabetes mellitus (NIDDM), eight obese sub- from 0.64 ± 0.29 to 1.18 ± 0.48 pmol/(2 x 105 cells) x
jects with NIDDM were studied before and after weight (10 s 1 ) and 0.42 ± 0.20 to 1.04 ± 0.30 pmol/(2 x 109
reduction with posttreatment assessment after 3 wks of urn2) x (10 s- 1 ).
isocaloric (weight maintenance) refeeding. Finally, absolute serum insulin levels during oral glu-
After weight loss of 16.8 ± 2.7 kg (mean ± SE), the cose-tolerance tests and meal-tolerance tests were un-
fasting plasma glucose level decreased 55% from 277 changed by weight reduction, whereas plasma glucose
± 21 to 123 ± 8 mg/dl. The individual fasting glucose levels were markedly reduced.
levels were highly correlated with the elevated basal We conclude that in obese NIDDM, weight loss re-
rates of hepatic glucose output (HGO) (r = 0.91, P < sults in improved glucose homeostasis with 1) reduced
.001), which fell from 138 ± 11 to 87 ± 5 mg • n r 2 • basal HGO predominently responsible for the lowering
m i n 1 after weight loss. The change in fasting plasma of fasting glucose levels; 2) improved postprandial glu-
glucose also correlated significantly with the change in cose excursions with marked amelioration of peripheral
the basal rates of HGO (r = 0.74, P < .05). This was insulin resistance due to improved postreceptor insulin
associated with reduced fasting serum levels of gluca- action, which is at least partly due to enhanced glu-
gon (from 229 ± 15 to 141 ± 12 pg/ml), reduced free cose transport system activity; and 3) unchanged ab-
fatty acids (from 791 ± 87 to 379 ± 35 |xeq/L), and un- solute insulin levels in the face of markedly reduced
changed basal insulin levels (17 ± 4 to 15 ± 2 fill/ml). glycemia, indicative of enhanced p-cell sensitivity to in-
Peripheral glucose disposal, assessed by the eugly- sulinogenic stimuli. DIABETES 1986; 35:990-98.
cemic glucose-clamp technique, at insulin infusion
rates of 120 and 1200 mU rrr 2 • m i n 1 increased be-
tween 135 and 165%, from 128 ± 17 to 288 ± 24 mg
M
m~2 • m i n 1 during the 120-mU • rrr 2 • m i n 1 studies any patients with non-insulin-dependent diabe-
and from 159 ± 19 to 318 ± 24 mg rrr 2 • m i n 1 dur- tes mellitus (NIDDM) are obese, and numerous
ing the 1200-mU • rrr 2 • m i n 1 clamp studies, despite studies have demonstrated improved glucose
comparable steady-state serum insulin levels at each tolerance after weight loss.1-10 However, the
infusion rate before and after weight loss. HGO during mechanisms underlying the beneficial effects remain in-
the 120-mU rrr 2 m i n 1 clamp studies increased from completely understood because most studies have assessed
85% to complete (100%) suppression after treatment. the influence of weight loss on only one specific abnormality.
Adipocyte size was reduced 44% (851 ± 91 to 475 ± Furthermore, the changes reported after weight loss have
48 pi), whereas surface area decreased by 32% (4.30 x often been contradictory. Thus, although the hyperglycemia
104 to 2.92 x 104 (imVcell). Insulin binding to isolated
of NIDDM is the result of a combination of decreased insulin
adipocytes was unchanged, whereas basal in vitro
secretion, elevated rates of hepatic glucose output, and pe-
ripheral insulin resistance,11 the relative contribution of
From the Department of Medicine, University of California, San Diego, La Jolla, changes in each of these abnormalities to the improved gly-
CA; the San Diego Veterans Administration Medical Center, San Diego, CA; cemia after weight loss is not known.
and the Department of Medicine and Clinical Research Center, University of
Colorado Health Sciences Center, Denver, CO. Weight reduction in obese nondiabetic subjects leads to
Address reprint requests to Robert R. Henry, M.D., Veterans Administration improved tolerance to oral glucose and increased peripheral
Medical Center, Medical Research Service (V-111G), 3350 La Jolla Village
Drive, San Diego, CA 92161. sensitivity to insulin.12-15 In obese subjects with NIDDM, a
Received for publication 30 October 1985 and in revised form 10 March 1986. recent report indicates that the elevated basal hepatic glu-
990 DIABETES, VOL. 35, SEPTEMBER 1986
cose output (HGO) is reduced after weight loss,8 whereas stituted to a liquid with ~8 oz of water per serving. It contains
the insulin secretory response to various stimuli is improved 55% carbohydrate, mainly from lactose and fructose, 42%
in some studies13-61016"18 and unchanged in others.89 Even protein, primarily of milk and soy origin, and 3% milk fat, with
the few studies that have measured peripheral insulin re- the essential fatty acids linoleic acid and linolenic acid, and
sistance in NIDDM have been conflicting, with reports of both micronutrients to meet the daily requirements advised by the
improvement9 and no change8 after weight loss. National Research Council. After weight loss and for at least
To help clarify these issues, we have measured insulin 3 wk before restudy, subjects were placed on a solid weight-
secretion, HGO, and insulin resistance in a group of obese maintenance diet (30 kcal/kg) of the same composition as
NIDDM subjects before and after weight loss during a period the previously detailed liquid-formula diet.
of weight-maintenance refeeding. Studies of insulin secretion. On separate days after a 12-
h overnight fast and at least 30 min after the placement of
MATERIALS AND METHODS an indwelling venous cathether, an oral glucose-tolerance
Subjects. This study was conducted on eight obese subjects test and a meal-tolerance test were performed. For the oral
with NIDDM19 who were otherwise healthy. After written, vol- glucose-tolerance test, the subjects were given a 75-g load
untary, informed consent, all subjects were admitted to the at 8 AM consumed over a 15-min period, and blood samples
clinical research ward and underwent a complete medical were obtained at 0,30,60,120, and 180 min for measurement
history, physical examination, electrocardiogram, and chest of glucose and insulin levels. During the meal-tolerance tests,
X-ray. The next day, after an overnight fast, a chemistry panel blood samples were obtained in the fasting state and at
and urinalysis were done. Subjects were screened to ensure hourly intervals throughout the day for measurements of glu-
a stable body weight for at least 2 mo before admission. cose and insulin levels while the subject ingested, over a 30-
During diet therapy, subjects were encouraged to maintain min period, the allotted morning and noon portions of the
their usual level of physical exercise. During initial weight liquid-formula diet described above. The morning portion
reduction, all subjects were hospitalized from 10 to 40 days (1/5 of total calories) was ingested immediately after the
and then followed as outpatients. The duration of the weight- fasting sample was obtained and the noon meal (2/5 of total
reduction period ranged from 60 to 380 days. Seven subjects calories) was taken immediately after the 4-h sample was
had been on oral hypoglycemic agents, one was untreated, drawn. These studies were conducted before and then after
and none had ever been on insulin therapy. Oral hypogly- weight loss.
cemic agents were withdrawn at least 3 wk before entry into Euglycemic glucose-clamp studies. In vivo insulin sensi-
the study. One subject was on maintenance thyroxine ther- tivity was measured by a modification of the euglycemic glu-
apy, and no medications were taken that were known to affect cose-clamp technique as previously described.20 At 0700 h
carbohydrate metabolism. Additional characteristics of the after a 12-h overnight fast, an intravenous catheter was
study subjects are shown in Table 1. placed in an antecubital vein for infusion of insulin, glucose,
Diets. For at least 3 days before and then during each series [3-3H]glucose (New England Nuclear, Boston, MA), and po-
of studies, subjects consumed a weight-maintenance liquid- tassium phosphate. Another catheter was placed in a ret-
formula diet (30 kcal/kg) containing 45% carbohydrate, 40% rograde manner into a dorsal hand vein and kept in a warming
fat, and 15% protein given in three feedings of 1 /5, 2/5, and device at 70°C. After insertion of the catheters, [3-3H]glucose
2/5 of the total daily calories at 0800, 1200, and 1700 h, was infused for at least 120 min before initiating the insulin
respectively. Between study periods, the subjects consumed infusion. A priming dose of insulin was administered during
a very low-calorie diet (MediBase, Advanced Healthcare, Pa- the initial 10 min in a logrithmically decreasing manner to
cific Grove, CA), ranging from 330 to 600 kcal/day given in acutely raise serum insulin to the desired level, where it was
three servings. This diet is a powdered preparation recon- maintained for the duration of the study by a continuous
TABLE 1
Clinical and metabolic characteristics
Before treatment After treatment
Age (yr) 54 ± 4
Sex 5 male, 3 female
Duration of diabetes, yr 7± 2
Therapy of diabetes 7 on hypoglycemic agents,
1 on no treatment
Weight, kg 102.9 ± 5.1 86.1 ± 3.7*
Body mass index (kg/m2) 34.4 ± 1.8 28.7 ± 1.0*
Fasting plasma glucose (mg/dl) 277 ± 21 123 ± 8 t
Total glycosylated hemoglobin (%) 11.9 ± 0 . 8 7.5 ± 0.4*
Fasting plasma cholesterol (mg/dl) 181 ± 14 135 ± 12*
Fasting plasma triglyceride (mg/dl) 351 ± 124 125 ± 22*
Fasting serum free fatty acids (|xeq/!_):}: 791 ± 87 379 ± 35§
Fasting serum insulin (^ll/ml) 17±4 15 ± 2
Fasting serum glucagon (pg/ml) 229 ± 15 141 ±12t
Adipocyte cell volume (pi) 851 ± 91 475 ± 48f
Adipocyte cell surface area ( x 10" n,m2) 4.30 ± 0.31 2.92 ± 0.20f
Values are expressed as means ± SE (except for age, expressed as mean ± SD).
*P < .01. fP < 001. $N = 5. §P < .05.
DIABETES, VOL. 35, SEPTEMBER 1986 991
insulin infusion. The amount of insulin given during the initial performed on the day before the initial glucose clamp in all
10-min period was roughly twice that given during each sub- subjects both before and after weight loss. The methods of
sequent 10-min interval by continuous infusion. Plasma glu- these procedures have been described in detail previously.23
cose was maintained between 80 and 90 mg/dl throughout Analytic methods. Blood for glucose determinations was
the study period, with a coefficient of variation of <5%, by drawn, and the plasma was immediately separated with a
means of a variable rate of infusion of 20% dextrose. After Beckman microfuge (Beckman, Palo Alto, CA) and measured
initiation of insulin infusion, the serum glucose level was al- by the glucose oxidase method with a Beckman glucose
lowed to fall to - 1 0 0 mg/dl before initiating the infusion of analyzer (Beckman, Fullerton, CA). Blood for the determi-
20% glucose. Based on plasma glucose measurements done nation of serum insulin and free fatty acids was collected in
at 5-min intervals, the glucose infusion was adjusted as untreated tubes and allowed to clot at room temperature.
needed to maintain the plasma glucose level at —85 mg/dl Blood for glucagon determination was collected in tubes con-
during the period the measurements were made. Potassium taining a protease inhibitor (Trasylol, 500 KlU/ml; F.B.A. Phar-
phosphate was administered at a rate of 15-20 meq/h to maceuticals, New York, NY) and allowed to clot at room tem-
maintain the serum potassium level between 3.5 and 4.5 perature. Blood for glucose sp act was collected in tubes
meq/L and thus avoid hypokalemia or hypophosphatemia. containing potassium oxalate and sodium fluoride and im-
Each subject was studied at an infusion rate of 120 mil • mediately placed on ice until spun. These specimens were
rrr 2 • min~1 to allow assessment of both submaximal pe- then centrifuged, and the serum was removed and stored at
ripheral glucose disposal and suppressibility of HGO. In ad- -20°C until the determinations were made. Blood for total
dition, on a different day each subject was studied at an cholesterol and triglycerides was collected in EDTA-treated
insulin rate of 1200 mU • m~2 • min~1 to assess maximum tubes, centrifuged, and refrigerated at 4°C until assayed.
stimulation of overall glucose disposal. Because suppression Serum insulin levels were measured by a specific double-
of HGO might not be complete in diabetic subjects during antibody radioimmunoassay according to the method of Des-
the insulin infusion rate of 120 mU • rrr 2 • min~\ the overall buquois and Aurbach.24 Serum glucagon was assayed by
glucose disposal rate was determined isotopically (see next the charcoal-separation method of Unger and Faloona.25 Free
section) for each 20-min interval after achieving euglycemia. fatty acids were determined in serum by an enzymatic col-
Endogenous glucose production is completely suppressed orimetric method kit (Wako Pure Chemical Industries, Osaka,
during the 1200-mU • m~2 • min~1 insulin infusion so that the Japan). Total cholesterol and triglyceride were measured en-
total glucose disposal rate is equal to the exogenous glucose zymatically.2627 Total glycosylated hemoglobin was deter-
infusion rate. Due to variability in the length of time required mined in heparinized blood by the Fast Hemoglobin Test
to attain steady-state insulin effects on glucose kinetics, all System kit (Isolab, Akron, OH).
studies were carried out for at least 180 min or for 60 min Data analysis. All calculations and statistical analyses were
after stable glucose infusion rates at euglycemia. The data performed on a Digital P.D.P. 11/24 computer (Digital, May-
for the last three 20-min intervals of each study were sub- nard, MA) with the CLINFO data-base management and anal-
sequently meaned and used as the data point for that par- ysis program (Bolt, Beranek, and Newman, Cambridge, MA).
ticular study. Glucose-clamp studies were carried out for the For statistical comparisons, analysis of variance (ANOVA) for
identical duration of time before and after weight loss, with repeated measures and the Student's t test for paired data
comparison of the same 20-min data points. Urinary glucose were used. The Wilcoxon signed-rank test was used for anal-
loss was not measured with all studies conducted under ysis when the data was not normally distributed. Correlation
euglycemic conditions. All subjects were restudied in an coefficients were calculated by the method of least squares.
identical manner after weight loss. Data are expressed as means ± SE unless otherwise stated.
Hepatic glucose output. The rate of glucose appearance
(Ra) and the rate of overall glucose disappearance (Rd) were
quantitated in both the basal (postabsorptive) state and dur- RESULTS
ing the 120-mU • rrr 2 • min" 1 glucose-clamp studies by in- The mean values of several metabolic variables measured
fusion of [3-3H]glucose in a primed continuous manner.2021 before and after weight loss and refeeding are listed in Table
With this technique, 30 ixCi of the tracer was injected as a 1. The mean weight of the group fell from 102.9 ± 5.1 to 86.1
bolus, followed by a continuous infusion rate of 0.60 ixCi/ ± 3.7 kg (P < .001), resulting in a total weight loss of 16.8
min. Blood samples were obtained at 20-min intervals for the ± 2.7 kg (range, 3.2-26.4 kg) or 16.4% of the initial mean
determination of both the concentration and sp act of glu- body weight. Similarly the mean body-mass index, which
cose. Ra and Rd were then calculated from the Steele correlates well with the degree of obesity, fell from 34.4 ±
equations22 in their modified derivative form21 because the 1.8 to 28.7 ± 1.0 kg/m 2 (normal values: <25 for males, <27
tracer exhibits non-steady-state kinetics under these condi- for females19). The mean fasting plasma glucose level de-
tions. The rate of HGO was then calculated, knowing that Ra creased from 277 ± 21 to 123 ± 8 mg/dl (P < .001), and
represents the sum of HGO and the rate of infusion of ex- the total glycosylated hemoglobin fell from 11.9 ± 0.8 to 7.5
ogenous glucose. Measurements of HGO were made before ± 0.4% (P < .001). Significant reductions also occurred in
diet therapy and again after 3 wk of weight-maintenance the fasting levels of plasma cholesterol, from 181 ± 14 to
refeeding. 135 ± 12 mg/dl (P < .01); plasma triglyceride, from 351 ±
Adipocyte insulin binding and 3-O-methylglucose trans- 124 to 125 ± 22 mg/dl (P < .01); and serum free fatty acids,
port. Isolated adipocytes for insulin binding and 3-O-meth- from 791 ± 87 to 379 ± 35 ixeq/L (P < .05). The mean
ylglucose transport were prepared from tissue obtained from fasting serum insulin concentration was not significantly dif-
open biopsy of the lower abdominal wall. The biopsy was ferent at 17 ± 4 and 15 ± 2 |i,U/ml (P, not significant),
992 DIABETES, VOL. 35, SEPTEMBER 1986
A
400 - * * * p<0.001
T T 150 • Before Diet
* /~ O After Diet
300 /
o
uli
o
o -
a 200
E ^ ^
s^ * •
Y 50
100
FIG. 1. Plasma glucose response (A) and serum
1 1 1 1 insulin response (B) to 75-g oral glucose-toler-
ance test before ( • ) and after (O) diet therapy.
30 60 120 180 30 60 120 180 Results are plotted as means ± SE. Shaded area
Time (minutes) Time (minutes) indicates normal range (means ± SE).
whereas the fasting serum glucagon level fell from 229 ± 15 reduced (P < .001) at all times, while the corresponding
to 141 ± 12 pg/ml (P < .001). There was a highly significant insulin levels (Fig. 28) remained unchanged [P(F15 = 1.67),
reduction in both adipocyte cell volume, from 851 ± 91 to not significant, ANOVA].
475 ± 48 pi (P < .001), and surface area, from 4.30 ± 0.31 As shown in Fig. 3/4, the total area under the glucose curve
x 104 |xm2 to 2.92 ± 0.20 x 104 |xm2 (P < .001), after weight after the oral glucose challenge was reduced 44% from
loss. 62,151 ± 4362 before diet therapy to 34,519 ± 2936 mg •
Plasma glucose and insulin levels. The plasma glucose dh 1 • min^1 after weight loss (P < .001). The total area under
and serum insulin responses after oral glucose and test meals the glucose curve during the meal-tolerance test was also
of mixed composition are shown before and after diet therapy significantly reduced after weight loss, falling 58% from
in Figs. 1 and 2, respectively. After weight loss, the mean 160,196 ± 12,706 to 67,425 ±4534 mg • dh 1 • min~1 (P <
plasma glucose levels after the 75-g oral glucose load were .001). The incremental areas under the glucose curve were
significantly reduced (P < .001) at all times measured (Fig. not significantly different after the oral glucose-tolerance tests
1/4). In contrast, the corresponding serum insulin levels (Fig. (from 13,596 ± 1006 to 10,534 ± 1425 mg • dl" 1 • mirr 1 , P,
16) were not significantly different at any time interval [P(F1i7 not significant) but were significantly reduced (from 41,760
= 1.11), not significant, ANOVA]. The glucose levels after ± 3420 to 18,480 ± 3060 mg • dh 1 • m i n \ P < .001) during
the morning and noon mixed meals are shown in Fig. 2k. the meal-tolerance tests. The total areas under the insulin
Again, the mean plasma glucose levels were significantly curve (Fig. 36) were not significantly different before and
* p < 0.001
400
• Before Diet
150 o After Diet
D) 300
•= 100
200
50
100
FIG. 2. Plasma glucose response {A) and serum
insulin response (B) to test meals of mixed com-
position before ( • ) and after (O) diet therapy. Re-
8 9 10 11 Noon 1 2 3 10 11 Noon 1 3 sults are plotted as means ± SE. Shaded area in-
AM PM AM PM dicates normal range (means ± SE).
DIABETES, VOL. 35, SEPTEMBER 1986 993
OGTT MTT OGTT MTT
16 T 20
*p < 0.001
15
10
FIG. 3. Total (D) and incremental ( 0 ) glucose re-
sponse (A) and total (D) and incremental ( 0 ) in-
sulin response (B) expressed as area under curve
during oral glucose-tolerance tests (OGTT) and
meal-tolerance tests (MTT) before and after diet
therapy. Results are plotted as means ± SE. Before After Before After Before After Before After
Diet Diet Diet Diet
after diet therapy after either the oral glucose-tolerance tests suppression of basal HGO was calculated from the values
(4374 ± 881 vs. 5571 ± 531 |xU • ml" 1 • mirr 1 , P, not sig- obtained for residual HGO during the 120-mU • m~2 • min- 1
nificant) or the meal-tolerance tests (17,276 ± 3726 vs. insulin infusion euglycemic clamp. The mean basal HGO was
19,091 ±2649(xU • ml" 1 • mirr 1 , P, not significant). Similarly, 21 ± 6 mg • irr 2 • min" 1 , or 85% suppressed, before diet
the incremental areas under the insulin curves were un- therapy and fell to - 4 4 ± 21 mg • rrr 2 • min" 1 after weight
changed during the oral glucose-tolerance tests (1584 ± 349 loss. For the purpose of this study, these negative values
vs. 2893 ± 440 |xU • ml" 1 • min~1, P, not significant) and the were assumed to indicate complete (100%) suppression of
meal-tolerance tests (9480 ± 2580 vs. 13,080 ± 1740 HGO. When the basal HGO and the corresponding basal
(xU • ml" 1 • rnirn1, P, not significant). The areas under the plasma glucose levels of individual subjects were compared
glucose and insulin curves were greater during the meal- both before and after weight loss, a very strong correlation
tolerance tests than during the oral glucose-tolerance tests was present (r = 0.91, P < .001). A significant correlation
due to the different duration of study times (7 vs. 3 h, re- was also present between the change in basal HGO and the
spectively). change in the basal plasma glucose level (r = 0.74, P <
Glucose-clamp studies. The individual and mean steady- .05) after weight loss.
state rates of total glucose disposal and the corresponding Insulin-binding studies. Figure 6 shows the competition
mean serum insulin levels attained during insulin infusions of curves for insulin binding to adipocyte receptors before and
120 and 1200 mil • nrr2 • min~1 are shown before and after after weight loss. When expressed on the basis of cell number
weight loss in Fig. 4, A and B, respectively. After weight loss, (Fig. 6A), it is apparent that these curves are essentially
the glucose disposal rate increased an average of 165%, identical, implying that insulin binding to receptors is unaf-
from 128 ± 17 to 288 ± 24 mg -irr 2 • min" 1 (P < .001), fected by weight loss. However, after weight loss it may be
during the 120-mU • nrr2 • min 1 studies and 136%, from 159 more appropriate to express the data per unit of cell surface
± 19 to 318 ± 24 mg • m" 2 • min- 1 (P < .001), during the area, because adipocyte volume was reduced 44% (from
1200-mU • rrr 2 • min" 1 clamp studies. The percent of maximal 851 ± 91 to 475 ± 48 pi) after weight loss, whereas surface
effect on total glucose disposal achieved during the 120-mU area decreased by 32% (from 4.30 x 104 to 2.92 x 104|Arrr7
• m~2 • min" 1 studies remained essentially unchanged: 83% cell). When the insulin-binding data were normalized to cell
before diet therapy and 87% after weight loss (P, not signif- surface area (Fig. 66), the binding curves were still com-
icant). The serum insulin levels at each infusion rate were parable with a significant increase in bindng (P < .05) noted
similar before and after weight loss at 353 ± 30 and 302 ± only at an insulin concentration of 5 ng/ml.
27 |xU/ml, respectively (P, not significant), during the 120- Adipocyte glucose transport. Basal and maximal rates of
mU • rrr 2 • min" 1 studies and 8,699 ± 754 and 9,611 ± 437 3-O-methylglucose transport in isolated adipocytes are
(xU/ml, respectively (P, not significant), during the 1200-mU shown in Fig. 7. When glucose transport is expressed per
• nrr2 • min" 1 study. cell number (Fig. 7A), the basal rate does not increase sig-
Basal hepatic glucose production rates. As shown in Fig. nificantly after weight loss [from 0.33 ± 0.20 to 0.62 ± 0.28
5, the mean basal rate of HGO before diet therapy was 138 pmol/(2 x 105 cells) x 10 s" 1 (P, not significant)], whereas
± 1 1 mg • m~2 • min~1 and fell to 87 ± 5 mg • irr 2 • min" 1 the maximal rate does [from 0.64 ± 0.29 to 1.18 ± 0.48 pmol/
(P < .001) after weight loss, which is comparable to that (2 x 105 cells) x 10 s~1 ( P < .05)]. When glucose transport
determined in nondiabetic control subjects (79 ± 3 mg • irr 2 is expressed per unit surface area (Fig. 78), both basal [from
• min"1). (One subject did not have basal HGO determined 0.21 ± 0.13 to 0.53 ± 0.24 pmol/(2 x 109 |xm2) x 10 S"1
during the study.) The mean basal plasma glucose level at (P < .005)] and maximal [from 0.42 ± 0.20 to 1.04 ± 0.30
these rates of HGO was 263 ± 21 mg/dl before diet therapy pmol/(2 x 109 n,m2) x 10 S"1 (P < .005)] rates of glucose
and 118 ± 6 mg/dl after weight loss (P < .001). The percent transport increased significantly.
994 DIABETES, VOL. 35, SEPTEMBER 1986
R R HENRY P WALLACE. AND
nrr2 • min~1) as compared with nondiabetic controls (79 ±
3 mg • m~2 • min 1 ) and that a close relationship exists be-
tween basal HGO and the fasting plasma glucose level in
400 - individual subjects both before and after weight loss (r =
0.91, P < .001). Weight loss led to a 40% reduction in the
basal rate of HGO [from 138 ± 11 to 87 ± 5 mg • rrr 2 • min~1
en (P < .001)] with significantly increased sensitivity to insulin's
~ 300 h
0) suppressive effects during the euglycemic clamp studies
[from 85 to 100% suppression (P < .001)]. Furthermore, the
change in HGO was well correlated to the change in fasting
glucose level, indicating that the effect of weight reduction
to lower fasting glycemia was due to the fall in basal HGO.
We have previously shown that resistance to the in vivo
action of insulin to promote glucose disposal in NIDDM is
due to a combination of receptor and postreceptor defects.20
Before initiating dietary therapy, the peripheral uptake of glu-
cose determined by the euglycemic clamp technique at
steady-state serum insulin concentrations of 300 and 10,000
ixU/ml was markedly reduced compared with normal control
subjects (see Fig. 4). After weight loss and 3 wk of weight
stabilization, the mean in vivo glucose disposal rates ap-
12,000
10,000 A
* p < 0.001
T 300
8,000
3. ^ \
200 -
*==
100 -
400
T
T
T
200
150 ~
Before After Normal Before After Normal
Diet Range Diet Range
FIG. 4. Individual and mean glucose disposal rates {A) and corre-
sponding means ± SE of serum insulin levels (B) during 120-mU - m 2
• min' 1 insulin infusion rates (D) and 1200-mU m" 2 min 1 insulin 2 100 -
infusion rates (E3) before and after diet therapy. Glucose disposal rates
(means ± SE) in nondiabetic subjects are included for comparison,
(D
DISCUSSION
Despite numerous studies demonstrating the merits of weight
reduction in obese NIDDM,1-1016-18 little is known about the
mechanisms underlying this improvement. Our study docu-
ments that modest weight loss in obese NIDDM subjects
results in greatly improved carbohydrate metabolism asso-
ciated with improved insulin secretion, reduced basal HGO,
and enhanced in vivo and in vitro insulin action. These Before Diet After Diet
beneficial metabolic effects were not simply due to the
FIG. 5. Individual and mean fasting plasma glucose levels {A) and
hypocaloric state during weight loss because the post- hepatic glucose output (B) during the basal state (D) and during
weight-reduction studies were carried out after a weight- 120-mU • m 2 • min 1 insulin-infusion euglycemic clamp study (E) be-
maintenance refeeding period of 3 wk. fore and after diet therapy. Hepatic glucose output during euglycemic
clamp study is expressed as mean ± SE. Normal range (mean ± SE)
We demonstrated, as have others,28-32 that the basal HGO for basal hepatic glucose output in nondiabetic subjects is included for
is elevated in untreated diabetic subjects (138 ± 11 mg • comparison.
DIABETES, VOL. 35, SEPTEMBER 1986 995
5 1.0
FIG. 6. Insulin binding to isolated adipocytes ex-
pressed as percentage 12sl-labeled insulin bound
per cell number {A) and per unit surface area (B)
before ( • ) and after (O) diet therapy. All data are
corrected for nonspecific binding and are plotted
Insulin Concentration (ng/ml) as means ± SE.
proached those of normal subjects (increasing between 135 jects after weight loss. Similarly, caloric restriction in obese
and 165%), clearly demonstrating the effects of weight re- NIDDM subjects has been associated with increased insulin
duction to ameliorate peripheral insulin resistance in NIDDM. receptors on circulating monocytes.510 The reasons for these
Earlier studies have shown improved insulin sensitivity after differences are not entirely clear but may indicate that obese
weight loss in obese subjects with normal or impaired glu- NIDDM subjects do not respond to weight loss as obese
cose tolerance.12-15 However, the effects of weight loss on nondiabetic subjects do or may reflect differences in cell type
insulin resistance in NIDDM have been more controversial. responsiveness (note that monocytes are not a recognized
Thus, Hughes et al.9 demonstrated improved insulin-me- target tissue for insulin as are adipocytes). In addition, a
diated glucose uptake after weight loss in obese NIDDM. In unique aspect of our study that might explain the discrepancy
contrast, however, Bogardus et al.,8 using the euglycemic in insulin binding is the prolonged period of weight-mainte-
clamp technique, documented significant improvement of nance refeeding (3 wk) after weight loss. Nevertheless, if one
peripheral insulin resistance in obese NIDDM only when diet assumes that insulin binding to adipocytes reflects insulin
therapy was combined with physical exercise, not with weight binding to other target tissues (particularly skeletal muscle),
loss from diet therapy alone. However, these workers studied then it follows that the marked improvement in peripheral
subjects with less severe diabetes (pretreatment fasting glucose disposal must be due to enhanced postbinding
plasma glucose 167 ± 28 mg/dl), who lost considerably less steps in insulin action. This concept is quite supportive of
weight (9.9 ± 1.3 kg) than those in our study (16.8 ± 2.7 earlier studies by ourselves20 and others36 demonstrating that
kg)- the major cause of the insulin resistance in NIDDM is due to
The mechanisms underlying this improvement in insulin's a postbinding defect in insulin action.
effect to stimulate peripheral glucose disposal are partially Thus, we have previously suggested that a defect in glu-
elucidated by our study. Thus, insulin binding to isolated cose transport activity is an important contributor to the post-
adipocytes was measured before and after weight reduction, binding defect in in vivo insulin action observed in NIDDM
and little if any change was noted (see Fig. 6). The lack of subjects.37 Accordingly, in our studies we have measured
any effect of weight loss on insulin binding in our study is adipocyte glucose transport before and after weight loss and
discordant with previous reports of increased insulin recep- have found substantial increases in glucose transport activ-
tors on both monocytes33 and adipocytes3435 in obese sub- ity. In combination, these results demonstrating correspond-
r A
* p < 0.05
<2 1.5 1.5
E 1-0 .1.0
0.5 80.5
FIG. 7. Basal and maximal rates (means ± SE) of
3-O-methylglucose transport in isolated adipo-
Before After Before After Before After Before After cytes expressed per cell number (A) and per unit
Diet Diet surface area (0) before and after diet therapy.
996 DIABETES, VOL. 35, SEPTEMBER 1986
ing increases in in vivo glucose disposal and in vitro adi- jects,324142 it is conceivable that the postbinding defect in
pocyte glucose transport with no change in insulin binding insulin action that characterizes the untreated diabetic state
certainly support the concept of decreased glucose transport results from insulin deficiency. However, our study does not
as a major factor underlying insulin resistance before weight support this hypothesis because peripheral insulin levels did
reduction and of increased glucose transport activity playing not increase after treatment, despite marked improvement of
an important role in the amelioration of peripheral insulin re- insulin resistance. This raises the possibility that hypergly-
sistance induced by weight loss. cemia itself leads to insulin resistance, possibly through long-
Plasma insulin levels after either oral glucose or mixed term effects impairing glucose transport system activity.
meals did not change significantly after weight reduction. Indirect support for this comes from our previous studies
Although it is possible that unchanged insulin levels could showing that the magnitude of the in vivo postreceptor
result from counterbalancing changes in insulin secretion defect20 as well as the in vitro decrease in glucose transport
and hepatic insulin extraction, this seems highly unlikely be- activity37 is closely correlated with the height of the fasting
cause the steady-state plasma insulin levels were compa- plasma glucose concentration in subjects with NIDDM. An
rable during the euglycemic glucose-clamp studies (see Fig. additional possibility is that elevated free fatty acid concen-
4) before and after weight loss. Therefore, it seems most trations contribute to the pre-weight-reduction insulin-re-
probable that the amount of insulin secreted to both oral sistant state. According to the "glucose fatty-acid cycle"
glucose and mixed meals was quite similar before and after (Randle hypothesis), an inverse relationship exists between
weight reduction. However, plasma insulin levels cannot be circulating free fatty acid levels and insulin-mediated glucose
properly interpreted without considering the prevailing levels disposal.43 It has recently been directly demonstrated that
of glycemia. Glycemia was markedly reduced after weight artificial elevation of free fatty acid levels in normal humans
loss, and because the effects of hyperglycemia to potentiate results in reduced insulin-stimulated glucose disposal rates.44
insulin secretion are well known,3839 the presence of un- By inference, the reduction of free fatty acid levels after
changed insulin levels in the face of reduced glucose levels weight loss (see Table 1) could lead to the opposite effect,
after weight loss are indicative of enhanced fJ-cell function. with an improvement in peripheral insulin action.
In other words, weight loss apparently leads to improved Our studies are also quite relevant to an understanding of
sensitivity of (S-cells to insulinogenic stimuli, allowing the pan- the pathogenesis of hyperglycemia in NIDDM. Thus, the fact
creas to secrete similar amounts of insulin after weight loss that plasma glucose levels were markedly reduced, despite
in the presence of much lower ambient glucose levels. no change in plasma insulin levels at the same time that basal
The total areas under the plasma glucose curves after oral HGO and peripheral insulin resistance were improved, dem-
glucose or meals were greatly reduced after weight loss, and onstrates that the proximate cause of the hyperglycemia in
this was predominantly due to the reduction in the fasting the untreated state was the elevated HGO rates and the
plasma glucose level. The incremental glucose levels were peripheral insulin resistance. As previously reviewed,45 fast-
either unchanged (after oral glucose) or lower (after mixed ing hyperglycemia is predominantly due to elevated rates of
meals) after weight loss, and this indicates an improvement basal HGO, whereas postprandial hyperglycemia is mostly
in insulin-mediated postprandial glucose uptake. Thus, the due to decreased insulin-mediated glucose disposal. Thus,
rate of glucose uptake is increased not only by insulin but the weight-reduction-mediated fall in basal HGO was the
also by hyperglycemia. The higher the plasma glucose level, major cause of reduced fasting glucose levels, whereas the
the greater the mass-action effect of glucose to promote its improved peripheral insulin action led to the enhanced post-
own uptake. Therefore, if insulin action is constant, the post- prandial glucose disposal. This line of reasoning should not
prandial increments to a comparable incoming glucose load be construed to imply that impaired insulin secretion is un-
will be lower the higher the ambient glucose level. At the time important to the pathogenesis of NIDDM. Clearly, many in-
of the post-weight-reduction studies, plasma glucose levels dividuals with insulin resistance, due to obesity or other
were reduced by - 5 0 % indicating a twofold greater mass- causes, maintain relatively normal glucose homeostasis by
action effect of glucose to promote its own uptake before augmenting insulin secretion with the development of hy-
compared with after weight loss. Despite this, the postpran- perinsulinemia. By analogy, it seems most likely that if NIDDM
dial glucose increments after weight loss are either un- subjects were able to mount a hyperinsulinemic fJ-cell re-
changed (oral glucose-tolerance test) or lower (meal-toler- sponse, hyperglycemia could be largely or partly avoided,
ance test), demonstrating that the effects of insulin to even in the face of increased basal HGO and peripheral
stimulate glucose uptake in the postprandial state are mark- insulin resistance. With this line of reasoning, when insulin
edly improved. This is consistent with the enhanced glucose requirements increase due to advancing insulin resistance
disposal rates during the euglycemic clamp studies and fur- and/or increased HGO, then NIDDM subjects (or those des-
ther demonstrates that the improved peripheral insulin re- tined to become diabetic) are unable to meet target tissue
sistance measured during these clamp studies has a phys- demands because of restricted (3-cell function, allowing hy-
iologically relevant manifestation, i.e., lowered postprandial perglycemia to develop or worsen. With this scenario the
plasma glucose levels. Just as hyperglycemia potentiates three metabolic defects—peripheral insulin resistance, im-
insulin secretion, it also potentiates glucose uptake, and it is paired insulin secretion, and increased basal HGO—all work
quite notable that after weight loss the system resets itself in concert, resulting in the NIDDM state.
so that postprandial increments of both insulin and glucose
are fairly similar to the values before weight loss. ACKNOWLEDGMENTS
Because insulin therapy can ameliorate the insulin resis- We thank the laboratory staffs and nurses of the Special
tance in alloxan-diabetic dogs40 as well as in NIDDM sub- Diagnostic and Treatment Unit at the San Diego Veterans
DIABETES, VOL. 35, SEPTEMBER 1986 997
Administration Medical Center, the University of California, sugar and insulin levels in patients with maturity-onset diabetes mellitus. Lan-
cet 1975; 1:1263-66.
San Diego, and the University of Colorado Clinical Research 19
National Diabetes Data Group: Classification and diagnosis of dia-
Center for assistance; Paul Shragg, CLINFO system man- betes mellitus and other categories of glucose intolerance. Diabetes 1979;
ager, for statistical advice and assistance; and Elizabeth Mar- 28:1039-57.
20
Kolterman, O. G., Gray, R. S., Griffin, J., Burstein, P., Insel, J., Scarlett,
tinez for excellent secretarial assistance in preparing the J. A., and Olefsky, J. M.: Receptor and postreceptor defects contribute to the
manuscript. insulin resistance in non-insulin-dependent diabetes mellitus. J. Clin. Invest.
1981; 68:957-69.
This work was supported by funds from the Medical Re- 21
Chiasson, J. L , Liljenquist, J. E., Lacy, W. W., Jennings, A. S., and
search Council of Canada and the Medical Research Service Cherrington, A. D.: Gluconeogenesis: methodological approaches in vivo. Fed.
of the Veterans Administration, NIAMDD Grants AM-26180 Proc. 1977; 3 6 : 2 2 9 - 3 5 .
22
Steele, R.: Influence of glucose loading and injected insulin on hepatic
and AM-31317, NIH Clinical Research Center Branch Grants glucose output. Ann. NY Acad. Sci. 1959; 82:420-30.
RR-00051 and RR-00827, and a grant from the Cambridge 23
Ciaraldi, T. P., Kolterman, O. G., Siegel, J. A., and Olefsky, J. M.:
Quest Foundation. Insulin stimulated glucose transport in human adipocytes. Am. J. Physiol. 1979;
236:E621-25.
Dr. Henry is the recipient of a Medical Research Council 24
Desbuquois, B., a n d Aurbach, A. D.: Use of polyethylene glycol to
of Canada Fellowship Award. separate free a n d antibody-bound peptide hormones in radioimmunoassays.
J. Clin. Endocrinol. Metab. 1971; 3 3 : 7 3 2 - 3 8 .
This work was presented in abstract form at the annual 25
Faloona, G. R., a n d Unger, R. H.: Methods of hormone radioimmu-
meeting of the American Diabetes Association, Baltimore, noassay. In G l u c a g o n . Jaffe, B. M., a n d Behrman, H. R., Eds. New York,
MD, June, 1985. Academic, 1975:317-30.
26
D o w Chemical C o m p a n y : Triglycerides Determination. Midland, Ml,
1978.
27
Richmond, W.: Preparation a n d properties of a cholesterol oxidase
REFERENCES from Nocardia Sp. a n d its application to the enzymatic assay of total cholesterol
1
Hadden, D. R., Montgomery, D. A. D., Skelley, R. J., Tremble, E. R., in serum. Clin. Chem. 1973; 19:1350-56.
28
Weaver, J. A., Wilson, E. A., and Buchanan, K. D.: Maturity onset diabetes Bowen, H. F, a n d Moorhouse, J. A.: Glucose turnover and disposal
mellitus: response to intensive dietary management. Br. Med. J. 1975; 3 : 2 7 6 - in maturity-onset diabetes. J . Clin. Invest. 1973; 5 2 : 3 0 3 3 - 4 5 .
29
78. Best, J. D., J u d z e w i t s c h , R. G., Pfeifer, M. A., Beard, J. C , Halter,
2
Bistrian, B. R., Blackburn, G. L , Flatt, J., Sizer, J., Scrimshaw, N. S., J. B., and Porte, D., Jr.: The effect of chronic sulfonylurea therapy on hepatic
and Sherman, M.: Nitrogen metabolism and insulin requirements in obese glucose production in non-insulin-dependent diabetes. Diabetes 1982;
diabetic adults on a protein-sparing modified fast. Diabetes 1976; 2 5 : 4 9 4 - 31:333-38.
30
504. Revers, R. R., Fink, R., Griffin, J., Olefsky, J. M., and Kolterman,
3
Genuth, S. M.: Insulin secretion in obesity and diabetes: an illustrative O. G.: Influence of h y p e r g l y c e m i a o n insulin's in vivo effects in type II diabetes.
case. Ann. Intern. Med. 1977; 87:714-16. J. Clin. Invest. 1984; 7 3 : 6 6 4 - 7 2 .
4 31
Savage, P. J., Bennion, L. J., and Bennett, P. K : Normalization of insulin Kolterman, O. G., Gray, R. S., Shapiro, G., Scarlett, J . A., Griffin, J.,
and glucagon secretion in ketosis-resistant diabetes mellitus with prolonged and Olefsky, J. M.: The acute a n d chronic effects of sulfonylurea therapy in
diet therapy. J . Clin. Endocrinol. Metab. 1979; 4 9 : 8 3 0 - 3 3 . type II diabetes. Diabetes 1984; 3 3 : 3 4 6 - 5 4 .
5 32
Savage, P. J., Bennion, L. J., Flock, E. V., Nagulesparan, M., Mott, D., Garvey, W. T., Olefsky, J. M., Griffin, J., H a m m a n , R. F, a n d Koiterman,
Roth, J., Unger, R. H., and Bennett, P. H.: Diet-induced improvement of ab- 0. G.: The effect of insulin treatment on insulin secretion and insulin action in
normalities in insulin and glucagon secretion and in insulin receptor binding type II diabetes mellitus. Diabetes 1985; 34:222-34.
33
in diabetes mellitus. J. Clin. Endocrinol. Metab. 1979; 48:999-1007. Bar, R. S., G o r d e n , P., Roth, J., Kahn, C. R., a n d DeMeyts, P.: Fluc-
6
Nagulesparan, M., Savage, P. J., Bennion, L. J., Unger, R. H., and tuations in the affinity and concentration of insulin receptors on circulating
Bennett, P. H.: Diminished effect of caloric restriction on control of hypergly- monocytes of obese patients. J. Clin. Invest. 1976; 58:1123-35.
34
cemia with increasing known duration of type II diabetes mellitus. J. Clin. Kolterman, 0. G., Saekow, M., and Olefsky, J. M.: The effects of acute
Endocrinol. Metab. 1981; 5 3 : 5 6 0 - 6 8 . and chronic starvation on insulin binding to isolated human adipocytes. J. Clin.
7
Fitz, J. D., Sperling, E. M., and Fein, H. G.: A hypocaloric high-protein Endocrinol. Metab. 1979; 48:836-42.
35
diet as primary therapy for adults with obesity-related diabetes: effective long- Pedersen, 0., Hjollund, E., and Sorensen, N. S.: Insulin receptor bind-
term use in a community hospital. Diabetes Care 1983; 6:328-33. ing and insulin action in human fat cells: effects of obesity and fasting. Me-
8
Bogardus, C , Ravussin, E., Robbins, D. C , Wolfe, R. R., Horton, tabolism 1982; 31:82-93.
36
E. S., a n d Simms, E. A. H : Effects of physical training and diet therapy on Rizza, R. A., Mandarino, L. J., and Gerich, J. E.: Mechanism and
carbohydrate metabolism in patients with glucose intolerance and non-insulin- significance of insulin resistance in noninsulin-dependent diabetes mellitus.
dependent diabetes mellitus. Diabetes 1984; 33:311-18. Diabetes 1981; 30:990-95.
9 37
Hughes, T. A., Gwynne, J. T., Switzer, B. R., Herbst, C , and White, Ciaraldi, T. P., Kolterman, 0. G., Scarlett, J. A., Kao, M., and Olefsky,
G.: Effects of caloric restriction and weight loss on glycemic control, insulin J. M.: Role of glucose transport in the postreceptor defect of non-insulin-
release and resistance, and atherosclerotic risk in obese patients with type II dependent diabetes mellitus. Diabetes 1982; 31:1016-22.
38
diabetes mellitus. Am. J. Med. 1984; 77:7-17. Halter, J. B., Graf, R. J., a n d Porte, D., Jr.: Potentiation of insulin
10
Beck-Nielsen, H., Pedersen, O., andSorensen, N.S.: Effects of dietary secretory responses b y p l a s m a g l u c o s e levels in man: evidence that hyper-
changes on cellular insulin binding and in vivo insulin sensitivity. Metabolism glycemia in diabetes c o m p e n s a t e s for impaired glucose potentiation. J. Clin.
1980; 2 9 : 4 8 2 - 8 7 . Endocrinol. Metab. 1979; 48:946-54.
11 39
DeFronzo, R. A., Ferrannini, E., and Koivisto, V : New concepts in the Ward, W. K., Bolgiano, D. C , McKnight, B., Halter, J. B., a n d Porte,
pathogenesis a n d treatment of noninsulin-dependent diabetes mellitus. A m . D., Jr.: Diminished B cell secretory c a p a c i t y in patients with noninsulin-de-
J. Med. 1983; 74 (Suppl.):52-81. pendent diabetes mellitus. J . Clin. Invest. 1984; 7 4 : 1 3 1 8 - 2 8 .
12 40
Olefsky, J . , Reaven, G. M., and Farquhar, J. W.: Effects of weight Reaven, G. M., S a g e m a n , W. S., a n d Swenson, R. S.: Development
reduction on obesity. Studies of lipid and carbohydrate metabolism in normal of insulin resistance in normal d o g s following alloxan-induced insulin defi-
and hyperlipoproteinemic subjects. J. Clin. Invest. 1974; 5 3 : 6 4 - 7 6 . • ciency. Diabetologia 1977; 13:459-62.
13 41
Kalkhoff, R. K., Kim, H. J., Cerletty, J., and Ferrou, C. A.: Metabolic Scarlett, J. A., Gray, R. S., Griffin, J., Olefsky, J. M., and Kolterman,
effects of weight loss in obese subjects. Diabetes 1971; 2 0 : 8 3 - 9 1 . 0. G.: Insulin treatment reverses the insulin resistance of type II diabetes
14
Kosaka, K., Hagura, R., Odagiri, R., Saito, F, and Kuzuya, T : Effect mellitus. Diabetes Care 1982; 5:353-63.
42
of weight changes on serum insulin response in subjects with normal oral Scarlett, J. A., Kolterman, 0. G., Ciaraldi, T. P., Kao, M., and Olefsky,
glucose tolerance. J. Clin. Endocrinol. Metab. 1972; 3 5 : 6 5 5 - 5 8 . J. M.: Insulin treatment reverses the postreceptor defect in adipocyte 3-0-
15
Berkowitz, D.: Metabolic changes associated with obesity before and methylglucose transport in type II diabetes mellitus. J. Clin. Endocrinol. Metab.
after weight reduction. JAMA 1964; 187:399-403. 1983; 56:1195-201.
16 43
Stanik, S., and Marcus, M.: Insulin secretion improves following di- Randle, P. J., Garland, P. B., Hales, C. N., and Newsholme, E. A.:
etary control of plasma glucose in severely hyperglycemic obese patients. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic
Metabolism 1980; 2 9 : 3 4 6 - 5 0 . disturbances of diabetes mellitus. Lancet 1963; 1:785-89.
17 44
Kosaka, K., Kuzuya, T., Akanuma, Y., and Hagura, R.: Increase in Ferrannini, E., Barrett, E. J., Bevilacqua, S., and DeFronzo, R. A.:
insulin response after treatment of overt maturity-onset diabetes is independent Effect of fatty acids on glucose production and utilization in man. J. Clin. Invest.
of the mode of treatment. Diabetologia 1980; 18:23-28. 1983; 72:1737-47.
18 45
Doar, J. W. H., Thompson, M. E., Wilde, C. E., and Sewell, P. F. J.: Olefsky, J. M.: Introduction: pathogenesis of insulin resistance and
Influence of treatment with diet alone on oral glucose-tolerance test and plasma hyperglycemia in NIDDM. Am. J. Med. 1985; 79:1-11.
998 DIABETES, VOL. 35, SEPTEMBER 1986
You might also like
- A6 MF 1Document2 pagesA6 MF 1hidraulic100% (1)
- Itl 512 Learning Map Final DraftDocument5 pagesItl 512 Learning Map Final Draftapi-483586558No ratings yet
- Prophecies in Islam & Prophet Muhammad - A.aliDocument82 pagesProphecies in Islam & Prophet Muhammad - A.aliZaky MuzaffarNo ratings yet
- Naplex Complete Study Outline A Topic-Wise Approach DiabetesFrom EverandNaplex Complete Study Outline A Topic-Wise Approach DiabetesRating: 4 out of 5 stars4/5 (3)
- Cardiff Council Planning Committee Agenda For Wednesday February 13, 2013.Document222 pagesCardiff Council Planning Committee Agenda For Wednesday February 13, 2013.jessica_best2820No ratings yet
- Noninsulin-Dependent Diabetes: Effects of Insulin Peripheral Splanchnic Glucose Metabolism in 11)Document7 pagesNoninsulin-Dependent Diabetes: Effects of Insulin Peripheral Splanchnic Glucose Metabolism in 11)agiekNo ratings yet
- The Effect of Glimepiride On Glycemic Control and Fasting Insulin LevelsDocument3 pagesThe Effect of Glimepiride On Glycemic Control and Fasting Insulin Levelszahrosofi ahmadahNo ratings yet
- Effects of Glucose Ingestion On Postprandial LipemiaDocument6 pagesEffects of Glucose Ingestion On Postprandial LipemiaBram KNo ratings yet
- Глюкагон мен глюкоза, инсулинDocument5 pagesГлюкагон мен глюкоза, инсулинArsen IzbanovNo ratings yet
- Am J Clin Nutr 1979 Anderson 2312 21Document10 pagesAm J Clin Nutr 1979 Anderson 2312 21Riza Haida WardhaniNo ratings yet
- HFD MiceDocument5 pagesHFD Micechar462No ratings yet
- Obesity and Type 2 Diabetes Impair Insulin-Induced Suppression of Glycogenolysis As Well As GluconeogenesisDocument11 pagesObesity and Type 2 Diabetes Impair Insulin-Induced Suppression of Glycogenolysis As Well As GluconeogenesisFauzi RahmanNo ratings yet
- Effect of Insulin Analog Initiation Therapy On LDL/ HDL Subfraction Profile and HDL Associated Enzymes in Type 2 Diabetic PatientsDocument11 pagesEffect of Insulin Analog Initiation Therapy On LDL/ HDL Subfraction Profile and HDL Associated Enzymes in Type 2 Diabetic PatientsAan AlawiyahNo ratings yet
- Insulin Secretion and FunctionDocument8 pagesInsulin Secretion and FunctionWendy EscalanteNo ratings yet
- Beneficial Effects of Viscous Dietary Fiber From Konjac-Mannan in Subjects With The Insulin Resistance Syndro M eDocument6 pagesBeneficial Effects of Viscous Dietary Fiber From Konjac-Mannan in Subjects With The Insulin Resistance Syndro M eNaresh MaliNo ratings yet
- CHAPTER 7 - Inpatient Management of Diabetes and HyperglycemiaDocument6 pagesCHAPTER 7 - Inpatient Management of Diabetes and HyperglycemiaenesNo ratings yet
- Jurnal DMDocument5 pagesJurnal DMratihparmadiniNo ratings yet
- Glycemic and IRDocument10 pagesGlycemic and IRAyman AleemNo ratings yet
- Diabeties and CamelmilkDocument4 pagesDiabeties and Camelmilkarib syedNo ratings yet
- Gracia Ramos2016Document8 pagesGracia Ramos2016Rahmanu ReztaputraNo ratings yet
- Ratner 2011 Preprandial Vs Postprandial GlulisineDocument7 pagesRatner 2011 Preprandial Vs Postprandial GlulisineKusumaNo ratings yet
- Premixed Insulin Analogue Compared With Basal-Plus Regimen For Inpatient Glycemic ControlDocument9 pagesPremixed Insulin Analogue Compared With Basal-Plus Regimen For Inpatient Glycemic ControlAbraham RamonNo ratings yet
- Defining Insulin Resistance From HyperinsulinemicDocument19 pagesDefining Insulin Resistance From HyperinsulinemicWita Ferani KartikaNo ratings yet
- Glycemic Variability and Hypoglycemia Before and ADocument7 pagesGlycemic Variability and Hypoglycemia Before and ALeonorNo ratings yet
- Chronic Effect of PferospermuDocument1 pageChronic Effect of PferospermuamritaryaaligarghNo ratings yet
- Weight Reduction Improves Markers of Hepatic Function and InsulinDocument6 pagesWeight Reduction Improves Markers of Hepatic Function and Insulinmummy23572No ratings yet
- Medip, IJAM-3354 RDocument6 pagesMedip, IJAM-3354 RweasrandomnicolasNo ratings yet
- 216 orDocument1 page216 orBALTAZAR OTTONELLONo ratings yet
- The Effect of Postprandial Exercise On MealrelatedDocument7 pagesThe Effect of Postprandial Exercise On MealrelatedLeonardoValenzuelaNo ratings yet
- Home-Based Exercise Training Improves Capillary Glucose Profile in Women With Gestational DiabetesDocument4 pagesHome-Based Exercise Training Improves Capillary Glucose Profile in Women With Gestational DiabetesdiahadnyaNo ratings yet
- NIH Public Access: Author ManuscriptDocument13 pagesNIH Public Access: Author ManuscriptPaul SimononNo ratings yet
- Gestational DiabetesDocument51 pagesGestational Diabeteskhadzx100% (2)
- Manejo de La DMGDocument5 pagesManejo de La DMGsandymejiaNo ratings yet
- Accelerated Intestinal Glucose Absorption in Morbidly Obese Humans: Relationship To Glucose Transporters, Incretin Hormones, and GlycemiaDocument9 pagesAccelerated Intestinal Glucose Absorption in Morbidly Obese Humans: Relationship To Glucose Transporters, Incretin Hormones, and GlycemiaNissa SissariNo ratings yet
- Administration of Resveratrol For 5 WK Has No Effect On Glucagon-Like Peptide 1 Secretion, Gastric Emptying, or Glycemic Control in Type 2 Diabetes: A Randomized Controlled TrialDocument5 pagesAdministration of Resveratrol For 5 WK Has No Effect On Glucagon-Like Peptide 1 Secretion, Gastric Emptying, or Glycemic Control in Type 2 Diabetes: A Randomized Controlled TrialAmanda TeacaNo ratings yet
- Ada WhoDocument7 pagesAda WhoHironmoy RoyNo ratings yet
- Dia Care 1987 Feinglos 72 5Document4 pagesDia Care 1987 Feinglos 72 5Kang Opik TaufikNo ratings yet
- Diabetic EssayDocument6 pagesDiabetic Essayreet kaurNo ratings yet
- Track 3 Eating Patterns and Behaviour P63Document15 pagesTrack 3 Eating Patterns and Behaviour P63Yuriko AndreNo ratings yet
- Insulin in DMDocument46 pagesInsulin in DMask1400No ratings yet
- Nutrition in DMDocument33 pagesNutrition in DMkero R.habibNo ratings yet
- Diabetes 2Document9 pagesDiabetes 2Lakshmipriya GopinathNo ratings yet
- 2814 Full PDFDocument2 pages2814 Full PDFAnaNo ratings yet
- Insulin Sensitivity and Glucose Tolerance Are Altered by Maintenance On A Ketogenic DietDocument17 pagesInsulin Sensitivity and Glucose Tolerance Are Altered by Maintenance On A Ketogenic DietSebastiano ViganòNo ratings yet
- Associations of Glucose Control with Insulin Sensitivity and Pancreatic β-Cell Responsiveness in Newly Presenting Type 2 DiabetesDocument6 pagesAssociations of Glucose Control with Insulin Sensitivity and Pancreatic β-Cell Responsiveness in Newly Presenting Type 2 DiabetesJoseph PositivoNo ratings yet
- Effects of Olanzapine and Haloperidol On The Metabolic Status of Healthy MenDocument8 pagesEffects of Olanzapine and Haloperidol On The Metabolic Status of Healthy MenBenjamin paulNo ratings yet
- Insulin Resistance IcuDocument18 pagesInsulin Resistance IcuLucero GutierrezNo ratings yet
- DMJ 37 465Document10 pagesDMJ 37 465sn4s7nyxcbNo ratings yet
- Toward Improved Management of NIDDM: A Randomized, Controlled, Pilot Intervention Using A Lowfat, Vegetarian DietDocument5 pagesToward Improved Management of NIDDM: A Randomized, Controlled, Pilot Intervention Using A Lowfat, Vegetarian DietpepeNo ratings yet
- Continuous Glucose Profiles in Healthy Subjects Under Everyday Life Conditions and After Different MealsDocument9 pagesContinuous Glucose Profiles in Healthy Subjects Under Everyday Life Conditions and After Different MealsAsose TuNo ratings yet
- Insulin Analogs Versus Human Insulin in The Treatment of Patients With Diabetic KetoacidosisDocument6 pagesInsulin Analogs Versus Human Insulin in The Treatment of Patients With Diabetic KetoacidosisrosieveliaNo ratings yet
- Mitigating Effects of Vildagliptin in Experimental Diabetes With Metabolic SyndromeDocument9 pagesMitigating Effects of Vildagliptin in Experimental Diabetes With Metabolic Syndromerajesh sumanNo ratings yet
- DR - Rihab Pediatrics 02.pediatric DM Part TwoDocument7 pagesDR - Rihab Pediatrics 02.pediatric DM Part TwoMujtaba JawadNo ratings yet
- Original Article: A. Kashiwagi, H. Takahashi, H. Ishikawa, S. Yoshida, K. Kazuta, A. Utsuno & E. UeyamaDocument9 pagesOriginal Article: A. Kashiwagi, H. Takahashi, H. Ishikawa, S. Yoshida, K. Kazuta, A. Utsuno & E. UeyamadbcooperdkNo ratings yet
- Estudo CarboidratosDocument5 pagesEstudo CarboidratosSemi Chung AzekaNo ratings yet
- NONE BDocument11 pagesNONE BPincelito21No ratings yet
- Effect of A High-Protein, Low-Carbohydrate Diet On Blood Glucose Control in People With Type 2 DiabetesDocument8 pagesEffect of A High-Protein, Low-Carbohydrate Diet On Blood Glucose Control in People With Type 2 DiabetesFajar AgNo ratings yet
- Clinical: Influence of Pharmacokinetics Bioavailability Highly Purified Beef DependentDocument5 pagesClinical: Influence of Pharmacokinetics Bioavailability Highly Purified Beef Dependentsstrumello7395No ratings yet
- Pharmacological Management of Type 1 DiabetesDocument6 pagesPharmacological Management of Type 1 DiabetesMI RFNo ratings yet
- Carbohydrate-Last Meal Pattern Lowers Postprandial Glucose and Insulin Excursions in Type 2 DiabetesDocument5 pagesCarbohydrate-Last Meal Pattern Lowers Postprandial Glucose and Insulin Excursions in Type 2 DiabetesClaudio Soares da SilvaNo ratings yet
- Reduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic PatientsDocument5 pagesReduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic PatientsRoger CNo ratings yet
- Efficacy of Autologous Bone Marrow DerivDocument10 pagesEfficacy of Autologous Bone Marrow DerivsulaymanwaquarNo ratings yet
- 6992 Birkeland Diabetes Care 2011Document14 pages6992 Birkeland Diabetes Care 2011Pharmatopia CorpNo ratings yet
- Complementary and Alternative Medical Lab Testing Part 18: PsychiatryFrom EverandComplementary and Alternative Medical Lab Testing Part 18: PsychiatryRating: 5 out of 5 stars5/5 (1)
- Local Wound Care and Topical Management of Hidradenitis SupurativaDocument7 pagesLocal Wound Care and Topical Management of Hidradenitis SupurativaChristine Yohana SianturiNo ratings yet
- Flowchart PKTCRDDocument2 pagesFlowchart PKTCRDChristine Yohana SianturiNo ratings yet
- Jurnal Bahasa InggrisDocument8 pagesJurnal Bahasa InggrisChristine Yohana SianturiNo ratings yet
- Hasil StatistikDocument8 pagesHasil StatistikChristine Yohana SianturiNo ratings yet
- PDZVBB - 18 Jun 2018Document12 pagesPDZVBB - 18 Jun 2018Christine Yohana SianturiNo ratings yet
- Family Wellness Plan Keluarga Bapak GunawanDocument2 pagesFamily Wellness Plan Keluarga Bapak GunawanChristine Yohana SianturiNo ratings yet
- Comparative Widal and Blooc Culture 2014Document19 pagesComparative Widal and Blooc Culture 2014Christine Yohana SianturiNo ratings yet
- Hasil StatistikDocument8 pagesHasil StatistikChristine Yohana SianturiNo ratings yet
- Myopia Control: A Review: Jeffrey J. WallineDocument6 pagesMyopia Control: A Review: Jeffrey J. WallineChristine Yohana SianturiNo ratings yet
- V34n1a22 Which FactorsDocument13 pagesV34n1a22 Which FactorsChristine Yohana SianturiNo ratings yet
- Za2 PDFDocument7 pagesZa2 PDFChristine Yohana SianturiNo ratings yet
- PDZVBB - 18 Jun 2018 PDFDocument1 pagePDZVBB - 18 Jun 2018 PDFChristine Yohana SianturiNo ratings yet
- Materi Medical Emergency Response Plan (Kedok. Unila 2015)Document38 pagesMateri Medical Emergency Response Plan (Kedok. Unila 2015)Christine Yohana SianturiNo ratings yet
- Patient Safety Topic 11Document21 pagesPatient Safety Topic 11Christine Yohana SianturiNo ratings yet
- Hidung: - Anatomi - Fisiologi - Kelainan Kongenital - Nasobifida - Dermoid Cyst - Atresia/StenosisDocument27 pagesHidung: - Anatomi - Fisiologi - Kelainan Kongenital - Nasobifida - Dermoid Cyst - Atresia/StenosisChristine Yohana SianturiNo ratings yet
- Flave5 PDFDocument12 pagesFlave5 PDFChristine Yohana SianturiNo ratings yet
- EBTax Tax Simulator Webcast 16 Jul 14pdfDocument49 pagesEBTax Tax Simulator Webcast 16 Jul 14pdfM.MedinaNo ratings yet
- The MoonDocument6 pagesThe MoonjaudreytuyNo ratings yet
- Hybrid Clutch Installation GuideDocument2 pagesHybrid Clutch Installation Guidebrucken1987No ratings yet
- DLP TLE Grade7 Carpentry 5S JH 012319Document1 pageDLP TLE Grade7 Carpentry 5S JH 012319Jonathan Magtibay HusainNo ratings yet
- 1-Module 1 Introduction 20181114 RCGDocument46 pages1-Module 1 Introduction 20181114 RCGRoger ChiuNo ratings yet
- Management Accounting Costing and BudgetingDocument31 pagesManagement Accounting Costing and BudgetingnileshdilushanNo ratings yet
- Unit-7: Iot SecurityDocument21 pagesUnit-7: Iot SecuritySAMPANo ratings yet
- Amol Sanap Teacher ResumeDocument3 pagesAmol Sanap Teacher ResumeAmol SanapNo ratings yet
- Distortion in Music ProductionDocument306 pagesDistortion in Music ProductionjansolostarNo ratings yet
- GC - Interview QuestionsDocument7 pagesGC - Interview QuestionsSasiNo ratings yet
- Strategic Intent Envisioning and MissionDocument23 pagesStrategic Intent Envisioning and Mission14122001riya2001No ratings yet
- Bonding QuizDocument7 pagesBonding Quiz卜一斐No ratings yet
- Controllogix FestoDocument43 pagesControllogix FestoEdwin Ramirez100% (1)
- IPC2022 87194 Enhancing MFL A Ultra UtilizingFEM JSpille FinalDocument5 pagesIPC2022 87194 Enhancing MFL A Ultra UtilizingFEM JSpille FinalOswaldo MontenegroNo ratings yet
- Curriculum Till DE-39 PDFDocument173 pagesCurriculum Till DE-39 PDFubaid umarNo ratings yet
- Datasheet: Delastic Preformed Pavement SealsDocument1 pageDatasheet: Delastic Preformed Pavement SealsUnited Construction Est. TechnicalNo ratings yet
- Contemporary World FinalDocument22 pagesContemporary World FinalApril Grace Genobatin BinayNo ratings yet
- Bagrut Module D Answers To The Treasure of Lemon BrownDocument8 pagesBagrut Module D Answers To The Treasure of Lemon BrownMohammed ArarNo ratings yet
- Major Fractures of The Pilon The Talus and The Calcaneus Current Concepts of TreatmentDocument241 pagesMajor Fractures of The Pilon The Talus and The Calcaneus Current Concepts of TreatmentPaul Radulescu - FizioterapeutNo ratings yet
- Schallert 2019Document7 pagesSchallert 2019Dian Putri NingsihNo ratings yet
- Experiment 1 Safety Measures: Use and Preparation of Chemical Substance 1.1 ObjectivesDocument8 pagesExperiment 1 Safety Measures: Use and Preparation of Chemical Substance 1.1 ObjectivesMaldini JosnonNo ratings yet
- Research ProposalDocument9 pagesResearch Proposalsyed arslan aliNo ratings yet
- Mwn-Wapr150n User GuideDocument116 pagesMwn-Wapr150n User GuideDan WrysinskiNo ratings yet
- All About Zookeeper and ClickHouse KeeperDocument45 pagesAll About Zookeeper and ClickHouse KeeperomyeudaihiepNo ratings yet
- Ashok Mali ReportDocument1 pageAshok Mali ReportAshok MaliNo ratings yet
- C16ac3 - 60T LoadcellDocument2 pagesC16ac3 - 60T LoadcellAmit AGRAWALNo ratings yet