Professional Documents
Culture Documents
ADA 2015 Summary PDF
ADA 2015 Summary PDF
Uploaded by
Lady MuffinsOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ADA 2015 Summary PDF
ADA 2015 Summary PDF
Uploaded by
Lady MuffinsCopyright:
Available Formats
2015 American Diabetes Association (ADA) Diabetes Guidelines 1
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
1. Diabetes Diagnosis
Criteria for Diabetes Diagnosis: 4 options
A1C ≥6.5%*
Perform in lab using NGSP-certified method and standardized to DCCT assay
FPG ≥126 mg/dL (7.0 mmol/L)*
Fasting defined as no caloric intake for ≥8 hrs
2-hr PG ≥200 mg/dL (11.1 mmol/L) during OGTT (75-g)*
Performed as described by the WHO, using glucose load containing the equlivalent
of 75g anhydrous glucose dissolved in water
Random PG ≥200 mg/dL (11.1 mmol/L)
In persons with symptoms of hyperglycemia or hyperglycemic crisis
*In the absence of unequivocal hyperglycemia results should be confirmed using repeat testing
Unless clinical diagnosis is clear, same test to be repeated using a new blood sample for confirmation
2 discordant results? Result above cutpoint should be repeated
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 2
Summary Recommendations from NDEI
Testing for Type 2 Diabetes and Prediabetes in Asymptomatic Adults
Type 2 diabetes testing should be done in all adults who are overweight or obese (BMI ≥25 or ≥23 in Asian
Americans) who have ≥1 diabetes risk factor (see box):
Diabetes Risk Factors
Physical inactivity
First-degree relative with diabetes
High-risk race/ethnicity
Women who delivered a baby >9 lb or were diagnosed with GDM
HDL-C <35 mg/dL ± TG >250 mg/dL
Hypertension (≥140/90 mm Hg or on therapy)
A1C ≥5.7%, IGT, or IFG on previous testing
Conditions associated with insulin resistance: severe obesity, acanthosis
nigricans, PCOS
CVD history
Testing should begin at age 45, especially if the individual is overweight or obese
If normal results: repeat testing in ≥3-yr intervals
Prediabetes testing can be done using A1C, FPG, or 2-h PG after 75-g OGTT.
Identify and treat (if appropriate) other CVD risk factors
Prediabetes testing should be considered in children and adolescents who are overweight/obese and have ≥2 diabetes
risk factors (see box above)
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 3
Summary Recommendations from NDEI
Frequency of A1C Testing
Perform A1C test
At least 2 times each year Quarterly
in individuals who are meeting treatment targets in individuals whose therapy has changed
and have stable glycemic control or who are not meeting glycemic targets
Point-of-care A1C testing allows for more timely treatment changes
BMI=body mass index; CVD=cardiovascular diseaseDCCT=Diabetes Control and Complications Trial; FPG=fasting plasma glucose;
GDM=gestational diabetes mellitus; HDL-C=high-density lipoprotein cholesterol; IFG=impaired fasting glucose; IGT=impaired glucose tolerance;
NGSP=national glycohemoglobin standardization program; OGTT=oral glucose tolerance test; PCOS=polycystic ovarian syndrome; PG=plasma
glucose; TG=triglycerides; WHO=World Health Organization
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
Visit NDEI.org for interactive summary
recommendations & downloadable slides
on the ADA 2015 guidelines.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 4
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
2. Glycemic Targets
Glycemic Targets for Nonpregnant Adults With Diabetes
A1C <7.0%
Lowering A1C below or around 7.0% has been shown to reduce:
Microvascular complications
Macrovascular disease*
Preprandial capillary PG 80-130 mg/dL (4.1-7.2 mmol/L)
<180 mg/dL (<10.0 mmol/L)
Peak postprandial capillary PG
Postprandial glucose measurements should be made 1-2 hours after the
beginning of the meal
Individualize targets based on: Age/life expectancy
Comorbid conditions
Diabetes duration
Hypoglycemia status
Individual patient considerations
Known CVD/advanced microvascular complications
More or less stringent targets may be appropriate if can be achieved
without significant hypoglycemia or adverse events
More stringent target (<6.5%) Less stringent target (<8%)
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 5
Summary Recommendations from NDEI
Short diabetes duration Severe hypoglycemia history
Long life expectancy Limited life expectancy
No significant CVD/vascular complications Advanced microvascular or macrovascular complications
Extensive comorbidities
Long-term diabetes in whom general A1C target difficult to
attain
Targets shown are for nonpregnant adults
*If implemented soon after diagnosis
Managing Hypoglycemia
At-risk individuals Ask about symptomatic and asymptomatic hypoglycemia at each
encounter
Preferred treatment: glucose (15-20 g)*
After 15 mins of treatment, repeat if SMBG shows continued hypoglycemia
When SMBG is normal, patient should consume meal or snack to prevent hypoglycemia recurrence
Reevaluate treatment regimen
Hypoglycemia unawareness or episode of severe Insulin-treated patients: raise glycemic targets for several
hypoglycemia weeks to partially reverse hypoglycemia unawareness and
reduce recurrence
Low or declining cognition Continually assess cognitive function with increased vigilance
for hypoglycemia
*Any form of glucose-containing carbohydrate can be used
CVD=cardiovascular disease; PG=plasma glucose; SMBG=self-monitoring of blood glucose
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 6
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
3. Strategies for Preventing or Delaying Type 2 Diabetes
Prevention/Delay of Type 2 Diabetes
Patients with IGT, IFG, or A1C 5.7%-6.4% Refer to intensive and diet and physical activity (lifestyle) behavior
counseling
Weight loss (7% of body weight)
Increased physical activity (≥150 min/week moderate activity)
Consider metformin therapy for type 2 diabetes Especially in the presence of:
prevention in patients with IGT, IFG,
or A1C 5.7%-6.4% BMI >35 kg/m2
Age <60 years
Women with prior GDM
Annual monitoring of individuals with prediabetes
Screening for and treatment of modifiable CVD risk factors (obesity, hypertension, and dyslipidemia) suggested
DSME and DSMS are appropriate for prediabetes to receive education and support to develop and maintain behaviors
that can prevent and delay type 2 diabetes
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 7
Summary Recommendations from NDEI
Diabetes Self-Management Education and Support
Provide at diabetes diagnosis and as needed thereafter
Measure and monitor effectiveness of self-management and quality of life as part of overall care
Programs should:
Address psychosocial issues
Provide education and support to persons with prediabetes to encourage behaviors that may prevent or delay
diabetes onset
BMI=body mass index; CVD=cardiovascular disease; DSME=diabetes self-management education; DSMS=diabetes self-management education
and support; IFG=impaired fasting glucose; IGT=impaired glucose tolerance
January 2015
This content was created by Ashfield Healthcare Communi1cations, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 8
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
4. Pharmacologic Therapy for Type 2 Diabetes
Medications for Hyperglycemia in Type 2 Diabetes
Metformin Preferred initial therapy (if tolerated and not contraindicated)
when lifestyle changes alone have not achieved or maintained
glycemic goals
Consider insulin therapy with or without other agents At outset in newly diagnosed patients with markedly
symptomatic and/or elevated blood glucose levels or A1C
Add 2nd oral agent, GLP-1 receptor agonist, or insulin If noninsulin monotherapy at maximal tolerated dose does not
achieve or maintain A1C target over 3 months
Choice of pharmacologic therapy should be based on patient-centered approach, considering
Efficacy
Cost
Potential side effects
Effects on weight
Comorbidities
Hypoglycemia risk
Patient preferences
Insulin is eventually needed for many patients due to the progressive nature of type 2 diabetes
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 9
Summary Recommendations from NDEI
Bariatric Surgery in Type 2 Diabetes
Consider for adults with BMI >35 kg/m2 In particular, if diabetes or associated comorbidities are difficult
to control with lifestyle and pharmacologic therapy
Lifelong lifestyle support and medical monitoring are necessary post-surgery
Insufficient evidence to recommend surgery with BMI <35 kg/m2 outside of a research protocol
Advantages Disadvantages
Achieves near or complete normalization of Costly
glycemia 2 years after surgery Variable outcomes depending on procedure
Youth, shorter diabetes duration, lower A1C, Long term vitamin/mineral deficiencies
higher insulin levels lead to higher post-surgery Osteoporosis
type 2 diabetes remission rates Severe hypoglycemia from insulin
BMI=body mass index; GLP-1=glucagon-like peptide-1
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 10
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
5. Pharmacologic Therapy for Type 1 Diabetes
Insulin Therapy Is Recommended for Most Individuals With Type 1 Diabetes
Treat with multiple-dose insulin injections (3-4 injections/day of basal and prandial insulin) or continuous
subcutaneous insulin infusion
Match prandial insulin dose to carbohydrate intake, premeal blood glucose, and anticipated activity
Use insulin analogs to reduce risk of hypoglycemia
Consider using sensor-augmented low glucose suspend threshold pump in patients with frequent nocturnal
hypoglycemia and/or hypoglycemia unawareness
Other Agents Investigational Agents
Pramlinitide (amylin analog) Metformin + insulin
Delays gastric emptying Reduces insulin requirements and improves metabolic
Blunts pancreatic secretion of glucagon control in obese/overweight subjects with poor glycemic
Enhances satiety control
Induces weight loss
Lowers insulin dose Incretins
Use only in adults
GLP-1 receptor agonists
DPP-4 inhibitors
SGLT-2 inhibitors
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 11
Summary Recommendations from NDEI
Type 1 Diabetes Screening
Inform individuals with type 1 diabetes of the opportunity to have relatives screened for risk
of type 1 diabetes in the clinical research setting
Early diagnosis may limit complications, extend long-term endogenous insulin productio
Widespread testing of asymptomatic low-risk persons: not recommended
Screen high-risk persons only in clinical research setting
DPP-4=dipeptidyl peptidase-4; GLP-1=glucagon-like peptide-1; SGLT2=sodium glucose co-transporter 2
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 12
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
6. Insulin & Glucose Monitoring
Self-Monitoring of Blood Glucose (SMBG)
Encourage for patients receiving multiple dose insulin or insulin pump therapy:
Prior to meals and snacks
Occasionally postprandially
At bedtime
Prior to exercise
When low blood glucose is suspected
After treating low blood glucose until normoglycemic
Prior to critical tasks (eg, driving)
Results may be useful for guiding treatment and/or self-management for patients using less frequent insulin injections or
noninsulin therapies
Provide ongoing instruction and regular evaluation of SMBG technique and results and patient’s ability to use data
to adjust therapy
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 13
Summary Recommendations from NDEI
Continuous Glucose Monitoring (CGM)
Useful for A1C lowering in select adults (aged ≥25 yrs) with type 1 diabetes requiring intensive insulin regimens
May be useful among children, teens, and younger adults*
Success related to adherence to ongoing use
May be a useful supplement to SMBG among patients with
Hypoglycemia unawareness and/or
Frequent hypoglycemic episodes
Variable adherence to CGM? Assess individual readiness for continuing prior
to prescribing
Robust diabetes education, training, and
support critical for optimal CGM implementation
*Evidence for A1C lowering less strong in these populations
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 14
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
7. Lifestyle Changes
Medical Nutrition Therapy (MNT)
The ADA acknowledges that there is no one-size-fits-all eating pattern for individuals with type 2 diabetes
Medical nutrition therapy is recommended for all patients with type 1 and type 2 diabetes as part of an overall treatment
plan, preferably provided by a registered dietition skilled in diabetes MNT
Goals of MNT:
Healthful eating pattern to improve overall health
Attain individualized glycemic, BP, and lipid goals
Achieve and maintain body weight goals
Delay or prevent diabetes complications
Physical Activity
Adults with diabetes
Exercise programs should include
≥150 min/wk moderate-intensity aerobic activity (50%-70% max heart rate), spread over
≥3 days/wk with no more than 2 consecutive days without exercise
Resistance training ≥2 times/wk (in absence of contraindications)*
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 15
Summary Recommendations from NDEI
Reduce sedentary time = break up >90 minutes spent sitting
Evaluate patients for contraindications prohibiting certain types of exercise before recommending exercise program†
Consider age and previous level of physical activity
Children with diabetes or prediabetes
≥60 min physical activity/day
*Adults with type 2 diabetes
†
Eg, uncontrolled hypertension, severe autonomic or peripheral neuropathy, history of foot lesions, unstable proliferative retinopathy
Physical Activity In Patients With Nonoptimal Glycemic Control
Hyperglycemia
Avoid vigorous activity with ketosis When individuals with type 1 diabetes are deprived of
insulin for 12-28 hours and are ketotic, exercise can
worsen hyperglycemia and ketosis
Hypoglycemia
If taking insulin secretagogues, physical activity can cause Added carbohydrates should be ingested
hypoglycemia if medication dose or carb consumption is not when pre-exercise glucose is <100 mg/dL
altered (5.6 mmol/L)
Physical Activity Considerations for Patients With Diabetes Complications
Retinopathy
If proliferative diabetic retinopathy or severe nonproliferative Vigorous aerobic or resistance exercise may be
diabetic retinopathy present contraindicated
Peripheral Neuropathy
Decreased pain sensation and a higher pain threshold in the All individuals with neuropathy should wear proper
extremities cause increased risk of skin breakdown footwear and examine feet daily for lesions
Foot injury or open sore: restricted to non-weight-
bearing activity
Autonomic Neuropathy
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 16
Summary Recommendations from NDEI
Physical activity can acutely increase urinary protein excretion No evidence that vigorous exercise increases rate of
progression of diabetic kidney disease
Exercise restrictions not required
Smoking Cessation
Advise patients with diabetes not to smoke or use tobacco products
Counsel on smoking prevention and cessation as part of routine care
Assess level of nicotine dependence
Offer pharmacologic therapy as appropriate
Adding pharmacologic therapy to counseling is more effective than either treatment alone
No evidence that e-cigarettes are a healthy alternative to smoking
Nor can they facilitate smoking cessation
BP=blood pressure
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 17
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
8. Cardiovascular Disease (CVD) & Diabetes
Management of Blood Pressure (Hypertension)
Screening Measure BP at every visit; confirm elevated BP at separate visit
Treatment targets Diabetes and hypertension: SBP <140 mm Hg
Lower SBP targets (eg, <130 mm Hg) may be appropriate in certain individuals
if can be achieved without treatment burden
Diabetes: DBP <90 mm Hg
Lower DBP (eg, 80 mm Hg) may be appropriate in certain individuals if can be
achieved without treatment burden
Treatment BP >120/80 mm Hg: lifestyle changes
Weight loss (if overweight)
DASH-style diet including sodium restriction and potassium increase
Moderate alcohol intake
Increased physical activity
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 18
Summary Recommendations from NDEI
BP >140/90 mm Hg: lifestyle changes + pharmacologic therapy
Diabetes and hypertension: ACEI or ARB*
≥2 agents at max doses, including thiazide-type diuretic, ACEI, or ARB, usually
required to achieve targets
Administer ≥1 agent at bedtime
ACEI, ARB, diuretic: monitor serum creatinine/eGFR and serum potassium
Treatment and targets for Diabetes and hypertension: 110-129/65-79 mm Hg target
pregnant women ACEI, ARB contraindicated
*If one class not tolerated, substitute other class
Management of Lipids (Dyslipidemia)
Treatment initiation and initial dose driven by risk status—not LDL-C level
Age Risk factors Statin intensity Monitoring
<40 0 N/A Annually or as needed to
CVD risk factors Moderate or high check adherence
Overt CVD High
40-75 0 N/A As needed to check
CVD risk factors Moderate or high adherence
Overt CVD High
>75 0 N/A As needed to check
CVD risk factors Moderate or high adherence
Overt CVD High
Screening at diabetes diagnosis, initial medical evaluation, and/or at age 40
Every 1-2 years thereafter
CVD risk factors: LDL-C ≥100 mg/dL (2.6 mmol/L), high blood pressure, smoking, overweight/obesity
Overt CVD: Invidivuals with prior cardiovascular events or acute coronary syndrome
At time of publication, combination therapy (statin + non-statin) for lipid lowering was not shown to provide incremental
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 19
Summary Recommendations from NDEI
CVD benefit. Results from IMPROVE-IT1 have since shown a 2% CV risk reduction with ezetimibe/statin vs statin alone.
1. Cannon CP, et al. Presented at the American Heart Association Scientific Sessions 2014; Chicago, Illinois.
Antiplatelet Therapy
Aspirin: Primary prevention 75-162 mg/day: type 1 and type 2 diabetes at increased CVD risk (10-yr risk >10%)*
Low-risk patients (10-yr risk <5%):† not recommended; potential for bleeds likely
offsets potential benefits
Men <50 yrs, women <60 yrs with multiple other risk factors (10-yr risk 5%-10%):
use clinical judgment
Aspirin: Secondary prevention 75-162 mg/day: diabetes and CVD history
CVD and aspirin allergy Clopidogrel 75 mg/day
Dual antiplatelet therapy Reasonable for ≤1 year after ACS
*Includes most men aged >50 yrs or women aged >60 yrs with ≥1 add’l major risk factor: family history of CVD, hypertension, smoking,
dyslipidemia, or albuminuria
†
Men aged <50 yrs and women aged >60 yrs with no major additional CVD risk factors
CVD Screening and Treatment
Screening Routine CAD screening in asymptomatic patients not recommended
Does not improve outcomes as long as CVD risk factors are treated
Treatment Lifestyle
Focus on weight loss (decreased calorie intake, increased physical activity
Improves glycemic control, fitness, some CVD risk factors
Overt CVD: consider ACEI, and use aspirin and statin to reduce CV event risk
If hypertensive with overt CVD: Aspirin, statin, ACEI or ARB unless contraindications
Prior MI: continue use of beta-blockers for ≥2 yrs after event
Symptomatic heart failure: avoid TZDs
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 20
Summary Recommendations from NDEI
Metformin
Stable heart failure: may use metformin in presence of normal renal function
Avoid metformin in unstable or hospitalized heart failure patients
ACEI=angiotensin-converting enzyme inhibitor; ACS=acute coronary syndrome; ARB=angiotensin receptor blocker; BP=blood pressure;
CAD=coronary artery disease; CVD=cardiovascular disease; DASH=Dietary Approaches to Stop Hypertension; DBP=diastolic blood pressure;
eGFR=estimated glomerular filtration rate; LDL-C=low-density lipoprotein cholesterol; MI=myocardial infarction; SBP=systolic blood pressure;
TZDs=thiazolidinediones
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 21
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
9. Microvascular Complications & Foot Care
Nephropathy Screening and Treatment
Optimize glucose and BP control to reduce the risk for or slow the progression of nephropathy
Screening Annually measure urine albumin excretion in type 1
patients with ≥5-yr diabetes duration, and all type 2
patients starting at diagnosis
Treatment
Normal BP and albumin excretion ACEI or ARB for primary prevention of kidney disease
<30 mg/g not recommended
Nonpregnant with modest elevations Use ACEI or ARB (but not in combination)
(30-299 mg/24 h) or higher levels (≥300 mg/24 h) of urinary
albumin excretion
When using ACEI, ARB, diuretic Monitor creatinine and potassium levels
Monitor urine albumin excretion continually to assess therapeutic response and disease progression
If eGFR <60 mL/min/1.73 m2 Evaluate and manage CKD complications
Consider specialist referral Conditions of uncertainty regarding kidney disease
etiology, difficult management issues, advanced kidney
disease
Avoid combined use of different inhibitors of the renin-angiotensin system (RAS)
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 22
Summary Recommendations from NDEI
Retinopathy Screening and Treatment
Optimize glucose and BP control to reduce the risk for or slow the progression of retinopathy
Screening Initial dilated and comprehensive eye exam by an ophthalmologist or optometrist
Adults with type 1 diabetes: within 5 yrs after diabetes onset
Patients with type 2 diabetes: shortly after diagnosis
If no retinopathy for ≥1 eye exam: consider exams every 2 yrs
If retinopathy: annual exam
Retinopathy progressing or sight threatening: more frequent exams
Fundus photographs should be considered a screening tool, not a substitute for
comprehensive exam
Pregnant women or women planning pregnancy with preexisting diabetes
Retinopathy counseling, eye exam in first trimester
Close follow-up throughout pregnancy and 1 yr postpartum
Treatment
Macular edema, severe NPDR, any PDR Refer to ophthalmologist specializing in retinopathy
Laser photocoagulation therapy Indicated to reduce risk of vision loss for high-risk PDR, clinically significant
macular edema, some cases of severe NPDR
Anti-VEGF therapy Indicated for diabetic macular edema
Retinopathy not a contraindication to aspirin therapy for cardioprotection
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 23
Summary Recommendations from NDEI
Neuropathy Screening and Treatment
Screening Screen all patients for distal symmetric polyneuropathy
Type 2 diabetes: at diagnosis
Type 1 diabetes: 5 yrs after diagnosis and at least annually thereafter
Consider screening for signs/symptoms of cardiovascular autonomic neuropathy (CAN)
with advanced disease
Screening for cardiovascular autonomic neuropathy
Type 2 diabetes: at diagnosis
Type 1 diabetes: 5 yrs after diagnosis
Tight glycemic control is the only method shown to prevent or delay the development
of DPN and CAN in type 1, and to slow neuropathy progression in type 2
Treatment Assess and treat to reduce DPN-related pain and symptoms of autonomic neuropathy
Foot Care
All individuals with diabetes Annual foot exam to identify risk factors predictive of
ulcers and amputations
Assessment of foot pulses, loss of protective sensation
(LOPS) testing
Provide foot self-care education
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 24
Summary Recommendations from NDEI
Patients with foot ulcers, high-risk feet (previous ulcer Use multidisciplinary approach
or amputation)
All individuals with insensate feet, foot deformities, or Examine feet every visit
history of foot ulcers
Refer to foot care specialist People who smoke
LOPS and structural abnormalities
History of prior lower-extremity complications
Include in initial PAD screening History for claudication and assessment of pedal pulses
Obtain ankle-brachial index (ABI)
Refer for further vascular assessment Patients with positive ABI, significant claudication
Consider exercise, medications, surgical options
ACEI=angiotensin-converting enzyme inhibitor; BP=blood pressure; ARB=angiotensin receptor blocker; CKD=chronic kidney disease;
DPN=diabetic peripheral neuropathy; eGFR=estimated glomerular filtration rate; NDPR=nonproliferative diabetic retinopathy; PAD=peripheral
artery disease; PDR=proliferative diabetic retinopathy; VEGF=vascular endothelia growth factor
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 25
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
10. Diabetes in Pregnancy (Gestational Diabetes—GDM)
Managing Diabetes in Pregnancy
Maintain A1C levels as close to <7.0% as possible before attempting conception
All women of childbearing potential Provide preconception counseling starting
at puberty
Evaluate and treat (if necessary) in women Retinopathy
contemplating pregnancy Nephropathy
Neuropathy
CVD
Avoid teratogenic medications in sexually active Contraindicated/not recommended in pregnancy
women of child-bearing potential
Statins
ACEIs
ARBs
Most noninsulin therapies
Manage GDM first with lifestyle Medications added as needed
Baseline opthalmology exam in first trimester Monitor every trimester as needed by degree
in women with pregestational diabetes
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 26
Summary Recommendations from NDEI
of retinopathy
A1C target in pregnancy is <6.0% If can be achieved without significant hypoglycemia
GDM may indicate undiagnosed type 2 diabetes Screen DM for persistent diabetes or prediabetes at 6-12
wks postpartum, and every 1-3 yrs thereafter
Screening Gestational Diabetes
Pregnant women with risk factors Screen for undiagnosed type 2 diabetes at first prenatal visit
Pregnant women without known prior diabetes Screen at 24-28 wks
Women with GDM Screen for persistent diabetes 6-12 wks postpartum using OGTT
and nonpregnancy diagnostic criteria
Women with GDM history Screen for diabetes or prediabetes every ≥3 yrs
Women with GDM history and prediabetes Lifestyle interventions or metformin for diabetes prevention
Strategies for Diagnosing Gestational Diabetes
No uniform approach for GDM diagnosis
Two options for women not previously diagnosed with overt diabetes:
“One-Step” Strategy “Two-Step” Strategy
75-g OGTT with PG measurement fasting and 50-g GLT (nonfasting) with PG measurement at 1 h (Step
at 1 h and 2 h, at 24-28 wks in women not 1), at 24-28 wks in women not previously diagnosed with
previously diagnosed with overt diabetes overt diabetes
Perform OGTT in the morning after overnight If PG at 1 h after load is ≥140 mg/dL
fast (≥8 h) (7.8 mmol/L), proceed to 100-g OGTT
GDM diagnosis made if PG values meet or (Step 2), performed while patient is fasting
exceed: GDM diagnosis made when two or more PG levels meet or
Fasting: 92 mg/dL (5.1 mmol/L) exceed:
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 27
Summary Recommendations from NDEI
1 h: 180 mg/dL (10.0 mmol/L) Fasting: 95 mg/dL or 105 mg/dL (5.3/5.8)
2 h: 153 mg/dL (8.5 mmol/L) 1 hr: 180 mg/dL or 190 mg/dL (10.0/10.6)
2 hr: 155 mg/dL or 165 mg/dL (8.6/9.2)
3 hr: 140 mg/dL or 145 mg/dL (7.8/8.0)
Glycemic Targets in Pregnancy
GDM targets for women without preexisting Targets for women with preexisting type 1 or 2 who
type 1 or 2 diabetes become pregnant
Preprandial: ≤95 mg/dL (5.3 mmol/L) and either Premeal, bedtime, overnight glucose:
60-99 mg/dL (3.3-5.4 mmol/L)
1-hr postmeal: ≤140 mg/dL (7.8 mmol/L) Peak postprandial glucose: 100-129 mg/dL (5.4-7.1 mmol/L)
2-hr postmeal: ≤120 mg/dL (6.7 mmol/) A1C: <6.0%
Insulin Use During Pregnancy
Insulin is the preferred medication
Noninsulin medications lack long-term safety data
Insulin management during pregnancy is complex
Requires frequent titration to match changing requirements
Referral to specialized center recommended
Most insulins are category B; glargine and glulisine are category C
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 28
Summary Recommendations from NDEI
Managing Hypertension During Pregnancy
Target BP SBP: 110-129 mm Hg
DBP: 65-79 mm Hg
ACEIs and ARBs are contraindicated
Safe antihypertensive medications:
Methyldopa
Labetalol
Diltiazem
Clonidine
Prazosin
ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; BP=blood pressure; CVD=cardiovascular disease;
DBP=diastolic blood pressure; GDM=gestational diabetes mellitus; GLT=glucose loading test; OGTT=oral glucose tolerance test; PG=plasma
glucose; SBP=systolic blood pressure
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 29
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
11. Management of In-Patient Glycemia
Treatment for Hospitalized Patients With Diabetes
Insulin is the preferred method of glycemic control
Sliding scale insulin (SSI) is strongly discouraged in the hospital setting as the sole method
of treatment
ICU, intravenous insulin is the preferred route of administration
Outside of critical care units:
Basal-bolus regimen for individuals with good nutritional intake
Basal plus correction insulin regimen for individuals with poor oral intake or who are not taking anything by mouth
(NPO)
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 30
Summary Recommendations from NDEI
Glycemic Targets for Critically Ill Patients With Diabetes
Insulin is the preferred method for glycemic control
Persistent hyperglycemia:
Initiate insulin starting at ≤180 mg/dL (≤10.0 mmol/L)
Once insulin started, 140-180 mg/dL (7.8-10.0 mmol/L) recommended glucose range for most patients
More stringent targets may be appropriate for certain patients providing it can be achieved without increasing
hypoglycemia risk
110-140 mg/dL (6.1-7.8 mmol/L)
IV insulin protocol with demonstrated efficacy and safety in achieving targets with no increased hypoglycemia risk
Glycemic Targets for Non-Critically Ill Patients With Diabetes
Insulin is the preferred method for glycemic control
Insulin-treated: premeal target <140 mg/dL (<7.8 mmol/L) with random blood glucose <180 mg/dL (<10.0 mmol/L)
More or less stringent targets may be appropriate
More stringent: stable patients with previous tight glycemic control
Less stringent: severe comorbidities
Basal plus correction insulin regimen is preferred for patients with poor oral intake or who are taking nothing by mouth
(NPO)
Good nutritional intake: basal-bolus regimen
Hypoglycemia management protocol is critical
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 31
Summary Recommendations from NDEI
Document and track each episode
Obtain A1C in patients with diabetes admitted to the hospital if result of testing in prior 3 months is unavailable
Patients with hyperglycemia in the hospital who do not have prior diabetes diagnosis: appropriate follow-up testing
and care documented at discharge
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
January 2015
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 32
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
12. Diabetes Care in Older Adults
Diabetes Care in Older Adults
Older adults who are Same treatment goals as younger adults
Functional
Cognitively intact
Expected to live long enough to reap benefits
Glycemic targets may be relaxed for some older adults based on individual criteria (eg, advanced complications, life-
limiting comorbidities, cognitive or functional impairment)
Avoid hyperglycemic complications
Treat cardiovascular risk factors considering:
Timeframe of benefit and individual patient characteristics
Hypertension treatment indicated in many older adults
Lipid and aspirin therapy may benefit patients whose life expectancy is equal to the timeframe of primary- or
secondary-prevention trials
Individualize screening for complications
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 33
Summary Recommendations from NDEI
Be mindful of complications that may lead to functional impairment
Age ≥65 is a high-priority poulation for depression screening and treatment
Pharmacologic Therapy for Older Adults With Diabetes
Cost may be a significant factor due to polypharmacy
Metformin may be contraindicated due to renal insufficiency or significant heart failure
Thiazolidinediones (TZDs) Use cautiously in individuals with, or at risk for, heart failure
Associated with fracture risk
Sulfonylureas Can cause hypoglycemia
Insulin
Insulin secretagogues
Insulin requires that patients or caregivers have good visual and motor skills and cognitive ability
GLP-1 receptor agonists Few side effects
DPP-4 inhibitors Cost may be a barrier
DPP-4: may increase hospitalization for heart failure
(saxagliptin)1
1. Scirica BM, et al. N Engl J Med. 2013;369:1317-1326.
DPP-4=dipeptidyl peptidase-4; GLP-1=glucagon-like peptide-1
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise
noted. Consult individual prescribing information for approved uses outside of the United States.
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest. January 2015
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 34
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
13. Children & Adolescents With Diabetes
Screening Children for Type 2 Diabetes and Prediabetes
Consider for all children who are overweight* and have ≥2 of the following risk factors:
Family history of type 2 diabetes in first- or second-degree relative
Native American, African American, Larino, Asian American, or Pacific Islander
†
Signs of insulin resistance or conditions associated with insulin resistance
Maternal history of diabetes or GDM during child’s gestation
Begin testing at age 10 yrs or onset of puberty
Test every 3 yrs using A1C beginning at age 10 or puberty onset
Children: age ≤18 yrs
*BMI >85th percentile for age and sex, weight for height >85th percentile, or weight >120% ideal for height
†
Acanthosis nigricans, hypertension, dyslipidemia, PCOS, or small-for-gestational-age birth weight
§
Sleep apnea, hepatic steatosis, orthopedic complications, psychosocial concerns
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 35
Summary Recommendations from NDEI
Glycemic Targets for Children and Adolescents With Type 1 Diabetes
Consider risk-benefit assessment, including hypoglycemia risk, when individualizing targets
A1C <7.5%
Plasma glucose before meals 90-130 mg/dL (5.0-7.2 mmol/L)
Plasma glucose at bedtime and overnight 90-150 mg/dL (5.0-8.3 mmol/L)
If on basal-bolus: measure postprandial PG to monitor glycemic values and if discrepancy between preprandial PG and
A1C: goals should be individualized. Glucose goals should be modified in children with frequent hypoglycemia or
hypoglycemia unawareness.
Managing Microvascular Complications in Children and Adolescents With Type 1 Diabetes
Nephropathy
Screening At least annual albuminuria screen with random spot urine sample for urine albumine-
to-creatinine ratio (UACR) in patients with 5-yr diabetes duration
Measure creatinine clearance and eGFR at initial evaluation and then based on age,
diabetes duration, and treatment
Treatment ACEI titrated to normalization of albumin excretion
If elevated ACR confirmed (>30 mg/g) over 6 mos, after efforts to control glucose,
normalize BP
Retinopathy
Screening Initial dilated and comprehensive eye exam
Aged ≥10 yrs or puberty onset (whichever occurs first) with 3-5–yr diabetes duration
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 36
Summary Recommendations from NDEI
Follow-up Yearly
Less frequently: per recommendation of eye care professional
ACEIs are not approved by the U.S. Food and Drug Administration (FDA) for treatment of nephropathy. Not all ACEIs are indicated for use in
children/adolescents by the FDA. Refer to full prescribing information for indications and uses in pediatric populations.
Managing High Blood Pressure in Children and Adolescents With Type 1 Diabetes
Screening Measure BP at every visit
Confirm elevated BP at separate visit
Treatment High-normal BP (SBP or DBP >90th percentile*)
Lifestyle changes (diet & exercise) Pharmacologic therapy
If target BP not met in 3-6 mos
ACEI or ARB: initial treatment†
Hypertension (SBP or DBP >95th percentile)
Target: <130/80 mm Hg or <90th percentile*
*For age, sex, height; †Provide counseling re: potential teratogenic effects
Not all ACEIs are indicated for use in children/adolescents by the U.S. Food and Drug Administration (FDA). Refer to full prescribing information
for indications and uses in pediatric populations.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 37
Summary Recommendations from NDEI
Managing Dyslipidemia in Children and Adolescents With Type 1 Diabetes
Obtain fasting lipids Aged ≥2 years post-diagnosis*
Lipid monitoring in all patients
Abnormal lipids: yearly every 5 yrs
LDL-C <100 mg/dL (<2.6 mmol/L)
Treatment
Initial Optimal glucose control
Medical nutrition therapy (MNT): decrease saturated fat intake
Aged ≥10 yrs Lifestyle changes and MNT
After lifestyle changes, add statin† if LDL-C
>160 mg/dL (>4.1 mmol/L) or >130 mg/dL
(>3.4 mmol/L) + ≥1 CVD risk factor
Target: LDL-C <100 mg/dL (<2.6 mmol/L)
*When glucose levels well controlled
†
Statins are approved by the U.S. Food and Drug Administration for treatment of heterozygous familial hypercholesterolemia in children and
adolescents. Not all statins are FDA approved for use under the age of 10 yrs; statins should generally not be used in children with type 1
diabetes before age 10. Refer to full prescribing information for indications and uses in pediatric populations. For postpubertal girls, pregnancy
prevention is important as statins are contraindicated in pregnancy.
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 38
Summary Recommendations from NDEI
Screening for Autoimmunities in Children and Adolescents With Type 1 Diabetes
Hypothyroidism
Post-diagnosis of type 1 diabetes consider Screening for
Antithyroid peroxidase antibodies
Antithyroglobulin antibodies
Measuring TSH (when metabolic levels well controlled)
Reassess every 1-2 yrs if normal
Celiac disease
Post-diagnosis of type 1 diabetes consider measuring IgA antitissue transglutaminase
Antiendomysial antibodies
Candidates for testing
Family history of celiac disease
Failure to grow or gain weight
Weight loss
Diarrhea or flatulence
Abdominal pain
Signs of malabsorption
Repeated hypoglycemia of unknown cause or decline in glycemic control
Asymptomatic with positive antibodies Gastroenterologist referral for confirmatory endoscopy and biopsy
If diagnosis confirmed Gluten-free diet; dietitian consultation
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 39
Summary Recommendations from NDEI
Monogenic Diabetes Syndromes
Neonatal diabetes • Maturity-onset diabetes of the young
Consider if:
Diabetes diagnosed within first 6 mos after birth
Strong diabetes family history; no typical features of type 2 diabetes
Mild fasting hyperglycemia,* esp if young and nonobese
Diabetes with negative autoantibodies, no signs of obesity or insulin resistance
*100-150 mg/dL (5.5-8.5 mmol/L)
Considerations for Children and Adolescents With Type 2 Diabetes
At diagnosis After diagnosis
Perform eye exam Similar screening and treatment as for type 1 diabetes for:
Measure risk factors
Blood pressure Hypertension
Fasting lipids Albumin excretion
Albumin secretion Dyslipidemia
Retinopathy
Other issues that may need to be addressed:
Polycystic ovarian disease
Pediatric obesity comorbidities: sleep apnea, hepatic steatosis, orthopedic complications, psychosocial concerns
Any pharmacologic agents discussed are approved for use in the United States by the U.S. Food and Drug Administration (FDA) unless otherwise noted. Consult individual
prescribing information for approved uses outside of the United States. This content was created by Ashfield Healthcare Communications, and was not associated with
funding via an educational grant or a promotional/commercial interest. January 2015
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 40
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
14. Immunizations
Immunization/Vaccine Recommendations
Influenza vaccine Annually in all patients with diabetes aged ≥6 mos
Pneumococcal polysaccharide vaccine 23 (PPSV23) All patients with diabetes aged ≥2 yrs
Aged >65 yrs previously vaccinated with PPSV23:
Administer pneumococcal conjugate vaccine 13 (PCV13)
followed by PPSV23 6-12 months after initial vaccination*
Aged >65 yrs previously vaccinated with PPSV23:
Follow-up vaccine in ≥12 months with PCV13
If additional doses of PPSV23 required, subsequent doses
given 6-12 months after PCV13 and ≥5 yrs since most
recent PPSV23 dose
Hepatitis B vaccine Unvaccinated adults with diabetes aged 19-59 yrs
Consider in unvaccinated adults aged ≥60 yrs
*Two doses should not be coadministered: minimum interval between dosing: 8 wks
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest. January 2015
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
2015 American Diabetes Association (ADA) Diabetes Guidelines 41
Summary Recommendations from NDEI
2015 American Diabetes Association (ADA) Diabetes Guidelines
Summary Recommendations from NDEI
Source: American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care.
2015;38(suppl 1):S1-S93.
Refer to source document for full recommendations, including level of evidence rating.
15. Psychosocial Care & Assessment
Immunization/Vaccine Recommendations
Reasonable to include psychological and social assessments of patient as part of diabetes management
Psychosocial screening and follow-up may include:
Attitudes about diabetes
Expectations for medical management and outcomes
Mood
Quality of life
Financial, social, emotional resources
Psychiatric history
Older adults (65 yrs)with diabetes should be considered a high-priority population for depression screening and treatment
Routinely screen for depression and diabetes-related distress, anxiety, eating disorders, and cognitive impairment
This content was created by Ashfield Healthcare Communications, and was not associated with funding via an educational grant or a
promotional/commercial interest. January 2015
Visit NDEI’s Slide Library at NDEI.org to download
the full 2015 ADA diabetes guidelines slide set
The National Diabetes Education Initiative® (NDEI®) is sponsored
by Ashfield Healthcare Communications, Lyndhurst, NJ.
Copyright © 2015 Ashfield Healthcare Communications. All rights reserved.
You might also like
- Clinical Dietetics Online Biochemistry Cheat SheetDocument5 pagesClinical Dietetics Online Biochemistry Cheat Sheettimea_ghenea100% (1)
- NICE Gestational Diabetes - 2021Document10 pagesNICE Gestational Diabetes - 2021Sajedah MahdiNo ratings yet
- Diabetes Mellitus CPGDocument157 pagesDiabetes Mellitus CPGejikieru03100% (3)
- Femoral Neck FractureDocument36 pagesFemoral Neck FractureAnonymous ekem7I100% (2)
- ADA 2015 Summary PDFDocument46 pagesADA 2015 Summary PDFHam SotheaNo ratings yet
- ADA Diabetes Management Guidelines 2015Document117 pagesADA Diabetes Management Guidelines 2015Fakhri Firman GunawanNo ratings yet
- Standarts of Medical CareDocument34 pagesStandarts of Medical CareLussiana Mercy MaramisNo ratings yet
- Meal Planning For DiabetesDocument29 pagesMeal Planning For DiabetesnurulahdiahNo ratings yet
- Clinical Practice Guidelines For Diabetes ManagementDocument5 pagesClinical Practice Guidelines For Diabetes ManagementIqbal Fida MaulanaNo ratings yet
- Diabetes 1Document13 pagesDiabetes 1n_catalinNo ratings yet
- DM MX GuidlineDocument22 pagesDM MX GuidlineSuritha VitharanaNo ratings yet
- ALGORITHM Pre-Diabetes Identification and InterventionDocument2 pagesALGORITHM Pre-Diabetes Identification and InterventionconisenopadangNo ratings yet
- Executive Summary RSSDI Recommendations 241019Document44 pagesExecutive Summary RSSDI Recommendations 241019Sunil MishraNo ratings yet
- Classification of DiabetesDocument94 pagesClassification of DiabetesTHAO DANGNo ratings yet
- Konsensus DM Ada Esda & PerkeniDocument31 pagesKonsensus DM Ada Esda & PerkeniWindy SuryaNo ratings yet
- Diabetes The Basics An Introduction: How Is Diabetes Managed?Document2 pagesDiabetes The Basics An Introduction: How Is Diabetes Managed?debabrata5976No ratings yet
- Primary Prevention of Diabetes Update 2011Document18 pagesPrimary Prevention of Diabetes Update 2011Putri AdimuktiNo ratings yet
- Screening and Management of Lipids: Patient Population: ObjectiveDocument20 pagesScreening and Management of Lipids: Patient Population: ObjectiveKatie Kroll BradyNo ratings yet
- Algoritmo Slide Set Diabetes 2012Document45 pagesAlgoritmo Slide Set Diabetes 2012flordeNo ratings yet
- How Is Diabetes Managed?: Type 1 and Type 2 Diabetes ORDocument2 pagesHow Is Diabetes Managed?: Type 1 and Type 2 Diabetes ORdebabrata5976No ratings yet
- Diabetes Care Guidelines - ADA 2014Document9 pagesDiabetes Care Guidelines - ADA 2014Manish Chandra PrabhakarNo ratings yet
- ADA Standards of Care For Diabetes 2013Document4 pagesADA Standards of Care For Diabetes 2013Syawal PratamaNo ratings yet
- Diabetes PPT FianlDocument31 pagesDiabetes PPT FianlUqba Mishal100% (1)
- Managing Diabetes in Primary Care in The CaribbeanDocument24 pagesManaging Diabetes in Primary Care in The CaribbeanAndre SookdarNo ratings yet
- Nfs 774 Case StudyDocument37 pagesNfs 774 Case Studyapi-533845626No ratings yet
- Diabetes Mellitus CPGDocument137 pagesDiabetes Mellitus CPGKristine-Joy Legaspi FrancoNo ratings yet
- Clinical Diabetes AbridgedDocument19 pagesClinical Diabetes AbridgedMagnus CarlsenNo ratings yet
- 2nd Case Session ToT CPG HPTDocument127 pages2nd Case Session ToT CPG HPThakimahsNo ratings yet
- Summary of IMBR-Saud Alzahrani-08-2020Document30 pagesSummary of IMBR-Saud Alzahrani-08-2020AHMAD ALROWAILYNo ratings yet
- (CLINPHAR) 2015 Clinical Practice Guidelines For The Management of Dyslipidemia in The Philippines - ExtractedDocument44 pages(CLINPHAR) 2015 Clinical Practice Guidelines For The Management of Dyslipidemia in The Philippines - ExtractedDenise Yanci DemiarNo ratings yet
- Presentation Indonesia Sep 8 2022Document88 pagesPresentation Indonesia Sep 8 2022yuni chanNo ratings yet
- Type 2 Diabetes MellitusDocument4 pagesType 2 Diabetes MellitusMazhar WarisNo ratings yet
- Glucose Bad 1Document3 pagesGlucose Bad 1Jean-Yves ESTINFILNo ratings yet
- Diabetes Mellitus Management of Gestational Diabetes - 280720Document12 pagesDiabetes Mellitus Management of Gestational Diabetes - 280720AddisNo ratings yet
- Executive Summary: Standards of Medical Care in Diabetesd2014Document9 pagesExecutive Summary: Standards of Medical Care in Diabetesd2014Juan Carlos Plácido OlivosNo ratings yet
- Diabetes PDFDocument86 pagesDiabetes PDFHari SubagiyoNo ratings yet
- Diabetes in Pregnancy Gestational Diabetes Risk Assessment Testing Diagnosis and ManagementDocument10 pagesDiabetes in Pregnancy Gestational Diabetes Risk Assessment Testing Diagnosis and Managementteana2No ratings yet
- Cardiovascular Disease Risk AssesmentDocument24 pagesCardiovascular Disease Risk Assesmentaldrin zamoraNo ratings yet
- LipidsupdateDocument23 pagesLipidsupdatedrshekarforyouNo ratings yet
- DM PDFDocument34 pagesDM PDFfani melindaNo ratings yet
- Standards of Medical Care in Diabetes: Merican Iabetes SsociationDocument33 pagesStandards of Medical Care in Diabetes: Merican Iabetes Ssociationdavid-lo-512No ratings yet
- Anexo 6. UpToDate - 2022 - PrevenciónDocument32 pagesAnexo 6. UpToDate - 2022 - PrevenciónRubiNo ratings yet
- Oral Glucose Tolerance TestDocument6 pagesOral Glucose Tolerance TestIka HabelNo ratings yet
- Estandares de Manejo AdaDocument38 pagesEstandares de Manejo AdaHERACLITONo ratings yet
- DiabetesDocument182 pagesDiabetesMiaMDNo ratings yet
- Guidelines For The Prevention and Management of DiabetesDocument261 pagesGuidelines For The Prevention and Management of DiabetesCaldwellNo ratings yet
- 02 Jones DouglasDocument27 pages02 Jones DouglasKarthik SNo ratings yet
- Ada 2019Document44 pagesAda 2019Oxide HelixNo ratings yet
- Guideline ADA 2010Document51 pagesGuideline ADA 2010Rivano Frits Henry PandalekeNo ratings yet
- Diabetes Interactive0412 PDFDocument83 pagesDiabetes Interactive0412 PDFrugbyrover_123091332No ratings yet
- A Practical Guide To Diagnosing Type 2 DiabetesDocument4 pagesA Practical Guide To Diagnosing Type 2 Diabeteselena_bautista_3No ratings yet
- Resumen Guias ADA 11Document7 pagesResumen Guias ADA 11Yolpa Figueroa PorrasNo ratings yet
- Diabetes: in All Asymptomatic People Whose Initial Result Suggests A Diagnosis of Diabetes, A ConfirmatoryDocument3 pagesDiabetes: in All Asymptomatic People Whose Initial Result Suggests A Diagnosis of Diabetes, A ConfirmatorytothomileNo ratings yet
- Case StudyDocument9 pagesCase StudyJulianne P. VillanuevaNo ratings yet
- LP HiperglikemiaDocument65 pagesLP HiperglikemiaLisna SulistyaniNo ratings yet
- Type 1 Diabetes Mellitus - Practice Essentials, Background, PathophysiologyDocument8 pagesType 1 Diabetes Mellitus - Practice Essentials, Background, PathophysiologyTrifosa Ika Septiana Eryani100% (1)
- 3-Diabetes Update PresentationDocument85 pages3-Diabetes Update PresentationLucy Naomi Besitimur100% (1)
- Diabetes Ready Reference for Nurse Practitioners: Clear, Concise Guidelines for Effective Patient CareFrom EverandDiabetes Ready Reference for Nurse Practitioners: Clear, Concise Guidelines for Effective Patient CareNo ratings yet
- Natural Remedies To Pre-Diabetes: Reverse Type 2 Diabetes Naturally in 90 DaysFrom EverandNatural Remedies To Pre-Diabetes: Reverse Type 2 Diabetes Naturally in 90 DaysNo ratings yet
- Faculty of Business - Report WritingDocument16 pagesFaculty of Business - Report WritingcuambyahooNo ratings yet
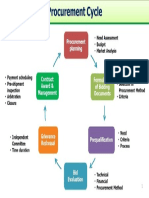
- Procurement Cycle PDFDocument1 pageProcurement Cycle PDFcuambyahoo100% (1)
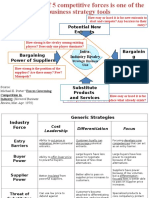
- Potential New Entrants: Strategic Business UnitDocument6 pagesPotential New Entrants: Strategic Business UnitcuambyahooNo ratings yet
- Immigration To Australia-StepbyStep Guide (Subclass 189&190) V3.0Document5 pagesImmigration To Australia-StepbyStep Guide (Subclass 189&190) V3.0cuambyahooNo ratings yet
- Aleppo and World ConscienceDocument28 pagesAleppo and World ConsciencecuambyahooNo ratings yet
- Impact of Demographic Changes On Inflation in Pakistan: A A J, F F F MDocument1 pageImpact of Demographic Changes On Inflation in Pakistan: A A J, F F F McuambyahooNo ratings yet
- Un-Employment Rates: Administrative 1981 Unit Both Sexes Male Female Census 1998-CensusDocument1 pageUn-Employment Rates: Administrative 1981 Unit Both Sexes Male Female Census 1998-CensuscuambyahooNo ratings yet
- Applications of OhmDocument1 pageApplications of OhmcuambyahooNo ratings yet
- AP1 Ch2studyguideDocument5 pagesAP1 Ch2studyguidecuambyahooNo ratings yet
- Daftar Pustaka ReferatDocument2 pagesDaftar Pustaka ReferatBetharlitha PurLikaNo ratings yet
- AnemiaDocument11 pagesAnemiadewiq_wahyuNo ratings yet
- Diabetes Mellitus Aticle ApthaDocument6 pagesDiabetes Mellitus Aticle ApthaRahul KirkNo ratings yet
- 14 - DR. S.K Haldar MAHC 3Document70 pages14 - DR. S.K Haldar MAHC 3Ashan SanNo ratings yet
- RCDSO Infection ControlDocument56 pagesRCDSO Infection ControlsnaniraqNo ratings yet
- Post Cardiac Arrest CareDocument28 pagesPost Cardiac Arrest CareAkib ArmanNo ratings yet
- Stok Opname: Tablet No Nama Obat SisaDocument7 pagesStok Opname: Tablet No Nama Obat SisajuleNo ratings yet
- Deep Margin ElevationDocument11 pagesDeep Margin Elevationjarodzee100% (4)
- Methodist Healthcare 6-26 PDFDocument172 pagesMethodist Healthcare 6-26 PDFTom Ling IINo ratings yet
- Disprin UploadDocument13 pagesDisprin Uploadgoved2100% (1)
- Sodium Stibogluconate Pentamidine IsetionateDocument10 pagesSodium Stibogluconate Pentamidine IsetionateThunder BoltNo ratings yet
- Diseases of The Spinal CordDocument15 pagesDiseases of The Spinal CordBai Aishwayra Haylee DalidigNo ratings yet
- NCP and CNPDocument37 pagesNCP and CNPDen TupasNo ratings yet
- Displacement of The Uterus: DR Sahar Anwar RizkDocument32 pagesDisplacement of The Uterus: DR Sahar Anwar RizkWida Ratna SariNo ratings yet
- Theory TestDocument18 pagesTheory TestKrisna PonggalungguNo ratings yet
- Ebola Virus Disease: Group 2 Adinda Pramestya Annisa Nurul Q Dewi Utami Salsabila Syah MirandaDocument20 pagesEbola Virus Disease: Group 2 Adinda Pramestya Annisa Nurul Q Dewi Utami Salsabila Syah Mirandadewi salsabillaNo ratings yet
- Shalamar Hospital Laboratory: LLFFLLLLLLLLLLLLLLLLLLDocument1 pageShalamar Hospital Laboratory: LLFFLLLLLLLLLLLLLLLLLLAfaq MehmoodNo ratings yet
- Metodologia de Admitere 2018 - Limba EnglezaDocument27 pagesMetodologia de Admitere 2018 - Limba EnglezaoscarapaNo ratings yet
- Clinical Efficacy of Panchamuladi Kaala Basti (Enema) in The Management of Amavata (Rheumatoid Arthritis)Document7 pagesClinical Efficacy of Panchamuladi Kaala Basti (Enema) in The Management of Amavata (Rheumatoid Arthritis)Jaldeep KarasaliyaNo ratings yet
- New PRC Case FormDocument5 pagesNew PRC Case Formgyds_17No ratings yet
- Aplastic AnemiaDocument60 pagesAplastic AnemiaAvi VermaNo ratings yet
- Intra Oral ExaminationDocument14 pagesIntra Oral ExaminationpriyaNo ratings yet
- New Advanced in RadiotherapyDocument49 pagesNew Advanced in RadiotherapyIndonesian Journal of CancerNo ratings yet
- Birth TraumaDocument44 pagesBirth TraumasreekalaNo ratings yet
- Anna Garcia Death Timeline 1Document19 pagesAnna Garcia Death Timeline 1api-245176779No ratings yet
- ProfessionalismDocument5 pagesProfessionalismMaria Elisa Valentim Pickler NicolinoNo ratings yet
- Doctors CV TemplateDocument3 pagesDoctors CV TemplateBoopathi KalaiNo ratings yet
- MS-SL Prelims FirstsemDocument17 pagesMS-SL Prelims Firstsemmcdonald 1234No ratings yet