Professional Documents
Culture Documents
Electrochemistry Page 2 PDF
Electrochemistry Page 2 PDF
Uploaded by
Nor HusseinOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrochemistry Page 2 PDF
Electrochemistry Page 2 PDF
Uploaded by
Nor HusseinCopyright:
Available Formats
UNIT KIMIA KEJURUTERAAN INTELECTUAL DISCOURSE
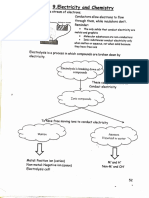
9.3 ELECTROLYTIC CELL
1. Cadangan kaedah penentuan ion yang terpilih untuk didiscajkan di anod dan katod :
Cathode Anode
(cation) (anion)
Inert electrode?
Group 1 or 2 metal ion?
yes no
no OH-, Cl-, Br-, I- present? electrode corrodes
yes
yes no
H2O is reduced More concentrated cation More concentrated H2O is reduced
is reduced anion is oxidised
Species
Solution Cations present
Species discharged
Solution Cations present
discharged
Pt in Na2SO4 SO42- and H20 H20
NaCl aqueous
Na+ and H20 H20
solution NO3- and H20 Zn is oxidised
Zn in Zn(NO3)2
CuSO4 aqueous Pt in NaCl
Cu+ and H20 Cu+ Cl- and H20 Cl-
solution 2 aqueous solution
Pt in NaCl dilute Cl- and H20 H20
solution
You might also like
- Slope Stability and Stabilization Methods Abramson-Sharma (2002)Document736 pagesSlope Stability and Stabilization Methods Abramson-Sharma (2002)supersalmon202000100% (3)
- Thermo HW SolsDocument61 pagesThermo HW Solsbra5100% (1)
- Redox MaterialDocument37 pagesRedox MaterialSuwahono, M.PdNo ratings yet
- ElectrolysisDocument3 pagesElectrolysisMohit RawatNo ratings yet
- MLP of Coordination Compound PDFDocument23 pagesMLP of Coordination Compound PDFAkash SureshNo ratings yet
- Note 21-Feb-2024 at 9 - 10 - 47 AMDocument6 pagesNote 21-Feb-2024 at 9 - 10 - 47 AMTaha BukhariNo ratings yet
- Aqueous Reactions and SolutionDocument53 pagesAqueous Reactions and SolutionsaneleNo ratings yet
- CH 17 PDFDocument15 pagesCH 17 PDFkrishnaNo ratings yet
- ElectrolysisDocument31 pagesElectrolysisVasavi VaasuNo ratings yet
- Electrolysis LessonDocument21 pagesElectrolysis LessonShenaya HewaNo ratings yet
- Hydrogen in 1 Shot - Class Notes - JEEDocument22 pagesHydrogen in 1 Shot - Class Notes - JEESaurabh KumarNo ratings yet
- Lecture 15.3 - Electrolytic CellsDocument10 pagesLecture 15.3 - Electrolytic CellsLiam DoranNo ratings yet
- EVT 637 Paper ReportDocument5 pagesEVT 637 Paper ReportAdleen SyahieraaNo ratings yet
- Electrochemistry Ol Notes 2Document7 pagesElectrochemistry Ol Notes 2Ahmed SherifNo ratings yet
- PCM Chapter 02Document7 pagesPCM Chapter 02Alif AzmirNo ratings yet
- Electrolysis Aqueous SolutionDocument40 pagesElectrolysis Aqueous SolutionVictor OkosunNo ratings yet
- TOPIC Products Obtained by ElectrolysisDocument2 pagesTOPIC Products Obtained by Electrolysisvivek daveNo ratings yet
- Net Electrochemical CellsDocument21 pagesNet Electrochemical CellsSourav DasNo ratings yet
- HYDROGEN - Class Notes - JEE Mind MapDocument18 pagesHYDROGEN - Class Notes - JEE Mind MapTanay1 MitraNo ratings yet
- Corrosion (All)Document59 pagesCorrosion (All)trongstaNo ratings yet
- Lab Report: Cmt555: Experiment 1: Galvanic & Electrolytic CellDocument11 pagesLab Report: Cmt555: Experiment 1: Galvanic & Electrolytic CellkuekNo ratings yet
- Voltaic Cell and Electrolytic Cell: by 5S3 Jocelyne LewDocument9 pagesVoltaic Cell and Electrolytic Cell: by 5S3 Jocelyne LewJocelyne LewNo ratings yet
- Exp 1Document4 pagesExp 1Jayendra JamadarNo ratings yet
- 10 2 JP3 Eurodia.Document30 pages10 2 JP3 Eurodia.huseyinelmalikiNo ratings yet
- ElectroDocument48 pagesElectroMang friesNo ratings yet
- 3 ELECTROCHEMISTRY Document - 2Document15 pages3 ELECTROCHEMISTRY Document - 2abdimoh7522No ratings yet
- Notes On ElectrolysisDocument3 pagesNotes On Electrolysisapi-3819012No ratings yet
- Electrochemistry NoteDocument3 pagesElectrochemistry NoteNaguib Zakaria100% (3)
- Chapter 6b Electrolysis of Aqueous SolutionDocument16 pagesChapter 6b Electrolysis of Aqueous SolutionKavitha ThayagarajanNo ratings yet
- 02 Group 2 NotesDocument6 pages02 Group 2 NotesAbdul RafayNo ratings yet
- Lab 2 - 555Document9 pagesLab 2 - 555Nurzawanah AkmarNo ratings yet
- Chlor Alkali Process: Membrane ElectrolysisDocument18 pagesChlor Alkali Process: Membrane ElectrolysisLuqmanNo ratings yet
- Chemical Effects of Electric Current - FinalDocument46 pagesChemical Effects of Electric Current - Finalartisatyam2323No ratings yet
- HYDROGEN - Class Notes - JEE MindmapDocument15 pagesHYDROGEN - Class Notes - JEE Mindmapadsaditya24No ratings yet
- 13.1 Compounds in Aqueous SolutionsDocument9 pages13.1 Compounds in Aqueous SolutionsOmar AlwaerNo ratings yet
- Experiment 2 Electrolytic Cell Nurul Husna Binti IbrahimDocument4 pagesExperiment 2 Electrolytic Cell Nurul Husna Binti IbrahimNurul HusnaNo ratings yet
- Gems Genesis: 9caieDocument4 pagesGems Genesis: 9caieBhavya darjiNo ratings yet
- Electrochemistry 1Document9 pagesElectrochemistry 1laila SheashaNo ratings yet
- Electrometallurgy 3: Laval University, Quebec City, Canada Fathi - Habashi@arul - Ulaval.caDocument28 pagesElectrometallurgy 3: Laval University, Quebec City, Canada Fathi - Habashi@arul - Ulaval.caBasilia YulianiNo ratings yet
- Class 10 Science Notes Chapter 2 Studyguide360Document18 pagesClass 10 Science Notes Chapter 2 Studyguide360Rohan RalliNo ratings yet
- Chem - Redox Formula Sheet (Never Completely Finished), Electrolytic Cells, Voltaic Cells, Electric PotentialsDocument2 pagesChem - Redox Formula Sheet (Never Completely Finished), Electrolytic Cells, Voltaic Cells, Electric PotentialsMark Riley100% (2)
- Chapter 8 ElectrochemistryDocument3 pagesChapter 8 Electrochemistrysitinur qahirahNo ratings yet
- Electrolysis Cheat Sheet: by ViaDocument2 pagesElectrolysis Cheat Sheet: by Viaaziz ahmadNo ratings yet
- Chapter 04 ISM Chang 14eDocument17 pagesChapter 04 ISM Chang 14elsytb2000No ratings yet
- Tutorial 9 - Level 1 Worked SolutionsDocument11 pagesTutorial 9 - Level 1 Worked SolutionsBloodCypherNo ratings yet
- BAB 6 ELEKTROKIMIA Elektrolisis Sebatian AkuesDocument5 pagesBAB 6 ELEKTROKIMIA Elektrolisis Sebatian AkuesNik Diana Hartika Nik HusainNo ratings yet
- Spot The Difference!: Molten Lead (II) Bromide Sodium Chloride Solution Figure A Figure BDocument9 pagesSpot The Difference!: Molten Lead (II) Bromide Sodium Chloride Solution Figure A Figure Baainaa86No ratings yet
- Electrolysis 1Document14 pagesElectrolysis 1cleohambiraNo ratings yet
- ElectrochemistryDocument38 pagesElectrochemistryShannon SmithNo ratings yet
- Chap 4 InstructorDocument26 pagesChap 4 InstructorOsama MohsinNo ratings yet
- A2 Group II NotesDocument6 pagesA2 Group II NotesZim Ahmed ZavianNo ratings yet
- Chem 1 FrontDocument1 pageChem 1 Frontvighneshdp174No ratings yet
- ELECTROLYSISDocument12 pagesELECTROLYSISKatlo KgosiyangNo ratings yet
- Electrochem 201516Document81 pagesElectrochem 201516Mohd AminudinNo ratings yet
- 2-L2SHL+Reactions of Cations 2Document3 pages2-L2SHL+Reactions of Cations 2lawandlatif36No ratings yet
- Ionic Equilibrium Notes Jee Main GuruDocument67 pagesIonic Equilibrium Notes Jee Main GuruAnonymous SFsecXafW0% (1)
- Topic Different Types of CellsDocument2 pagesTopic Different Types of Cellsvivek daveNo ratings yet
- Topic Different Types of CellsDocument2 pagesTopic Different Types of Cellsvivek daveNo ratings yet
- Electrochemistry Lab CH131Document24 pagesElectrochemistry Lab CH131Okana KofiNo ratings yet
- Electrolysis: of Molten Ionic CompoundsDocument16 pagesElectrolysis: of Molten Ionic CompoundsHafiz Abdul RehmanNo ratings yet
- New Frontiers in Asymmetric CatalysisFrom EverandNew Frontiers in Asymmetric CatalysisKoichi MikamiNo ratings yet
- Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and TestingFrom EverandCorrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and TestingNo ratings yet
- Module 3 Heat EffectsDocument41 pagesModule 3 Heat EffectsJatskinesisNo ratings yet
- GOLDEN Mono-Block Pumps (Domestic) PDFDocument3 pagesGOLDEN Mono-Block Pumps (Domestic) PDFkfctco100% (1)
- Comparison of 2D Versus 3D Modeling Approaches For The Analysis of The Concrete Face Rockfill Cokal DamDocument19 pagesComparison of 2D Versus 3D Modeling Approaches For The Analysis of The Concrete Face Rockfill Cokal DamSebastian PalaciosNo ratings yet
- Wee1964 N001Document9 pagesWee1964 N001Oliver RubioNo ratings yet
- DensityDocument15 pagesDensityapi-286079895No ratings yet
- Fluid Flow PhenomenaDocument13 pagesFluid Flow PhenomenaRaven ShadeNo ratings yet
- Agitator HW3 SolutionDocument7 pagesAgitator HW3 SolutionPrashant MalveNo ratings yet
- Black OilDocument70 pagesBlack OilsandaflorNo ratings yet
- Petronas Technical Standards: The Design of Glycol ContactorsDocument47 pagesPetronas Technical Standards: The Design of Glycol ContactorsadamNo ratings yet
- General Chemistry 2 Grade 12Document12 pagesGeneral Chemistry 2 Grade 12Ceejay IgnacioNo ratings yet
- Background of The StudyDocument22 pagesBackground of The StudyMaynard BaralNo ratings yet
- 2GDocument8 pages2GGaurav Panditrao UdanshivNo ratings yet
- JUAL Automatic Level Topcon AT-B4Document2 pagesJUAL Automatic Level Topcon AT-B4BayuPratamaNo ratings yet
- MAE 322 Machine Design: Dr. Hodge Jenkins Mercer UniversityDocument29 pagesMAE 322 Machine Design: Dr. Hodge Jenkins Mercer UniversityDuslerinalargaNo ratings yet
- Rust and Acid-Resistant Steels, Ferritic-AusteniticDocument3 pagesRust and Acid-Resistant Steels, Ferritic-AusteniticKiranNo ratings yet
- 081 - ME8594, ME6505 Dynamics of Machines - 2 MarksDocument20 pages081 - ME8594, ME6505 Dynamics of Machines - 2 Markssara vanaNo ratings yet
- Almeida & Neto-Effect of Void Content On The Strength of Composite LaminatesDocument10 pagesAlmeida & Neto-Effect of Void Content On The Strength of Composite LaminatesGustavo100% (1)
- CL 304 - CPT Ethylene and Propylene PolymerizationDocument23 pagesCL 304 - CPT Ethylene and Propylene PolymerizationRelaxation MasterNo ratings yet
- List & Properties of UK Strcutral Steel Shapes No Sheet DescriptionDocument65 pagesList & Properties of UK Strcutral Steel Shapes No Sheet DescriptionThắngg TrịnhhNo ratings yet
- Materials 14 02273Document20 pagesMaterials 14 02273Silas Sverre ChristensenNo ratings yet
- Nptel Learning Courses Structural Health Monitoring of CompositesDocument305 pagesNptel Learning Courses Structural Health Monitoring of Compositesaurora borealissNo ratings yet
- Dillidur Technical InformationDocument56 pagesDillidur Technical InformationoakleysteelNo ratings yet
- Poster-TIPURI IMB SUD, SIMBOLURI-engDocument1 pagePoster-TIPURI IMB SUD, SIMBOLURI-engLucian HoudiniNo ratings yet
- Precipitation ChapterDocument12 pagesPrecipitation ChapterMaricica Gorceag50% (2)
- Review Article: A Review On The Efficiency of Graphene-Based BHJ Organic Solar CellsDocument16 pagesReview Article: A Review On The Efficiency of Graphene-Based BHJ Organic Solar CellsJohn Lloyd GenerosoNo ratings yet
- Engineering Data Book: Si Version Sections 1-15Document16 pagesEngineering Data Book: Si Version Sections 1-15IBIKUNLENo ratings yet
- 24 - Stress Concentration PDFDocument9 pages24 - Stress Concentration PDFNaman ShuklaNo ratings yet
- Z Fxy X X T X XT y Yt Z Fxtyt T: 10.4 The Chain RuleDocument3 pagesZ Fxy X X T X XT y Yt Z Fxtyt T: 10.4 The Chain Ruleirene kimNo ratings yet