Professional Documents
Culture Documents
"Poison" "Poison": Substances Listed in Poison List "Poison List"
"Poison" "Poison": Substances Listed in Poison List "Poison List"
Uploaded by
nizam_ghaniOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
"Poison" "Poison": Substances Listed in Poison List "Poison List"
"Poison" "Poison": Substances Listed in Poison List "Poison List"
Uploaded by
nizam_ghaniCopyright:
Available Formats
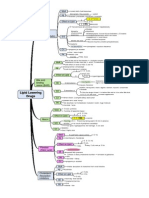
S20(a-b) by W/S which need w/s license S15→S26(1)
Substances listed in Poison List
&(2)(a)
"Poison list" S.20(a): →Lic Ph / Lic WS
S6: by Minister, assisted by Poison Board Sale/supply to whom
S.15(2)
How to record
Government hospital S.15(3)(a) record prior to supply - book prescribed
Procurement r26(1)PR'52
S20: Group A (Poisons Wholesale Sales Book - DRAW OUT the table)
Import
Do not need license as in S. 8(2)(c)PA52 How to label
r. 9(1)(b)PR52 "poison', red/red background, name of poison
No need label. Just receive the stock and
stored as it's labelled r. 10(b)PR52 …of the proportion which the poison contained
in such preparation…
Store as in r. 25PR52
r. 10(c)PR52 …in English, Malay, Chinese and Tamil
Wholesale
Do not need license to buy from licensed How to store: r. 6PR52 (a) → (d) explain each subregulations
wholesaler as in S.15(2)(i)PA52 s21 (c ) licensed pharmacist as a
dispensed medicine and via precription
Record as in S.15(3)(b) written sign order
{S. 21(1) (c)} at the licensed premises {S. 16(2)}
No need label. Just receive the stock and (2) Rx
stored as it's labelled
by reg MD, dentist, vet
Store as in r. 25 PR'52
Form of Rx
Supply Acknowledge/ endorse
be registered as a product upon the face of the prescription
r. 7(1)(a)CDCR84 registered product No code
r. 7(1)(b) CDCR84 must be licensed Obtain within 1 day
r. 12(1)(b) CDCR84 wholesale license (6) Rx book→S24: a book where the seller or supplier enter
particulars of the poison sold or supplied as a DISPENSED
Records of transactions S21: Group B MEDICINE
r. 27 (1) CDCR84 Part I (Disp Med)
(1) Details required
r. 27 (2) CDCR84 (2) - "Prescription", "Prescriber"
The date of sale/supply and serial number
1st The name of poison/proprietary name and quantity
(Poison List-S2)
The name of the patient if supply on prescription (Gp. B)
The name and address of the patient if without
prescription (Gp. C)
- Labelling R12 PR52
Schedule s21 (b) by regd. Medical practitioner regd dentist or vet officer
for medical treatment of his patient
- Labelling R12 PR52
as "dispensed medicine"
Supply of Group C poison must be by a licensed pharmacist
"Poison" either with/without prescriptions {S. 2} at the licensed
S22: Group C premises {S. 16(2)}
(Disp Med) Supply of dispensed medicine must be recorded in the
Prescription Book {S 24} on the day of supply with
particulars as {S 24(1)}
- Labelling R12 PR52
(2) Poison Book: a book where a seller records sale by retail
a group D poisons NOT as a dispensed medicine
S23: Group D Poison Book - buyer known introduced
by person known to the pharmacist S23(1)(b)
- Labelling R9, 10
Supply of Poison must comply with packaging, labelling and storing
provisions {S. 9}
Container:- impervious to the medicine {R 5}
Sale/supply to whom S.25(2)PA52 cleaning-preservation of building
Don't have to record Part II poison. In PA52, we merely have 3
How to record records, which are Poisons Wholesale Sales Book, Poison Book
Part II (group D) and Prescription Book.
r. 11(a) → (c)PR52
How to label
r. 9 PR52
How to store r. 6(a) → (c) PR52
Exempted
2nd Schedule Articles & preparations exempted from the provisions of this Act. (including: surgical dressings,
(S2 & S7) adhesive, ceramics, explosives, fireworks, glue, media culture
Psychotropic Substances
(includinging these DD status drugs:Alfentanil, Dihydrocodeine, Fentanyl,
Non-Poison Ketamin, Methadone, Morphine, Oxycodone, Pethidine, Sufentanil)
3rd Schedule i) Rx (90 days validity) with prescriber's tel no, patient's age
S7: Exemption from Required
the Poison Act (S30) ii) Rx register for Psychotropic Subs.
<RM 10K
Can be obtained without prescription
Penalty < 4 yrs imprison
Not applicable to Poison Act
Both
You might also like
- ASCO-SEP Sixth EditionDocument1,434 pagesASCO-SEP Sixth Editionshona nag100% (3)
- IncompatibilitiesDocument40 pagesIncompatibilitiesnizam_ghaniNo ratings yet
- Pharm Practice QuestionsDocument42 pagesPharm Practice QuestionsShannon Garcia100% (1)
- Summary of Document Revisions: 0 New Initial IssueDocument19 pagesSummary of Document Revisions: 0 New Initial IssueThomas JohnNo ratings yet
- IRC & Packing ListDocument17 pagesIRC & Packing ListHery MukhlisNo ratings yet
- Comparison of Commercial RT PCR Diagnostic Kits For COVID 19Document11 pagesComparison of Commercial RT PCR Diagnostic Kits For COVID 19Taufik NurhidayatNo ratings yet
- B224 CDU TPL 000 PP QD PR0059 0010 - CompressedDocument46 pagesB224 CDU TPL 000 PP QD PR0059 0010 - CompressedPRASHANTNo ratings yet
- Validation Process for Cleared Brgys KbDocument51 pagesValidation Process for Cleared Brgys Kbbarangaypatadon1974No ratings yet
- Checklist On ValidationDocument2 pagesChecklist On ValidationJamy Vab MontesNo ratings yet
- Pages From g446 0828 2810 Itp 0100 0046 Rev 05 Final Approved 2Document4 pagesPages From g446 0828 2810 Itp 0100 0046 Rev 05 Final Approved 2Vinay YadavNo ratings yet
- Agra-REP-JVTI-QAS-00176-E01 - Method Statement For Erection of Batching Plant - (R0) - AGCC02 - 221020Document2 pagesAgra-REP-JVTI-QAS-00176-E01 - Method Statement For Erection of Batching Plant - (R0) - AGCC02 - 221020shaikh iftekhar aliNo ratings yet
- HPD Jazeera Basic RenderDocument6 pagesHPD Jazeera Basic RenderAhmad BougeisNo ratings yet
- R272PFC RRX R PLN 1001 A2Document97 pagesR272PFC RRX R PLN 1001 A2Joemon T JoyNo ratings yet
- PRJ-R5481-401 Valve A2250Document2 pagesPRJ-R5481-401 Valve A2250Abhay KarandeNo ratings yet
- Spike-RBD-His: For Research Use Only, Not For Diagnostic or Therapeutic UseDocument2 pagesSpike-RBD-His: For Research Use Only, Not For Diagnostic or Therapeutic UsemwdhtirahNo ratings yet
- RF FVDB-05 Application For Veterinary Drug and Product Registration (CPR)Document5 pagesRF FVDB-05 Application For Veterinary Drug and Product Registration (CPR)jeffrey ignacioNo ratings yet
- GRP 08 TS000 Yuk Civ CRS 00026Document3 pagesGRP 08 TS000 Yuk Civ CRS 00026Usman SarwarNo ratings yet
- CTD Requirements 637774197954180858Document9 pagesCTD Requirements 637774197954180858prabu dassNo ratings yet
- C711 PDS2Document5 pagesC711 PDS2Jitendra ChaudhariNo ratings yet
- Badak Central Plant Exhibit 4 - Vendor Data Register List (VDRL)Document12 pagesBadak Central Plant Exhibit 4 - Vendor Data Register List (VDRL)admidakbarNo ratings yet
- Cover Sheet For MRB 8405-285a-A002-001Document3 pagesCover Sheet For MRB 8405-285a-A002-001SAHAYA RABINTOR M URK19ME10380% (1)
- 78 Placo Moisture Resistant BoardDocument8 pages78 Placo Moisture Resistant BoardTaoufik AzarkanNo ratings yet
- HPD Jazeera AfrwDocument6 pagesHPD Jazeera AfrwAhmad BougeisNo ratings yet
- R2B-P3-206-02-P-HD-00220 - DATASHEETS FOR PRESSURE REGULATING VALVE, U-21000 - Rev.1Document11 pagesR2B-P3-206-02-P-HD-00220 - DATASHEETS FOR PRESSURE REGULATING VALVE, U-21000 - Rev.1Diana Paula Echartea MolinaNo ratings yet
- HPD Jazeera Anticarbonation WBDocument6 pagesHPD Jazeera Anticarbonation WBAhmad BougeisNo ratings yet
- Su Tu Trang Full Field Development Phase 1 Project: Vendor Front SheetDocument6 pagesSu Tu Trang Full Field Development Phase 1 Project: Vendor Front SheetLe TranNo ratings yet
- Inspection and Test Plan: Company Name OR Logo CompanyDocument3 pagesInspection and Test Plan: Company Name OR Logo Companyhanif faisalNo ratings yet
- Reference Product - AssessmentDocument3 pagesReference Product - AssessmentBett KevinNo ratings yet
- HPD Jazeera Dethar AdhesiveDocument6 pagesHPD Jazeera Dethar AdhesiveAhmad BougeisNo ratings yet
- Health Product Declaration Rigips RB - RBI - RF - RFI - 1Document9 pagesHealth Product Declaration Rigips RB - RBI - RF - RFI - 1Dimitris KousoulasNo ratings yet
- 645 Evaluations EUA Submission GuidanceDocument11 pages645 Evaluations EUA Submission GuidanceAbd El-Rahman SayedNo ratings yet
- DefaultDocument5 pagesDefaultBrandon DietzNo ratings yet
- Project HSE PlanDocument16 pagesProject HSE PlanShaheer AhmedNo ratings yet
- IMMULITE 2000 Vitamin B12 United Kingdom DXDCM 09017fe980792d67-1670912585650Document26 pagesIMMULITE 2000 Vitamin B12 United Kingdom DXDCM 09017fe980792d67-1670912585650Ghaith MaaniNo ratings yet
- Vedanta: Vedanta L Mited C O GasDocument13 pagesVedanta: Vedanta L Mited C O GasfirozNo ratings yet
- V-2158-101-A-884 - 3 Inspection and Test Procedure For PumpsDocument80 pagesV-2158-101-A-884 - 3 Inspection and Test Procedure For PumpsMessaoud Goutas100% (1)
- Hassi Bir Rekaiz Field Development Phase1Document5 pagesHassi Bir Rekaiz Field Development Phase1ahmed.njahNo ratings yet
- 110 v2b Ew00 00001 - 00 - Vendor Documents Register List (VDRL)Document7 pages110 v2b Ew00 00001 - 00 - Vendor Documents Register List (VDRL)Basten M H SilitongaNo ratings yet
- ANDADocument12 pagesANDAManish NangaliaNo ratings yet
- Inspection and Test Plan For External Blasting & Coating As SP-1246 PCS-2A SystemDocument6 pagesInspection and Test Plan For External Blasting & Coating As SP-1246 PCS-2A SystemVinayaga MoorthiNo ratings yet
- Master Inventory Format PDFDocument2 pagesMaster Inventory Format PDFAnonymous otOtKtNo ratings yet
- Atmopshere EPA LabelAmendment Approval - 11-16-20Document19 pagesAtmopshere EPA LabelAmendment Approval - 11-16-20jramosalNo ratings yet
- Chemical Dosing Unit (Cdu) : Rabigh II Project Interconnecting Package (UO1)Document19 pagesChemical Dosing Unit (Cdu) : Rabigh II Project Interconnecting Package (UO1)dodonggNo ratings yet
- CD SO4Document7 pagesCD SO4Anisa SeptrianaNo ratings yet
- Danieli PSM PDF FreeDocument52 pagesDanieli PSM PDF FreeCharlie NguyenNo ratings yet
- Warning: 2.2: Non Flammable, Non Toxic GasDocument7 pagesWarning: 2.2: Non Flammable, Non Toxic Gass_13_sr20No ratings yet
- Hot Water Generator (HW) : Rabigh II Project Interconnecting Package (UO1)Document18 pagesHot Water Generator (HW) : Rabigh II Project Interconnecting Package (UO1)dodonggNo ratings yet
- Indra: Firm Up No.3 Api Oil Separator Project (Civil & Building Work)Document9 pagesIndra: Firm Up No.3 Api Oil Separator Project (Civil & Building Work)sajay2010No ratings yet
- Hassi Bir Rekaiz Field Development Phase1: Process Data Sheet Front End Engineering DesignDocument6 pagesHassi Bir Rekaiz Field Development Phase1: Process Data Sheet Front End Engineering Designahmed.njahNo ratings yet
- Weight Control Report For Regas Platform TopsidesDocument42 pagesWeight Control Report For Regas Platform TopsidesinnovativekarthiNo ratings yet
- Registrant Record of Controlled Substances DestroyedDocument2 pagesRegistrant Record of Controlled Substances DestroyedBaljinder SinghNo ratings yet
- Earthworks RBDocument3 pagesEarthworks RBGokulan Ponnikrishnan PNo ratings yet
- 0 25-Jan-2013 New Initial Issue: Page 2 of 18Document20 pages0 25-Jan-2013 New Initial Issue: Page 2 of 18Thomas JohnNo ratings yet
- Bndp3 in Cscec p3c El XX Ms El 00003 Method Statement For Equipment Installation Inside SubstationDocument42 pagesBndp3 in Cscec p3c El XX Ms El 00003 Method Statement For Equipment Installation Inside Substationsivalakshan96No ratings yet
- Book 2Document15 pagesBook 2dewayantiputriNo ratings yet
- B224 Cdu TPL 000 MP Ga PR0013 0001 0 - 2Document4 pagesB224 Cdu TPL 000 MP Ga PR0013 0001 0 - 2PRASHANTNo ratings yet
- Utility Nitrogen Generation System: 32-38 C 1,2 kg/m3 15 Mbarg (Assumption) Below 42 6,4Document1 pageUtility Nitrogen Generation System: 32-38 C 1,2 kg/m3 15 Mbarg (Assumption) Below 42 6,4Jofanny Ferdian RahmansyahNo ratings yet
- HPD Jazeera Concrete EffectDocument6 pagesHPD Jazeera Concrete EffectAhmad BougeisNo ratings yet
- LRC 125 CoronaVac C202105094 VLR099Document1 pageLRC 125 CoronaVac C202105094 VLR099driftailNo ratings yet
- QAP Roofing SheetDocument3 pagesQAP Roofing SheetAMRIT PAL SINGHNo ratings yet
- Contractor Monthly Audit ReportDocument7 pagesContractor Monthly Audit ReportMunaku TafadzwaNo ratings yet
- Chiyoda Malaysia Sdn. BHDDocument2 pagesChiyoda Malaysia Sdn. BHDmeeNo ratings yet
- CalculationDocument2 pagesCalculationnizam_ghani100% (1)
- Calculation in PharmacyDocument2 pagesCalculation in Pharmacynizam_ghaniNo ratings yet
- PP1 Solution ConceptDocument1 pagePP1 Solution Conceptnizam_ghaniNo ratings yet
- Individual Drug For Anti ArrhythmicDocument1 pageIndividual Drug For Anti Arrhythmicnizam_ghaniNo ratings yet
- 1 Mole Cacl 147 G: 1 Mole 2 Eq 1 Mole 1 Eq 1 Mole 1 Eq 2 Eq 2 EqDocument1 page1 Mole Cacl 147 G: 1 Mole 2 Eq 1 Mole 1 Eq 1 Mole 1 Eq 2 Eq 2 Eqnizam_ghaniNo ratings yet
- Eyes ManagementDocument1 pageEyes Managementnizam_ghaniNo ratings yet
- Lipid Lowering Drugs Lipid Lowering Drugs: HMG Coa Reductase Inhibitors (Statin)Document1 pageLipid Lowering Drugs Lipid Lowering Drugs: HMG Coa Reductase Inhibitors (Statin)nizam_ghaniNo ratings yet
- Heart Failure Heart Failure: Pressure Force/Area Pressure Force/AreaDocument1 pageHeart Failure Heart Failure: Pressure Force/Area Pressure Force/Areanizam_ghaniNo ratings yet
- Anti Arrhythmic Anti Arrhythmic: Class IDocument1 pageAnti Arrhythmic Anti Arrhythmic: Class Inizam_ghaniNo ratings yet
- Quality Assurance of Pharmaceuticals: Sharvini Farhana Chris FarhanDocument25 pagesQuality Assurance of Pharmaceuticals: Sharvini Farhana Chris Farhannizam_ghaniNo ratings yet
- Inforum EU PHARMA LAWMay2008Document4 pagesInforum EU PHARMA LAWMay2008Prasoon SimsonNo ratings yet
- Appendix 2 TallmanDocument1 pageAppendix 2 TallmanPria UtamaNo ratings yet
- TugasDocument16 pagesTugaselsarahmiNo ratings yet
- Advances in Chronic DiseasesDocument134 pagesAdvances in Chronic DiseasesCSaludSanJuanSalinasNo ratings yet
- Jiunkpe Ns s1 2007 36403066 6541 Rezeki Husada AppendicesDocument31 pagesJiunkpe Ns s1 2007 36403066 6541 Rezeki Husada AppendicesyuniNo ratings yet
- Community Pharmacy Symptoms Diagnosis AnDocument2 pagesCommunity Pharmacy Symptoms Diagnosis AnmnbNo ratings yet
- Fundamental Principles of EmulsionDocument17 pagesFundamental Principles of EmulsionClitor Fernandes de SouzaNo ratings yet
- Abul Kalam Lutful KABIR 2009Document9 pagesAbul Kalam Lutful KABIR 2009syed ali mesum rizviNo ratings yet
- Tablets Design and Manufacture Machines PHT 311 Lecture 3Document29 pagesTablets Design and Manufacture Machines PHT 311 Lecture 3Lê Quang DuyNo ratings yet
- Iontophoresis - A Potential Emerging DDSDocument8 pagesIontophoresis - A Potential Emerging DDSDodo6199No ratings yet
- Running Head: Group Case 2Document9 pagesRunning Head: Group Case 2raviNo ratings yet
- Banned Drugs in IndiaDocument3 pagesBanned Drugs in IndiaGottumukkala Venkateswara RaoNo ratings yet
- Formulation, Development and Evaluation of Fast Disintegrating Tablet of Piroxicam Using Solid Dispersion TechniqueDocument20 pagesFormulation, Development and Evaluation of Fast Disintegrating Tablet of Piroxicam Using Solid Dispersion TechniqueEditor IJTSRDNo ratings yet
- Glipizide - Uses, Dosage & Side Effects - DrugsDocument5 pagesGlipizide - Uses, Dosage & Side Effects - Drugsremyde07No ratings yet
- Amoxan 500 MG Intermoxil 500 MG Lapimox 500 MG Opimox 500 MGDocument16 pagesAmoxan 500 MG Intermoxil 500 MG Lapimox 500 MG Opimox 500 MG20.022 Ni Putu Ayu MelcianaNo ratings yet
- THE TRADE MARKS JOURNAL (No.763 AUGUST 1, 2014)Document196 pagesTHE TRADE MARKS JOURNAL (No.763 AUGUST 1, 2014)sailaroNo ratings yet
- Mindworks Testimonials S TitlesDocument3 pagesMindworks Testimonials S Titlesapi-244257330No ratings yet
- Chapter - 4 - Pharmaceutics Size ReductionDocument6 pagesChapter - 4 - Pharmaceutics Size ReductionABHISHEK JOHARINo ratings yet
- Clinical Trials Flow ProcessDocument77 pagesClinical Trials Flow ProcessAnonymous Qr9nZRb100% (2)
- WHOModelFormulary - Children 94 PDFDocument528 pagesWHOModelFormulary - Children 94 PDFCristianJiménezNo ratings yet
- State Board of PharmacyDocument66 pagesState Board of PharmacyAnonymous hF5zAdvwCC100% (1)
- GCC Data Requirements For Human Drugs Submission Version 1.1 PDFDocument81 pagesGCC Data Requirements For Human Drugs Submission Version 1.1 PDFBasha Yazn Anjak50% (2)
- BuscopanDocument2 pagesBuscopancen janber cabrillosNo ratings yet
- Sterlity Validation (Membrane Filtration Method) in Pharmaceuticals - Pharmaceutical GuidelinesDocument4 pagesSterlity Validation (Membrane Filtration Method) in Pharmaceuticals - Pharmaceutical GuidelinesDucNo ratings yet
- DR Reddy's Lab - 27-11-2019 - IC - ULJK PDFDocument21 pagesDR Reddy's Lab - 27-11-2019 - IC - ULJK PDFP VinayakamNo ratings yet
- English RPSDocument3 pagesEnglish RPSChichiFauziyahNo ratings yet
- From, Janaki Ballava NahakDocument4 pagesFrom, Janaki Ballava NahakRamboNo ratings yet
- AutoGenomics Intl-AACC, LADocument63 pagesAutoGenomics Intl-AACC, LAmohdkhairNo ratings yet