Professional Documents
Culture Documents
5.5.2 Mott Insulators Versus Charge Transfer Insulators
5.5.2 Mott Insulators Versus Charge Transfer Insulators
Uploaded by
KetanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
5.5.2 Mott Insulators Versus Charge Transfer Insulators
5.5.2 Mott Insulators Versus Charge Transfer Insulators
Uploaded by
KetanCopyright:
Available Formats
5.
5 Correlated Insulators 243
5.5.2 Mott Insulators versus
Charge Transfer Insulators
Materials like COO,or La&uO4, are insulators because an electron cor-
relation effect splits the partially filled d-band into a set of either com-
pletely filled, or empty, Hubbard subbands. However, these subbands
are imbedded into a sequence of wider bands which are derived from the
oxygen 2p and the transition metal'' 49 states. The character of the in-
sulator is decided by the lowest excitation energy which is sufficient for
creating a charge carrier: this will appear as the activation energy in the
Arrhenius law for the electrical conductivity. The Mott-Hubbard gap is
not necessarily the smallest of the gaps; it is possible that the relevant
excitation is a charge transfer process between a Hubbard subband and
a wide band.
4 9
> 3d (lower) U
%
holes
T
3 3d (low.)
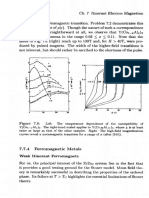
Figure 5.8: Shifting the relative positions of the Hubbard subbands and the 2p
band gives rise to different kinds of systems. In a Mott insulator (MI), the smallest
gap is between two Hubbard subbands. In a charge transfer insulator (CTI), the
lowest-energy process is the excitation of an electron from the 2p band to the upper
Hubbard subband. In both cases, the activation energy Aac is marked. In the right-
hand panel, the overlapping of the 21, band with the upper Hubbard subband gives
a metal (2pM) in which the dominant charge carriers are 2p holes. The horizontal
lines indicate the F'ermi level (FF.
Fig. 5.8 illustrates several of the possibilities, by inserting the Hub-
bard subbands into the midst of the wider bands. We should remember
'We do not bother to distinguish the noble metal Cu from transition metals; Cuat
is like the transition metal ions.
You might also like
- Magnetic Field of Air-Core ReactorsDocument8 pagesMagnetic Field of Air-Core Reactorshusa501100% (1)
- MemristorDocument18 pagesMemristorNaveed BashirNo ratings yet
- Correlated: InsulatorsDocument1 pageCorrelated: InsulatorsKetanNo ratings yet
- Inductors and Magnetic Properties: Jerry C. WhitakerDocument9 pagesInductors and Magnetic Properties: Jerry C. WhitakerArifNo ratings yet
- LearnEMC - Magnetic-Field Coupling (Inductive Coupling)Document3 pagesLearnEMC - Magnetic-Field Coupling (Inductive Coupling)danitranoster8512No ratings yet
- The Ideal Mos CapacitorDocument33 pagesThe Ideal Mos Capacitortennisbat91No ratings yet
- 3 - (2002) Thin Wire Representation in Finite Difference Time Domain Surge SimulationDocument8 pages3 - (2002) Thin Wire Representation in Finite Difference Time Domain Surge SimulationGuiNo ratings yet
- Chapter 12: Electrical Properties: Charge Carriers and ConductionDocument22 pagesChapter 12: Electrical Properties: Charge Carriers and ConductionJovan LlaneraNo ratings yet
- Dipole Antenna BasicsDocument3 pagesDipole Antenna BasicsEmil PatrickNo ratings yet
- Lecture 4: Interconnect RC: High Speed CMOS VLSI DesignDocument12 pagesLecture 4: Interconnect RC: High Speed CMOS VLSI DesignGyanaranjan NayakNo ratings yet
- Magnetic CircuitsDocument22 pagesMagnetic CircuitsHari NathNo ratings yet
- FormulaDocument7 pagesFormulahelenarajNo ratings yet
- Two Dimensional Electron Gas, Quantum Wells & Semiconductor SuperlatticesDocument41 pagesTwo Dimensional Electron Gas, Quantum Wells & Semiconductor SuperlatticesChang Jae LeeNo ratings yet
- Ae 212 Module 4Document9 pagesAe 212 Module 4kira arashiNo ratings yet
- Microwave Circuits: Stripline & MicrostripDocument29 pagesMicrowave Circuits: Stripline & MicrostripRocky BobNo ratings yet
- Chapter 2Document9 pagesChapter 2h_egNo ratings yet
- Circuit 2Document5 pagesCircuit 2richy launcherNo ratings yet
- VLSI2Document18 pagesVLSI2muzzammilanwarranchiNo ratings yet
- Ohmic ContactsDocument25 pagesOhmic Contactsbhaskar1026No ratings yet
- Analysis and Design of Transmission Line Structures by Means of The Geometric Mean Distance - IEEEDocument4 pagesAnalysis and Design of Transmission Line Structures by Means of The Geometric Mean Distance - IEEEJayson PatrickNo ratings yet
- Ground Wire OptimizationDocument20 pagesGround Wire OptimizationVăn Phạm NăngNo ratings yet
- Study of Wireless Charging Lane For Electric Vehicles: Jiongran Xiao, Eric Cheng, Norbert Cheung, Bo Zhang, J. F. PanDocument4 pagesStudy of Wireless Charging Lane For Electric Vehicles: Jiongran Xiao, Eric Cheng, Norbert Cheung, Bo Zhang, J. F. PanGaurav G PrabhuNo ratings yet
- 9.3 Brinkman-Rice Llansition 523 Not Affect The Global SymmetriesDocument1 page9.3 Brinkman-Rice Llansition 523 Not Affect The Global SymmetriesSupriyaNo ratings yet
- Br04 2005 Pes Shield Fem-McmDocument10 pagesBr04 2005 Pes Shield Fem-McmMiodrag MilutinovNo ratings yet
- 2-Metal Semiconductor ContactsDocument32 pages2-Metal Semiconductor ContactsSiddhesh GhodekarNo ratings yet
- Hand Out For Lab 1Document38 pagesHand Out For Lab 1tarekegn utaNo ratings yet
- Study Equivalent: On The Electrical Circuit Models of Polluted Outdoor InsulatorsDocument4 pagesStudy Equivalent: On The Electrical Circuit Models of Polluted Outdoor InsulatorsKamello AssisNo ratings yet
- Experiment5 SimulationStudyDocument12 pagesExperiment5 SimulationStudyRishikant KashyapNo ratings yet
- Shielded vs. Unshielded Square Magnetic Field Loops For EMI-ESD Design and TroubleshootingDocument26 pagesShielded vs. Unshielded Square Magnetic Field Loops For EMI-ESD Design and Troubleshootingagmnm1962No ratings yet
- Emi Properties of Passive Components: Crosstalk and CablingDocument30 pagesEmi Properties of Passive Components: Crosstalk and CablinggoliatcarroNo ratings yet
- Translate AndyDocument2 pagesTranslate Andybhye_anhNo ratings yet
- Objectives: To Learn The Fundamentals of Inductors and Varactors For RF CMOS Circuits, To LearnDocument13 pagesObjectives: To Learn The Fundamentals of Inductors and Varactors For RF CMOS Circuits, To LearnHubert Ekow AttahNo ratings yet
- Rosa 1908Document44 pagesRosa 1908joaomonterosNo ratings yet
- 11 - Nanoplasmonic Directional Couplers and Mach-Zehnder InterferometersDocument5 pages11 - Nanoplasmonic Directional Couplers and Mach-Zehnder InterferometersDr-Mandeep SinghNo ratings yet
- ch2 Raymond Winton Intermediate CircuitsDocument34 pagesch2 Raymond Winton Intermediate CircuitsTyler AnthonyNo ratings yet
- Practical Wide Frequency Approach For CalculatingDocument6 pagesPractical Wide Frequency Approach For Calculatingmichael gorgeNo ratings yet
- Single Electron Transistor (SET)Document8 pagesSingle Electron Transistor (SET)mokhaladNo ratings yet
- Doing Physics With Matlab: Static Magnetic Fields Biot-Savart Law Parallel WiresDocument21 pagesDoing Physics With Matlab: Static Magnetic Fields Biot-Savart Law Parallel WiresbeepeNo ratings yet
- Easy-to-Design Nano-Coupler Between Metal - Insulator-Metal Plasmonic and Dielectric Slab WaveguidesDocument6 pagesEasy-to-Design Nano-Coupler Between Metal - Insulator-Metal Plasmonic and Dielectric Slab WaveguidesDr-Mandeep SinghNo ratings yet
- Filter Inductor Design: Ac DCDocument28 pagesFilter Inductor Design: Ac DCkouroshNo ratings yet
- 2-D Magnetic Circuit Analysis For A Permanent Magnet Used in Laser Ablation Plume Expansion ExperimentsDocument11 pages2-D Magnetic Circuit Analysis For A Permanent Magnet Used in Laser Ablation Plume Expansion ExperimentsvijaymtrdcNo ratings yet
- Fundamentals of Electricity, Magnetism, and Circuits-Part 4: Cad/CamDocument13 pagesFundamentals of Electricity, Magnetism, and Circuits-Part 4: Cad/CamDedf †No ratings yet
- 2010 Stray Losses in Power Transformer Tank Walls and Construction Parts Miljavec Kralj Univ Ljubljana ICEMDocument4 pages2010 Stray Losses in Power Transformer Tank Walls and Construction Parts Miljavec Kralj Univ Ljubljana ICEMHaris RasoolNo ratings yet
- EMIC Unit 2 Part B Answers AVDocument30 pagesEMIC Unit 2 Part B Answers AVVigneswaran VigneshNo ratings yet
- P.S AssignDocument9 pagesP.S AssignYomifi AberaNo ratings yet
- Design of Hspice Model For Dual-Doped Memristor: Electronics and Communication EngineeringDocument6 pagesDesign of Hspice Model For Dual-Doped Memristor: Electronics and Communication EngineeringAshish JainNo ratings yet
- Transmission Lines CH 2Document12 pagesTransmission Lines CH 2Richard JoserNo ratings yet
- Alstom - Magnetic Field of Air-Core Reactors-Causes Effects and SolutionsDocument8 pagesAlstom - Magnetic Field of Air-Core Reactors-Causes Effects and SolutionsdenfordmuNo ratings yet
- 2 Introduction To Microwave Circuits and Transmission Line TheoryDocument15 pages2 Introduction To Microwave Circuits and Transmission Line TheoryYohannes NakachewNo ratings yet
- Twin Lead - ImpedanceDocument20 pagesTwin Lead - Impedancexerz_210% (1)
- AC Losses in High T Sub C SuperconductorsDocument6 pagesAC Losses in High T Sub C SuperconductorsJoseba BastarrarenaNo ratings yet
- Nbsbulletinv4n1p141 A2bDocument8 pagesNbsbulletinv4n1p141 A2bDiegoNo ratings yet
- Semiconductor StillDocument1 pageSemiconductor StillNurul Fahmi AriefNo ratings yet
- Accurate Analytic Formula For Calculation of Losses in Three-Core Submarine CablesDocument6 pagesAccurate Analytic Formula For Calculation of Losses in Three-Core Submarine Cablesgen liNo ratings yet
- Magnetization Processes in Wires in Orthogonal Fields: L.V.Panina, H.Katoh, and K.Mohri K.KawashimaDocument3 pagesMagnetization Processes in Wires in Orthogonal Fields: L.V.Panina, H.Katoh, and K.Mohri K.KawashimaAMS Lab.No ratings yet
- Shoc RectiferDocument3 pagesShoc RectiferAnand Satya KumarNo ratings yet
- Optimization of Wire Anode Distance From Pipe in Impressed Current Cathodic Protection SystemDocument9 pagesOptimization of Wire Anode Distance From Pipe in Impressed Current Cathodic Protection SystemsenthilkesavanpNo ratings yet
- Mathematical Model of High Voltage Sandwich Bus Duct: Vishal Ramnani, Mrunal Parekh, Vihang Dholakiya, Gaurang BuchDocument4 pagesMathematical Model of High Voltage Sandwich Bus Duct: Vishal Ramnani, Mrunal Parekh, Vihang Dholakiya, Gaurang BuchSaumyadeep DasNo ratings yet
- 2Document39 pages2Masood NizamiNo ratings yet
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- Digression: Symmetry Breaking (Or The Lack It) : (17, 3821: As We 1,2,. ADocument1 pageDigression: Symmetry Breaking (Or The Lack It) : (17, 3821: As We 1,2,. AKetanNo ratings yet
- 31 JLSL: 5 Mott InsulatorsDocument1 page31 JLSL: 5 Mott InsulatorsKetanNo ratings yet
- Ch. 5 Mott Insulators: (Cfatcfalcf6tcfblDocument1 pageCh. 5 Mott Insulators: (Cfatcfalcf6tcfblKetanNo ratings yet
- 7.7 Tkansition Metals and AlloysDocument1 page7.7 Tkansition Metals and AlloysKetanNo ratings yet
- Ofa As: !hasition andDocument1 pageOfa As: !hasition andKetanNo ratings yet
- The Problems: SolutionsDocument1 pageThe Problems: SolutionsKetanNo ratings yet
- G1, Glj2 Where ?-Spins Igl,. G L / 2) : 6.3 For EvenDocument1 pageG1, Glj2 Where ?-Spins Igl,. G L / 2) : 6.3 For EvenKetanNo ratings yet
- Solutions: ProblemsDocument1 pageSolutions: ProblemsKetanNo ratings yet
- '&xisition Metals: and AlloysDocument1 page'&xisition Metals: and AlloysKetanNo ratings yet
- I To A: Zlansition Metals and AlloysDocument1 pageI To A: Zlansition Metals and AlloysKetanNo ratings yet
- Itinerant Electron Magnetism: 1 of Is A A AsDocument1 pageItinerant Electron Magnetism: 1 of Is A A AsKetanNo ratings yet
- 7.7.2 LSDA Versus Lattice Fermion Models: Is ofDocument1 page7.7.2 LSDA Versus Lattice Fermion Models: Is ofKetanNo ratings yet
- Psat (442) (290) 1751: I Cannot Be The BareDocument1 pagePsat (442) (290) 1751: I Cannot Be The BareKetanNo ratings yet
- So We: ShouldDocument1 pageSo We: ShouldKetanNo ratings yet
- So Go: Itinerant Electron MagnetismDocument1 pageSo Go: Itinerant Electron MagnetismKetanNo ratings yet
- 7.7 Zhnsition Metals and Alloys 1 Also: So The Stoner Theory Is Not Valid in Its Original NaiveDocument1 page7.7 Zhnsition Metals and Alloys 1 Also: So The Stoner Theory Is Not Valid in Its Original NaiveKetanNo ratings yet
- 7.6 Spin: Problem 7.6Document1 page7.6 Spin: Problem 7.6KetanNo ratings yet
- Tiansition Metals and AlloysDocument1 pageTiansition Metals and AlloysKetanNo ratings yet
- 396 7 Itinerant Electron Magnetism: A So Pro-IsDocument1 page396 7 Itinerant Electron Magnetism: A So Pro-IsKetanNo ratings yet
- 33meaning The Nearest-Neighbour Hopping Model On A Hypercubic LatticeDocument1 page33meaning The Nearest-Neighbour Hopping Model On A Hypercubic LatticeKetanNo ratings yet
- 382 Ch. 7 Itinerant Electron Magnetism: RNQ (A) CosDocument1 page382 Ch. 7 Itinerant Electron Magnetism: RNQ (A) CosKetanNo ratings yet
- 7.7 Transition Metals and AlloysDocument1 page7.7 Transition Metals and AlloysKetanNo ratings yet
- L'Kansition Metals Alloys: &F-PKJDocument1 pageL'Kansition Metals Alloys: &F-PKJKetanNo ratings yet
- 7 Itinerant Electron Magnetism: (166) Felt Compelled To Conjecture That Any Ordering Away FromDocument1 page7 Itinerant Electron Magnetism: (166) Felt Compelled To Conjecture That Any Ordering Away FromKetanNo ratings yet
- 00396Document1 page00396KetanNo ratings yet
- Ch. 7 Itinerant Electron Magnetism: T,, U, U. TDocument1 pageCh. 7 Itinerant Electron Magnetism: T,, U, U. TKetanNo ratings yet
- 7.6 Spin Density Waves: (SDWI)Document1 page7.6 Spin Density Waves: (SDWI)KetanNo ratings yet
- Pm-Pi: Away From Half-FillingDocument1 pagePm-Pi: Away From Half-FillingKetanNo ratings yet
- 7.6 Spin Density: SDW MottDocument1 page7.6 Spin Density: SDW MottKetanNo ratings yet
- 7.6.3 Strong Coupling LimitDocument1 page7.6.3 Strong Coupling LimitKetanNo ratings yet